Valve-in-Valve-in-Valve: Degenerated Transcatheter Heart Valve within Degenerated Surgical Bioprosthetic Aortic Valve Treated with Second Transcatheter Heart Valve
Abstract
1. Introduction
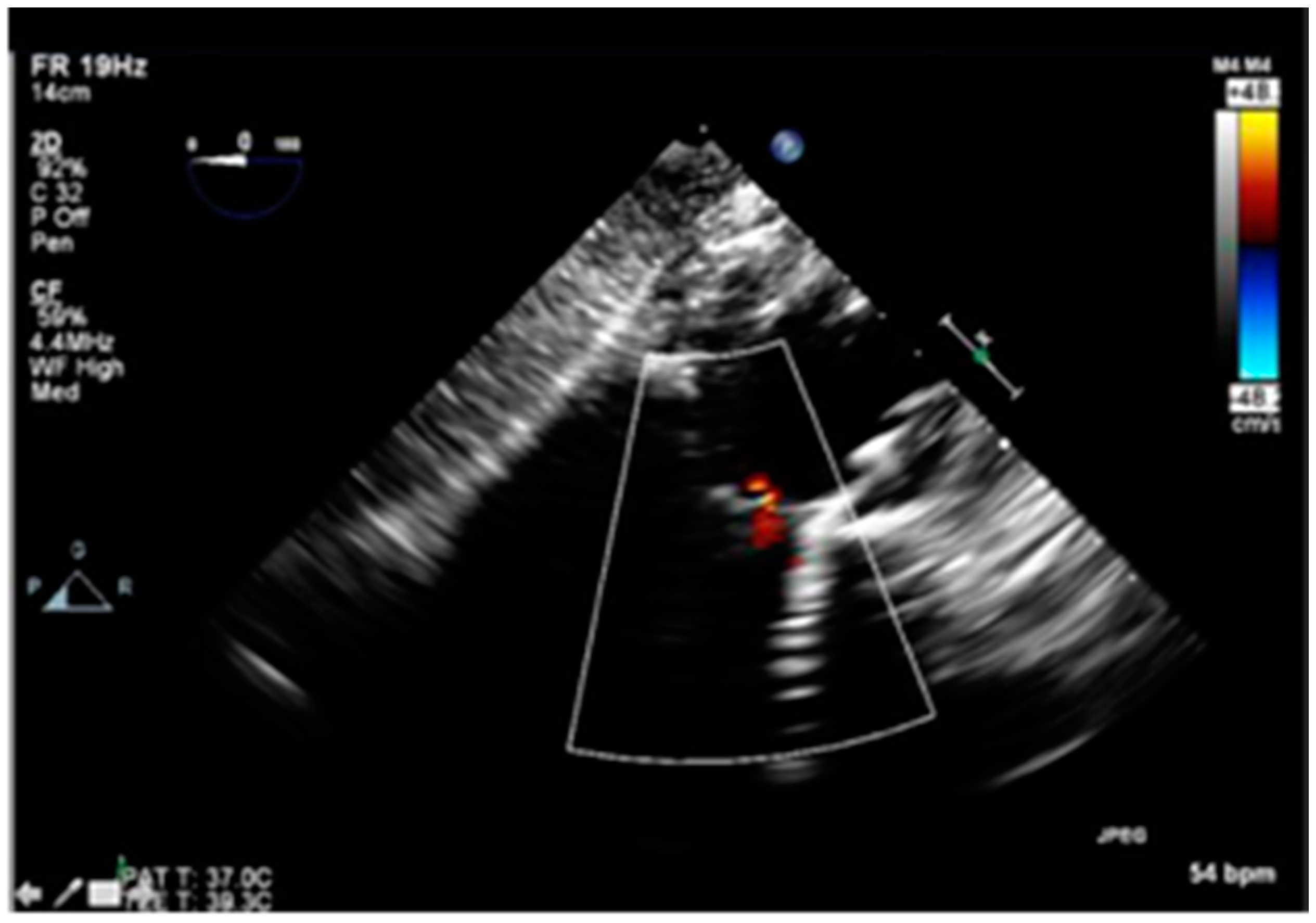
2. History
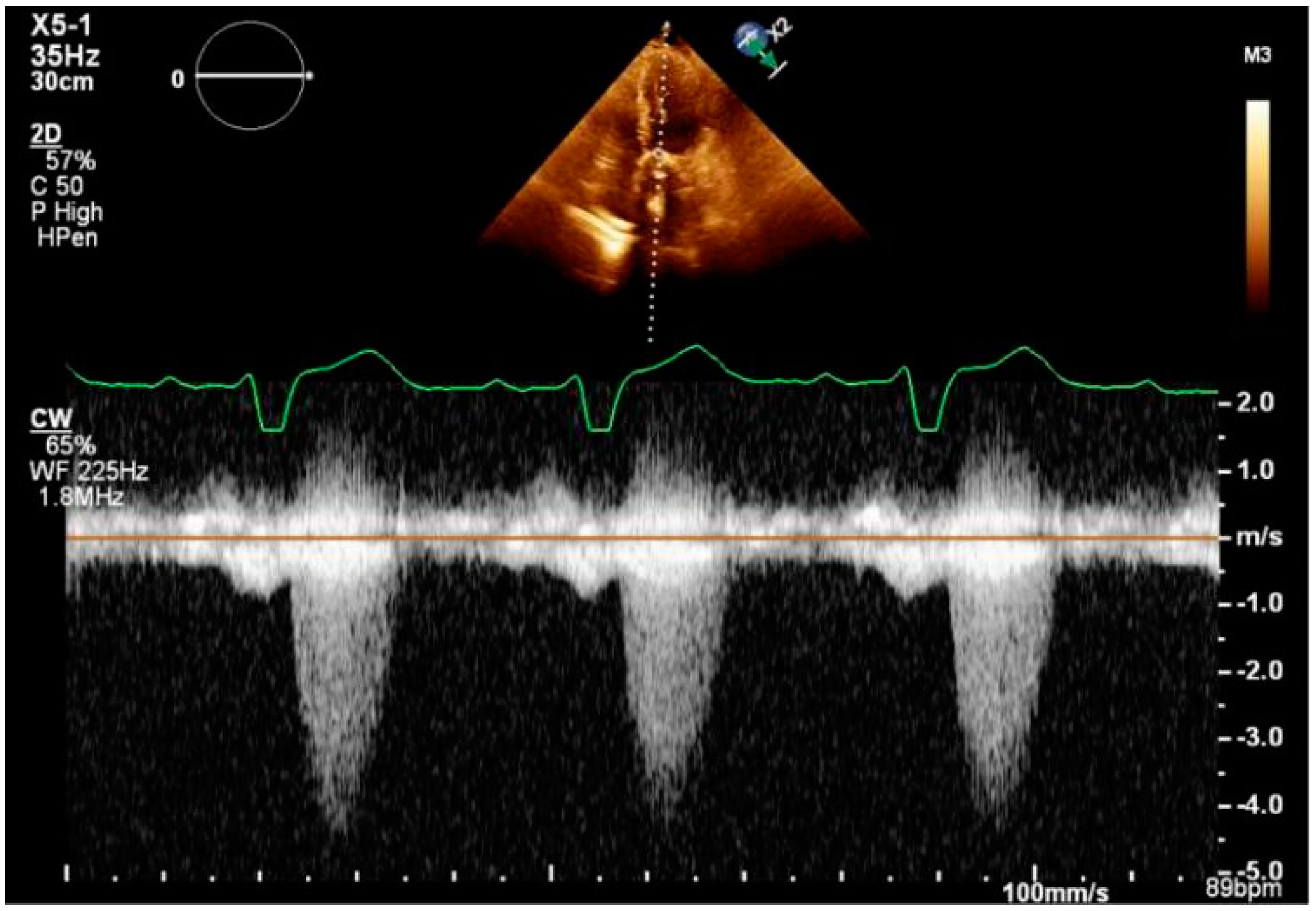
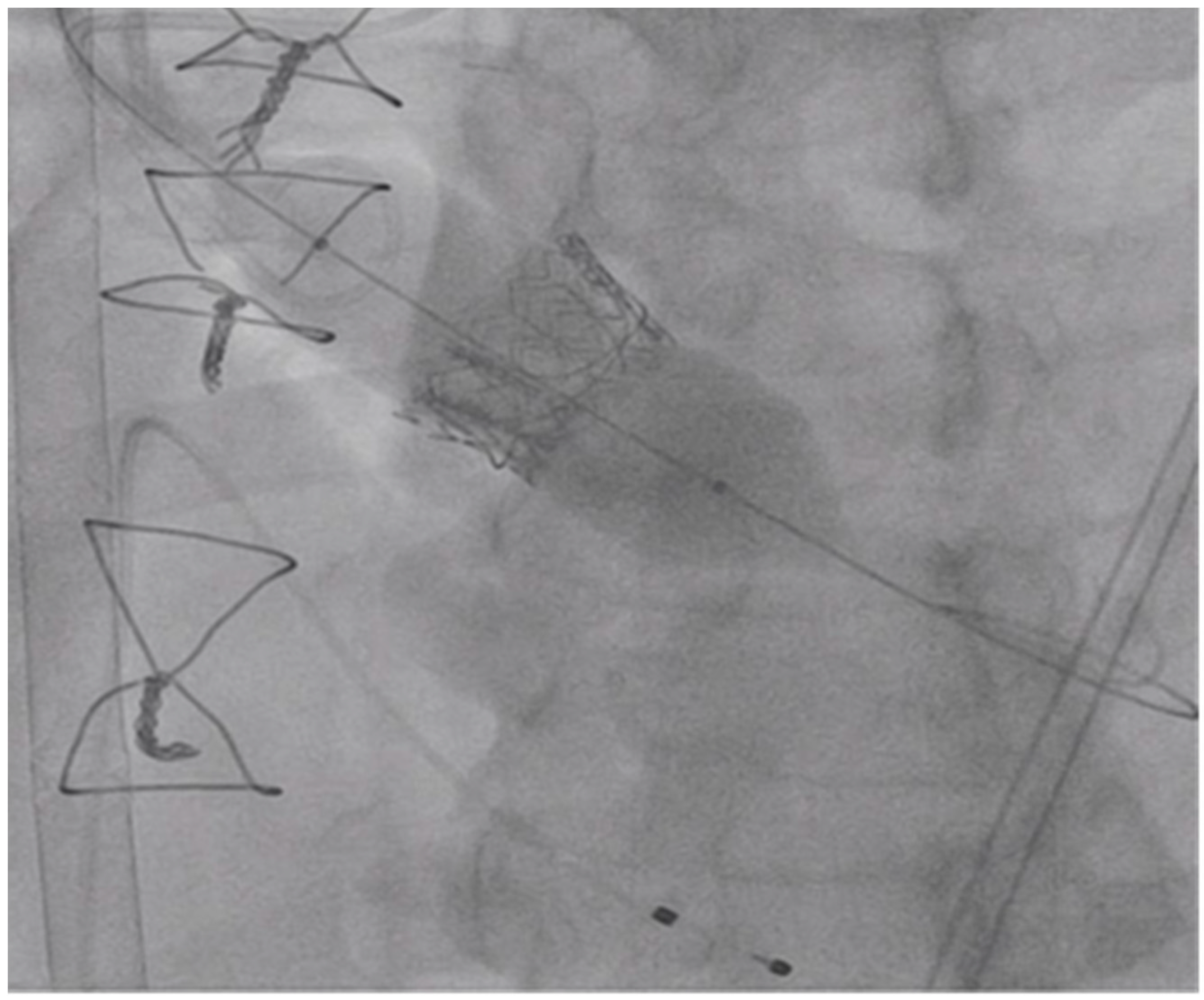
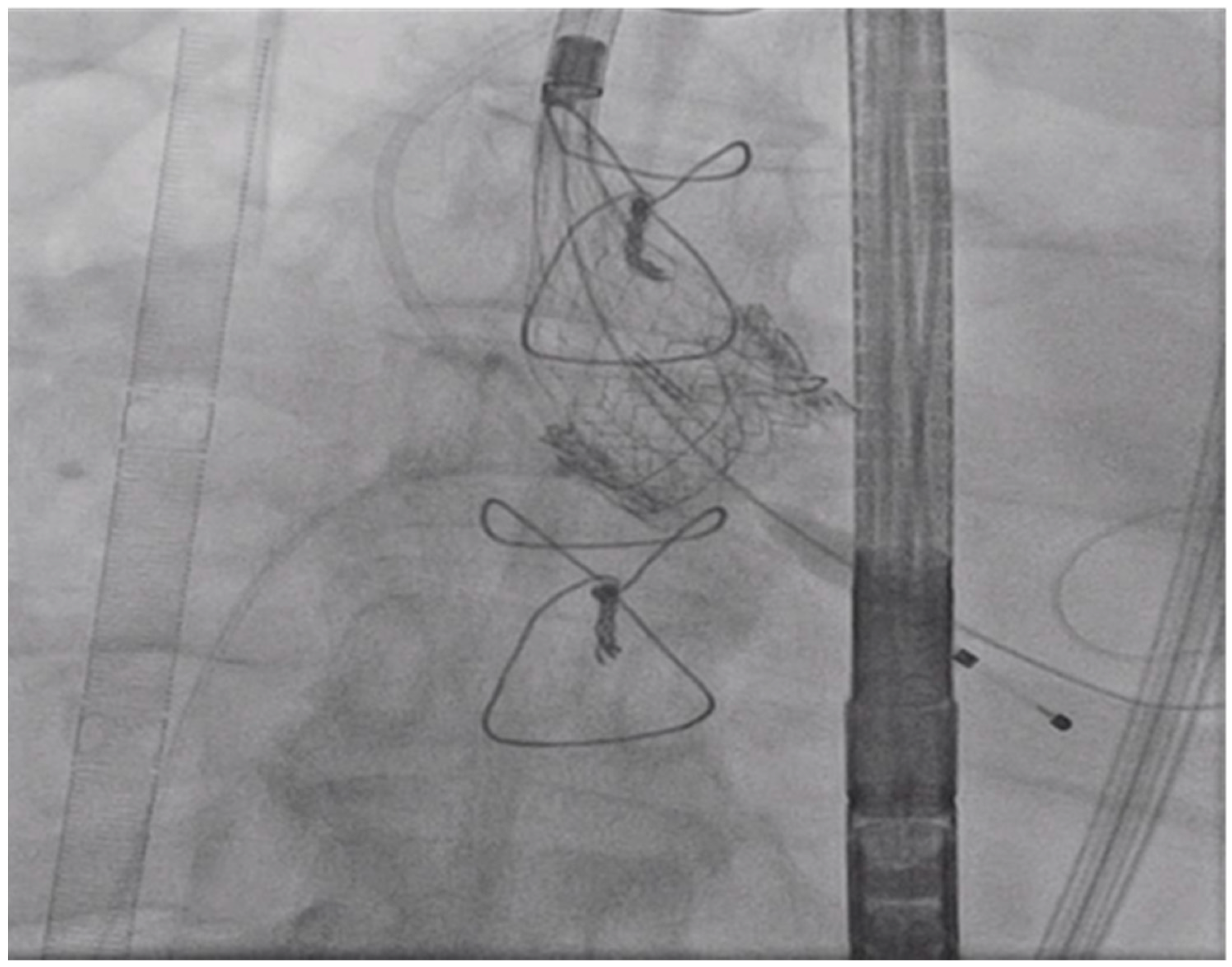
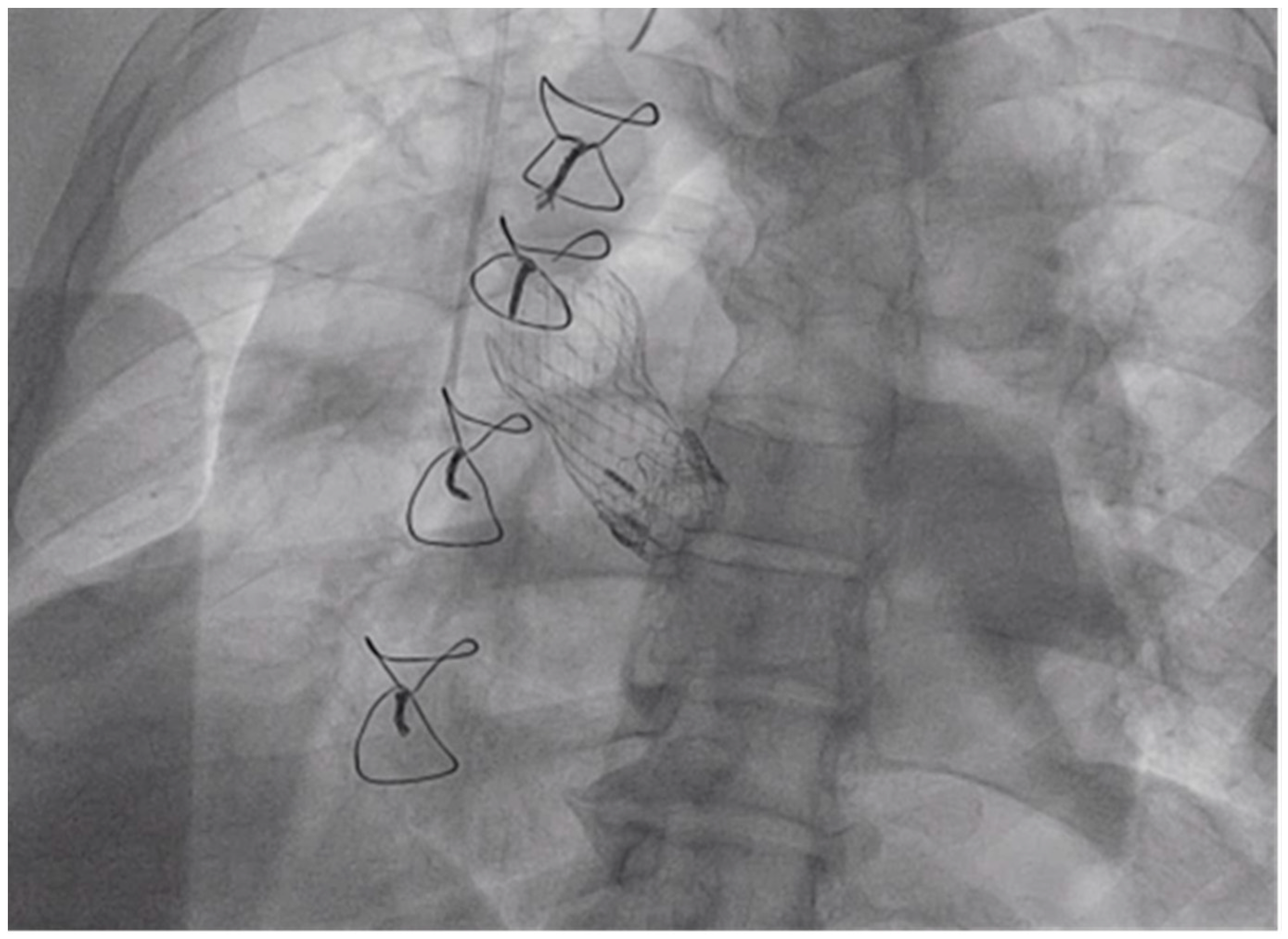
3. Procedure
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thourani, V.H.; Suri, R.M.; Gunter, R.L.; Sheng, S.; O’Brien, S.M.; Ailawadi, G.; Szeto, W.Y.; Dewey, T.M.; Guyton, R.A.; Bavaria, J.E.; et al. Contemporary real-world outcomes of surgical aortic valve replacement in 141,905 low-risk, intermediate-risk, and high-risk patients. Ann. Thorac. Surg. 2015, 99, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, A.J.; Shuhaiber, J.; Salemi, A.; Isom, O.W.; Sedrakyan, A. National trends in utilization and in-hospital outcomes of mechanical versus bioprosthetic aortic valve replacements. J. Thorac. Cardiovasc. Surg. 2015, 149, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.M.; Edwards, F.H.; Zhao, Y.; O’Brien, S.; Booth, M.E.; Dokholyan, R.S.; Douglas, P.S.; Peterson, E.D.; DEcIDE AVR (Developing Evidence to Inform Decisions about Effectiveness–Aortic Valve Replacement) Research Team. Long-term safety and effectiveness of mechanical versus biologic aortic valve prostheses in older patients: Results from the society of thoracic surgeons adult cardiac surgery national database. Circulation 2013, 127, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.G.; Mack, M.J.; White, J.M.; Dvir, D.; Blanke, P.; Herrmann, H.C.; Leipsic, J.; Kodali, S.K.; Makkar, R.; Miller, D.C.; et al. Transcatheter aortic valve implantation within degenerated aortic surgical bioprostheses, partner 2 valve-in-valve registry. J. Am. Coll. Cardiol. 2017, 69, 2253–2262. [Google Scholar] [CrossRef] [PubMed]
- Dvir, D. First look at long-term durability of transcatheter heart valves: Assessment of valve function up to 10 years after implantation. Available online: https://www.crtonline.org/presentation-detail/first-look-at-long-term-durability-of-transcathete (accessed on 1 March 2020).
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J. Am. Coll. Cardiol. 2014, 63, e57–e185. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Alfieri, O.; Andreotti, F.; Antunes, M.J.; Barón-Esquivias, G.; Baumgartner, H.; Borger, M.A.; Carrel, T.P.; de Bonis, M.; Evangelista, A.; et al. Guidelines on the management of valvular heart disease (version 2012): The joint task force on the management of valvular heart disease of the european society of cardiology (esc) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. J. Cardiothorac. Surg. 2012, 42, S1–S44. [Google Scholar]
- Rodriguez-Gabella, T.; Voisine, P.; Puri, R.; Pibarot, P.; Rodés-Cabau, J. Aortic bioprosthetic valve durability: Incidence, mechanisms, predictors, and management of surgical and transcatheter valve degeneration. J. Am. Coll. Cardiol. 2017, 70, 1013–1028. [Google Scholar] [CrossRef] [PubMed]
- Balsam, L.B.; Grossi, E.A.; Greenhouse, D.G.; Ursomanno, P.; Deanda, A.; Ribakove, G.H.; Culliford, A.T.; Galloway, A.C. Reoperative valve surgery in the elderly: Predictors of risk and long-term survival. Ann. Thorac. Surg. 2010, 90, 1195–1200, discussion 1201. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Loberman, D.; Gosev, I. Reoperative aortic valve replacement in the octogenarians-minimally invasive technique in the era of transcatheter valve replacement. J. Thorac. Cardiovasc. Surg. 2014, 147, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Vassileva, C.M.; Englum, B.; Kim, S.; Yammine, M.; Brennan, M.; Suri, R.M.; Thourani, V.H.; Jacobs, J.P.; Aranki, S. Contemporary outcomes of repeat aortic valve replacement: A benchmark for transcatheter valve-in-valve procedures. Ann. Thorac. Surg. 2015, 100, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Rahimtoola, S.H. The problem of valve prosthesis-patient mismatch. Circulation 1978, 58, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Saxon, J.T. Bioprosthetic valve fracture during valve-in-valve TAVR: Bench to bedside. Interv. Cardiol. Rev. 2018, 13, 20–26. [Google Scholar] [CrossRef]
- Allen, K.B.; Chhatriwalla, A.K.; Cohen, D.J.; Chhatriwalla, A.K. Bioprosthetic valve fracture to facilitate transcatheter valve-in-valve implantation. Ann. Thorac. Surg. 2017, 104, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Chhatriwalla, A.K.; Allen, K.B.; Saxon, J.T.; Cohen, D.J.; Aggarwal, S.; Hart, A.J.; Baron, S.J.; Dvir, D.; Borkon, A.M. Bioprosthetic valve fracture improves the hemodynamic results of valve-in-valve transcatheter aortic valve replacement. Circ. Cardiovasc. Interv. 2017, 10, e005216. [Google Scholar] [CrossRef] [PubMed]
- Nielsen-Kudsk, J.E.; Andersen, A.; Therkelsen, C.J.; Christensen, E.H.; Jensen, K.T.; Krusell, L.R.; Tang, M.; Terp, K.A.; Klaaborg, K.; Greisen, J.R.; et al. High-pressure balloon fracturing of small dysfunctional mitroflow bioprostheses facilitates transcatheter aortic valve-in-valve implantation. EuroIntervention 2017, 13, e1020–e1025. [Google Scholar] [CrossRef] [PubMed]
- Johansen, P.; Engholt, H.; Tang, M.; Nybo, R.F.; Rasmussen, P.D.; Nielsen-Kudsk, J.E. Fracturing mechanics before valve-in-valve therapy of small aortic bioprosthetic heart valves. EuroIntervention 2017, 13, e1026–e1031. [Google Scholar] [CrossRef] [PubMed]
- Dvir, D.; Webb, J.G.; Bleiziffer, S.; Pasic, M.; Waksman, R.; Kodali, S.; Barbanti, M.; Latib, A.; Schaefer, U.; Rodés-Cabau, J. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA 2014, 312, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; Zhao, D.F.; Wang, N.; Huo, Y.R.; di Eusanio, M.; Yan, T.D. Transcatheter valve-in-valve implantation versus reoperative conventional aortic valve replacement: A systematic review. J. Thorac. Dis. 2016, 8, E83–E93. [Google Scholar] [PubMed]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J. Am. Coll. Cardiol. 2017, 70, 252–289. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajmal, M.; Reddy, S.; Shetty, R.; Kazui, T.; Lotun, K. Valve-in-Valve-in-Valve: Degenerated Transcatheter Heart Valve within Degenerated Surgical Bioprosthetic Aortic Valve Treated with Second Transcatheter Heart Valve. Reports 2020, 3, 7. https://doi.org/10.3390/reports3020007
Ajmal M, Reddy S, Shetty R, Kazui T, Lotun K. Valve-in-Valve-in-Valve: Degenerated Transcatheter Heart Valve within Degenerated Surgical Bioprosthetic Aortic Valve Treated with Second Transcatheter Heart Valve. Reports. 2020; 3(2):7. https://doi.org/10.3390/reports3020007
Chicago/Turabian StyleAjmal, Muhammad, Sridhar Reddy, Ranjith Shetty, Toshinobu Kazui, and Kapildeo Lotun. 2020. "Valve-in-Valve-in-Valve: Degenerated Transcatheter Heart Valve within Degenerated Surgical Bioprosthetic Aortic Valve Treated with Second Transcatheter Heart Valve" Reports 3, no. 2: 7. https://doi.org/10.3390/reports3020007
APA StyleAjmal, M., Reddy, S., Shetty, R., Kazui, T., & Lotun, K. (2020). Valve-in-Valve-in-Valve: Degenerated Transcatheter Heart Valve within Degenerated Surgical Bioprosthetic Aortic Valve Treated with Second Transcatheter Heart Valve. Reports, 3(2), 7. https://doi.org/10.3390/reports3020007



