Gastro-Splenic Fistula Related to Large B Cell Lymphoma
Abstract
1. Introduction
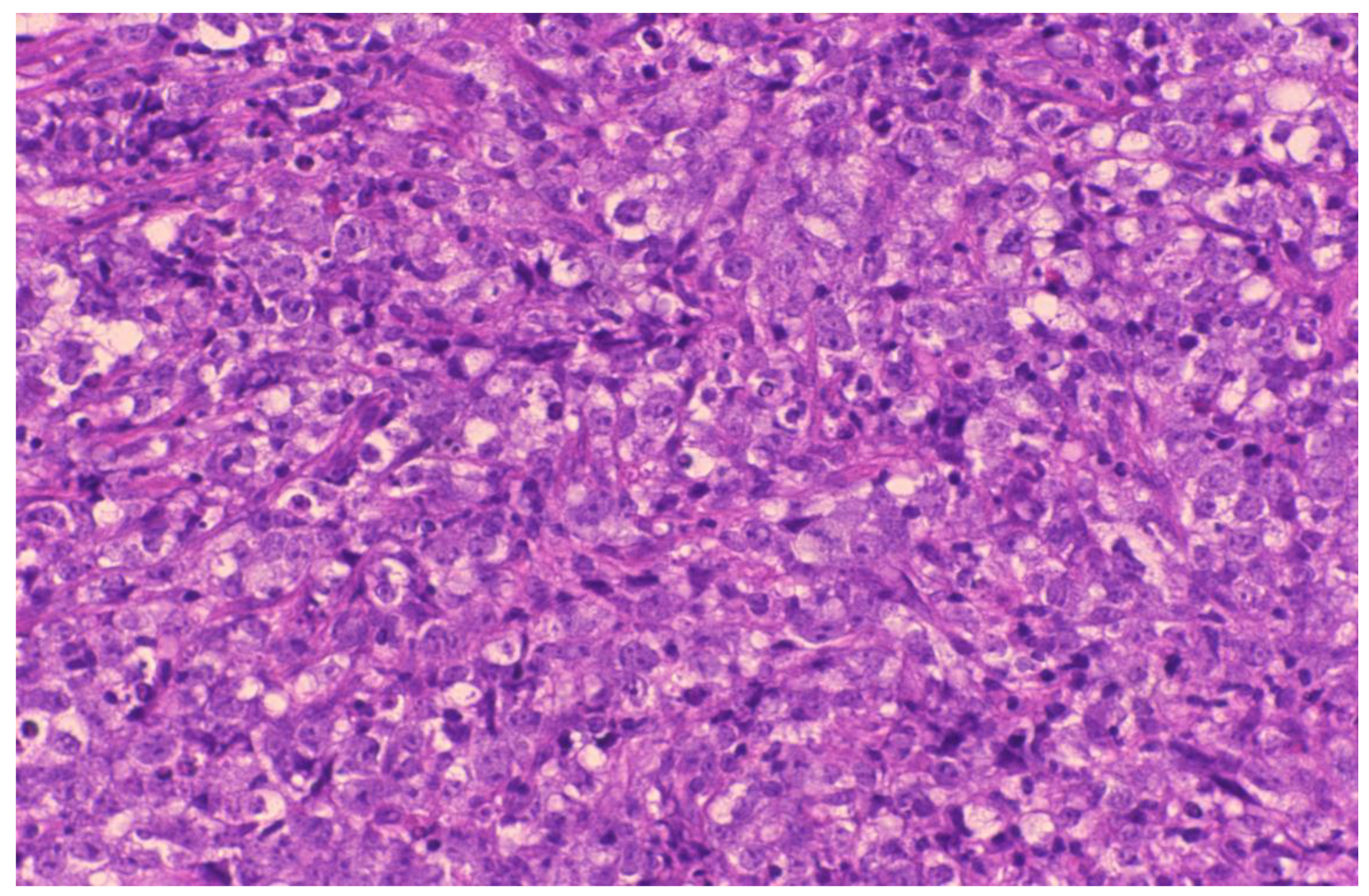
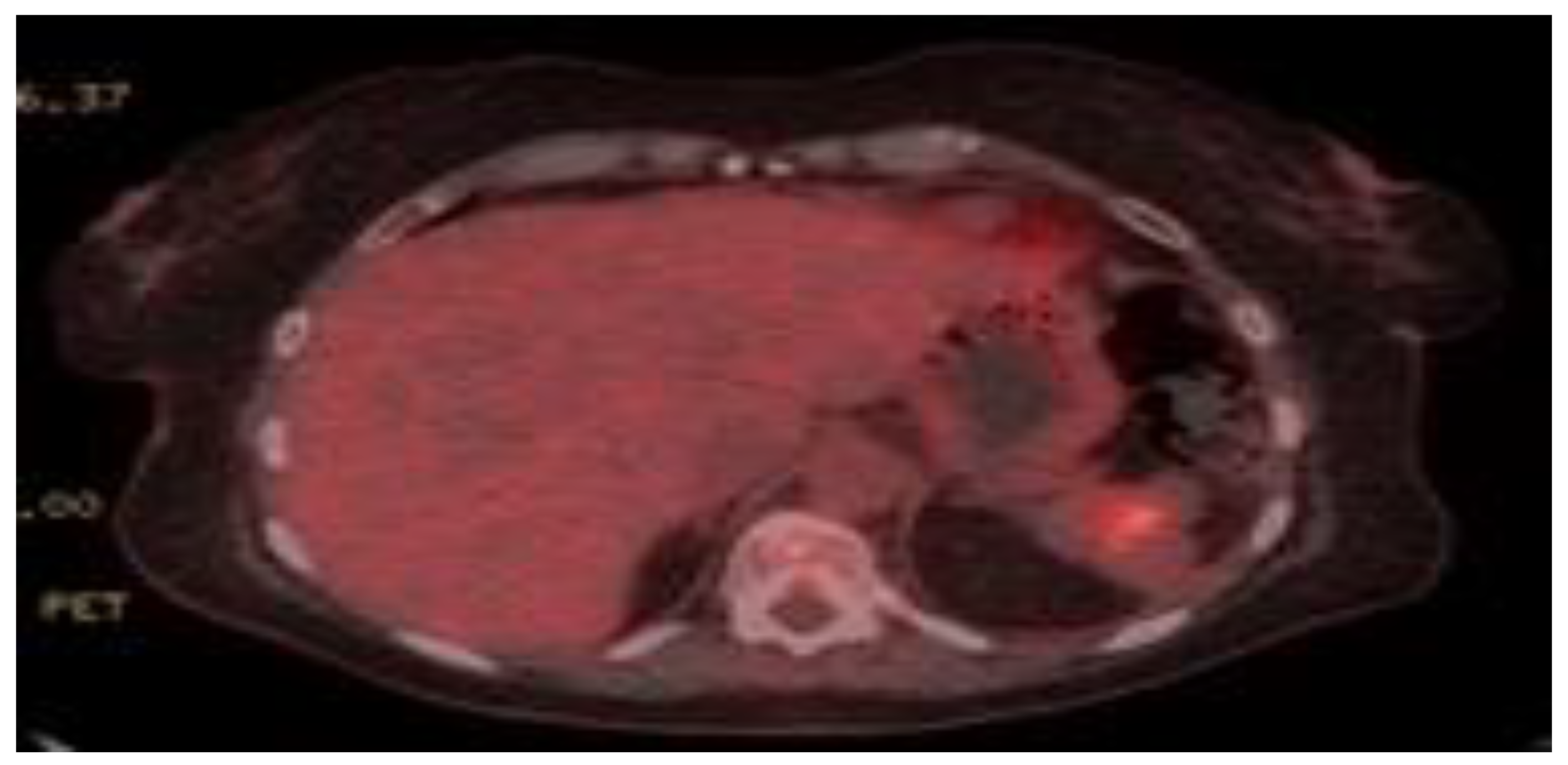
2. Case Presentation
3. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Scoville, A.; Bovy, P.; Demeester, P. Radiological splenomegaly by lymphosarcoma splenic necrosing with double fistulation in the digestive tract. Acta Gastroenterol. Belg. 1967, 30, 840–846. [Google Scholar]
- Ding, Y.-L.; Wang, S.-Y. Gastrosplenic fistula due to splenic large B-cell lymphoma. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2012, 17, 805–807. [Google Scholar]
- Kang, D.H.; Huh, J.; Lee, J.H.; Jeong, Y.K.; Cha, H.J. Gastrosplenic fistula occurring in lymphoma patients: Systematic review with a new case of extranodal NK/T-cell lymphoma. World J. Gastroenterol. 2017, 23, 6491–6499. [Google Scholar] [CrossRef] [PubMed]
- A Carolin, K.; Prakash, S.H.; Silva, Y.J. Gastrosplenic fistulas: a case report and review of the literature. Am. Surg. 1997, 63, 1007–1010. [Google Scholar] [PubMed]
- Aribaş, B.K.; Başkan, E.; Altinyollar, H.; Ungül, U.; Cengiz, A.; Erdil, H.F. Gastrosplenic fistula due to splenic large cell lymphoma diagnosed by percutaneous drainage before surgical treatment. Turk. J. Gastroenterol. 2008, 19, 69–70. [Google Scholar]
- Jain, V.; Pauli, E.; Sharzehi, K.; Moyer, M. Spontaneous gastrosplenic fistula secondary to diffuse large B-cell lymphoma. Gastrointest. Endosc. 2011, 73, 608–609. [Google Scholar] [CrossRef] [PubMed]
- Seib, C.D.; Rocha, F.G.; Hwang, D.G.; Shoji, B.T. Gastrosplenic Fistula from Hodgkin’s Lymphoma. J. Clin. Oncol. 2009, 27, e15–e17. [Google Scholar] [CrossRef]
- Marín, A.G.; García, L.B.; Rodríguez, A.V.; L, M.V.; Fuentes, F.T. Spontaneous gastrosplenic fistula secondary to primary gastric lymphoma. Rev. Esp. Enferm. Dig. 2009, 101, 76–78. [Google Scholar]
- Yang, S.E.; Jin, J.-Y.; Song, C.W.; Park, J.C.; Lee, J.I.; Kim, W.; Kim, J.; Lee, H.G. Gastrosplenic Fistula Complicated in a Patient with Non- Hodgkin’s Lymphoma. Cancer Res. Treat. 2002, 34, 153–156. [Google Scholar] [CrossRef]
- Moghazy, K. Gastrosplenic fistula following chemotherapy for lymphoma. Gulf J. Oncol. 2008, 3, 64–67. [Google Scholar]
- Puppala, S.; Williams, R.; Harvey, J.; Crane, M. Spontaneous gastrosplenic fistula in primary gastric lymphoma: case report and review of literature. Clin. Radiol. Extra 2005, 60, 20–22. [Google Scholar] [CrossRef]
- Maillo, C.; Bau, J. [Gastrosplenic and thoracosplenic fistula due to primary untreated splenic lymphoma]. Rev. Esp. Enferm. Dig. 2009, 101, 222–223. [Google Scholar] [PubMed]
- Palmowski, M.; Zechmann, C.M.; Satzl, S.; Bartling, S.; Hallscheidt, P. Large gastrosplenic fistula after effective treatment of abdominal diffuse large-B-cell lymphoma. Ann. Hematol. 2007, 87, 337–338. [Google Scholar] [CrossRef]
- Rothermel, L.D.; Chadwick, C.L.; Thambi-Pillai, T. Gastrosplenic fistula: etiologies, diagnostic studies, and surgical management. Int. Surg. 2010, 95, 270–272. [Google Scholar] [PubMed]
- A Bird, M.; Amjadi, D.; Behrns, K.E. Primary splenic lymphoma complicated by hematemesis and gastric erosion. South. Med. J. 2002, 95, 941–942. [Google Scholar] [CrossRef]
- Harris, N.L.; Aisenberg, A.C.; Meyer, J.E.; Ellman, L.; Elman, A. Diffuse large cell (Histiocytic) lymphoma of the spleen: Clinical and pathologic characteristics of ten cases. Cancer 1984, 54, 2460–2467. [Google Scholar] [CrossRef]
- Hiltunen, K.-M.; Airo, I.; Mattila, J.; Helve, O. Massively Bleeding Gastrosplenic Fistula Following Cytostatic Chemotherapy of a Malignant Lymphoma. J. Clin. Gastroenterol. 1991, 13, 478–480. [Google Scholar] [CrossRef]
- Kerem, M.; Sakrak, O.; Yilmaz, T.U.; Gultekin, F.A.; Bedirli, A.; Dursun, A. Spontaneous Gastrosplenic Fistula in Primary Gastric Lymphoma: Surgical Management. Asian J. Surg. 2006, 29, 287–290. [Google Scholar] [CrossRef]
- Choi, J.E.; Chung, H.J.; Lee, H.G. Spontaneous gastrosplenic fistula: a rare complication of splenic diffuse large cell lymphoma. Abdom. Imaging 2002, 27, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Bubenik, O.; Lopez, M.J.; Greco, A.O.; Kraybill, W.G.; Cherwitz, D.L. Gastrosplenic fistula following successful chemotherapy for disseminated histiocytic lymphoma. Cancer 1983, 52, 994–996. [Google Scholar] [CrossRef]
- Blanchi, A.; Bour, B.; Alami, O. Spontaneous gastrosplenic fistula revealing high-grade centroblastic lymphoma: Endoscopic findings. Gastrointest. Endosc. 1995, 42, 587–589. [Google Scholar] [CrossRef]
- Al-Ashgar, H.I.; Khan, M.Q.; Ghamdi, A.M.; Bamehriz, F.Y.; Maghfoor, I. Gastrosplenic fistula in Hodgkin’s lymphoma treated successfully by laparoscopic surgery and chemotherapy. Saudi Med. J. 2007, 28, 1898–1900. [Google Scholar] [PubMed]
- Cary, E.R.; Tremaine, W.J.; Banks, P.M.; Nagorney, D.M. Isolated Crohn’s disease of the stomach. Mayo Clin. Proc. 1989, 64, 776–779. [Google Scholar] [CrossRef]
- Ching, J.L.C.; Owen, M.J.; Heller, C.A. Radiological gastric filling defect due to penetration into the spleen by a large gastric ulcer. Br. J. Radiol. 1983, 56, 488–489. [Google Scholar] [CrossRef] [PubMed]
- Glick, S.N.; Levine, M.S.; Teplick, S.K.; Gasparaitis, A. Splenic penetration by benign gastric ulcer: Preoperative recognition with CT. Radiology 1987, 163, 637–639. [Google Scholar] [CrossRef]
- Joffe, N.; Antonioli, D.A. Penetration into spleen by benign gastric ulcer. Clin. Radiol. 1981, 32, 177–181. [Google Scholar] [CrossRef]
- Frenkel, A.; Bichovsky, Y.; Perry, Z.H.; Peiser, J.; Roy-Shapira, A.; Brotfain, E.; Koyfman, L.; Binyamin, Y.; Nalbandyan, K.; Klein, M. Management of gastrosplenic fistula in the emergency setting—A case report and review of the literature. Ann. Med. Surg. 2018, 29, 26–29. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triantafyllopoulou, D.; Gkikas, I.; Adiyodi, J.; Crossingham, I.; Al-Islam, S.; Alam, M.S.; Sahasrabudhe, N.; Kausar, A.; Ayub, A.B.; Cowburn, H.; et al. Gastro-Splenic Fistula Related to Large B Cell Lymphoma. Reports 2020, 3, 17. https://doi.org/10.3390/reports3020017
Triantafyllopoulou D, Gkikas I, Adiyodi J, Crossingham I, Al-Islam S, Alam MS, Sahasrabudhe N, Kausar A, Ayub AB, Cowburn H, et al. Gastro-Splenic Fistula Related to Large B Cell Lymphoma. Reports. 2020; 3(2):17. https://doi.org/10.3390/reports3020017
Chicago/Turabian StyleTriantafyllopoulou, Diana, Ioannis Gkikas, Jagdish Adiyodi, Iain Crossingham, Shofiq Al-Islam, Muhammad Shahbaz Alam, Neil Sahasrabudhe, Ambareen Kausar, Ali Bin Ayub, Hazel Cowburn, and et al. 2020. "Gastro-Splenic Fistula Related to Large B Cell Lymphoma" Reports 3, no. 2: 17. https://doi.org/10.3390/reports3020017
APA StyleTriantafyllopoulou, D., Gkikas, I., Adiyodi, J., Crossingham, I., Al-Islam, S., Alam, M. S., Sahasrabudhe, N., Kausar, A., Ayub, A. B., Cowburn, H., Fox, L., Punekar, M., Macheta, M., & Tooze, R. (2020). Gastro-Splenic Fistula Related to Large B Cell Lymphoma. Reports, 3(2), 17. https://doi.org/10.3390/reports3020017




