Pyrolysis Kinetics and Gas Evolution of Flame-Retardant PVC and PE: A TG-FTIR-GC/MS Study
Abstract
1. Introduction
- Existing research primarily focuses on conventional PVC and PE materials, with limited investigation into the pyrolysis behavior and volatile product release patterns of flame-retardant PVC and PE. In particular, there is a lack of systematic analysis of gas evolution at different pyrolysis stages.
- Current studies predominantly rely on single techniques such as TG or TG-FTIR. While these methods effectively analyze mass loss trends and some volatile product characteristics, they fall short in accurately identifying and quantifying complex gaseous products.
2. Materials and Methods
2.1. Materials
2.2. TG-FTIR-GC/MS Tests
2.3. Kinetic Methods
2.3.1. Model-Free Methods
2.3.2. Model-Fitting Methods
2.3.3. Classification and Regression Tree
3. Results and Discussion
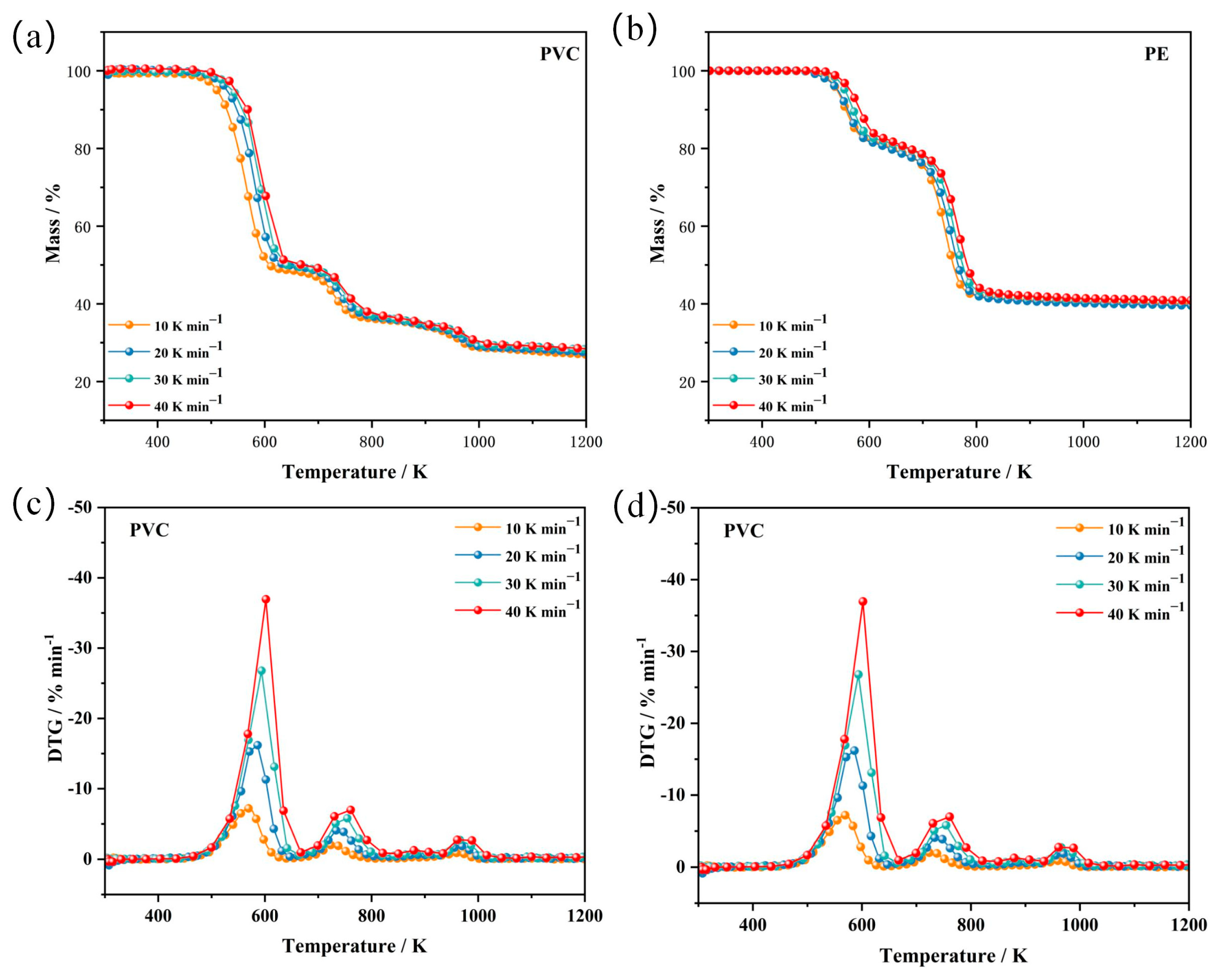
3.1. Thermogravimetric Analysis
3.2. Kinetic Analysis
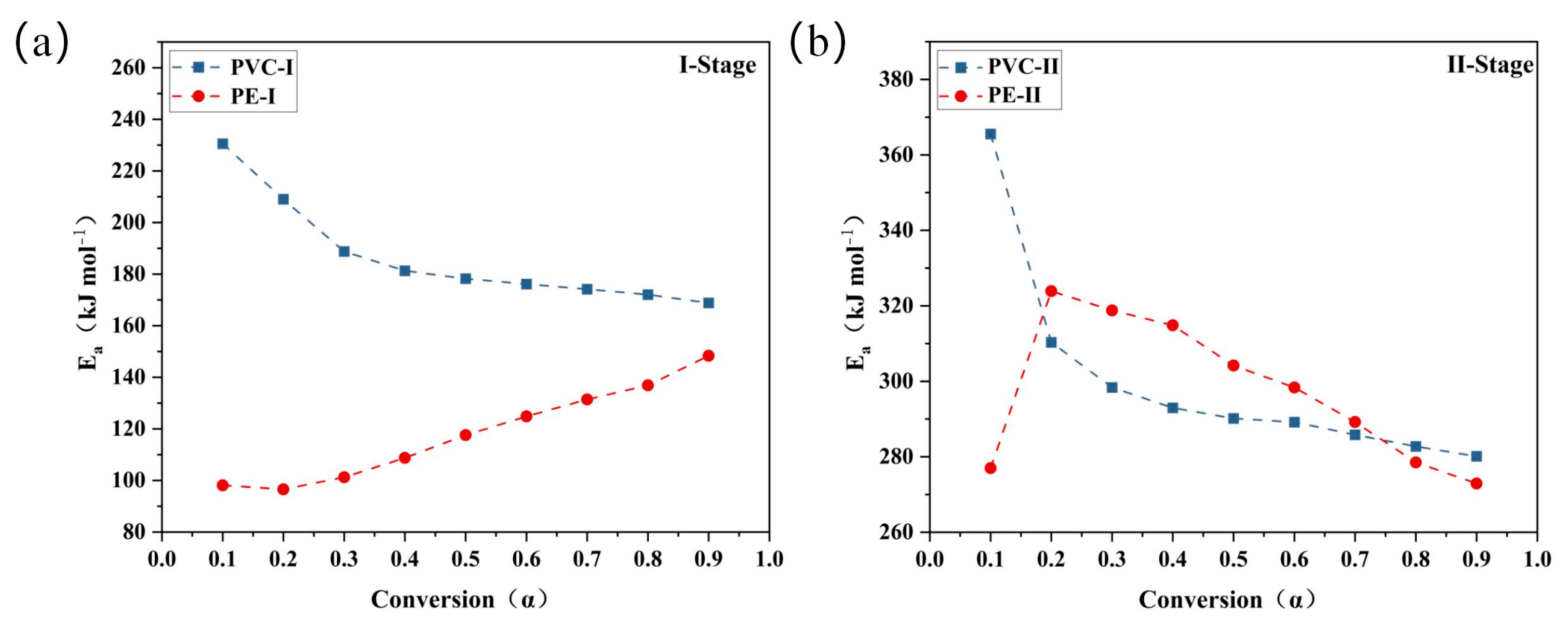
3.2.1. Kinetic Analysis by Model-Free Methods
3.2.2. Kinetic Analysis by Model-Fitting Method
3.3. Prediction of Thermal Decomposition Using the CART Model
3.4. Micro-Scale Pyrolysis Gas Analysis
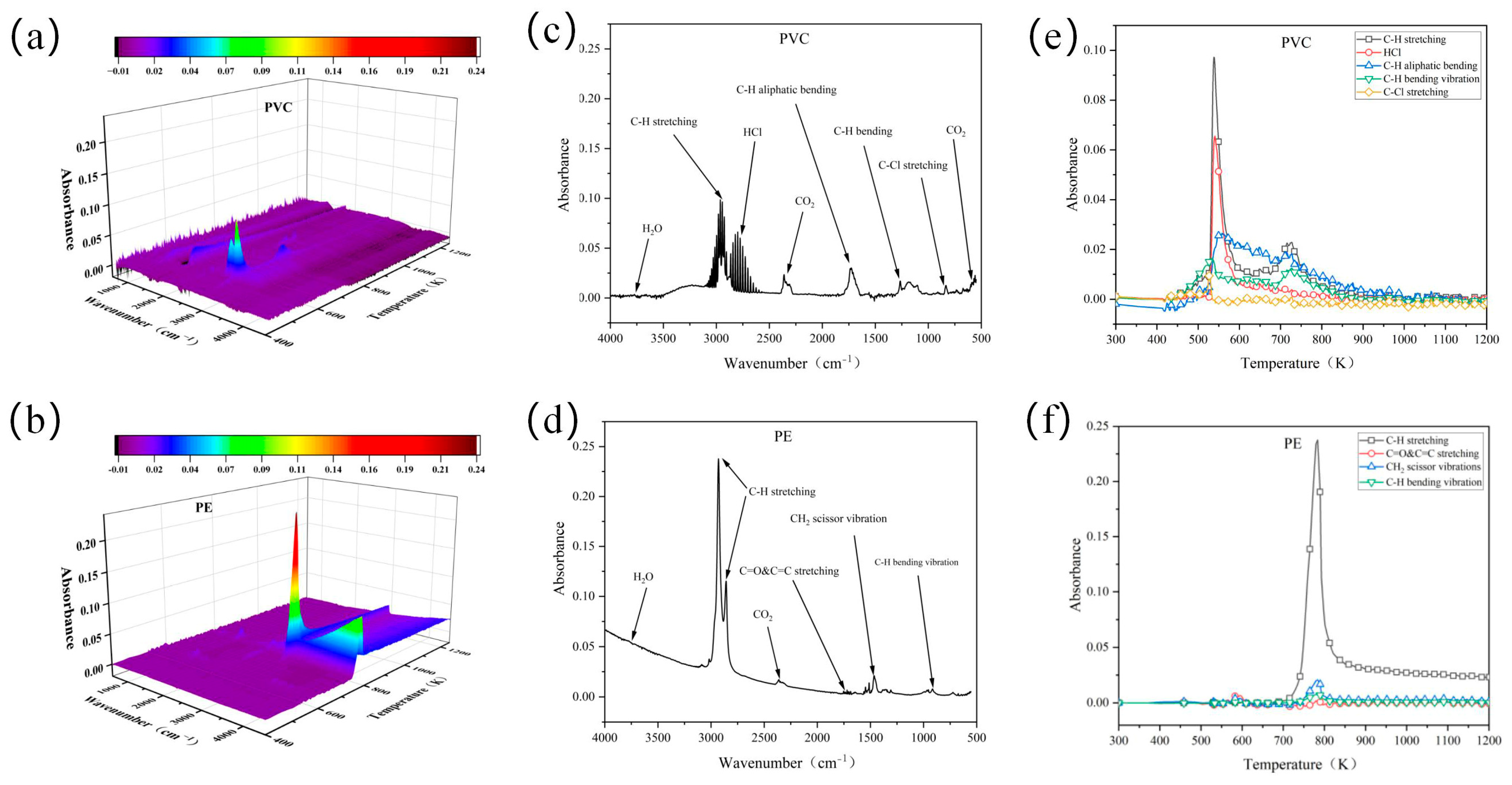
3.4.1. FTIR Analysis
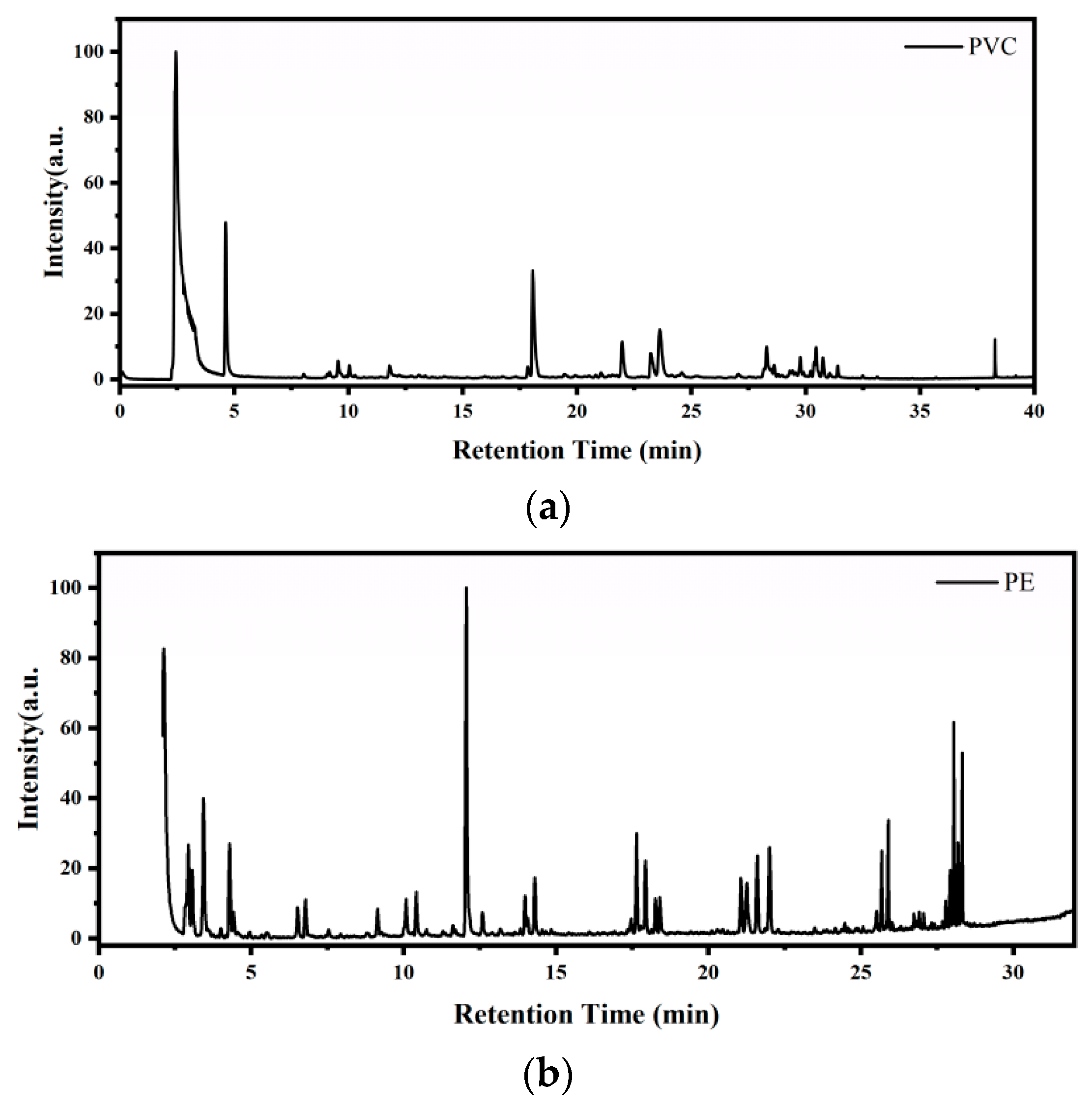
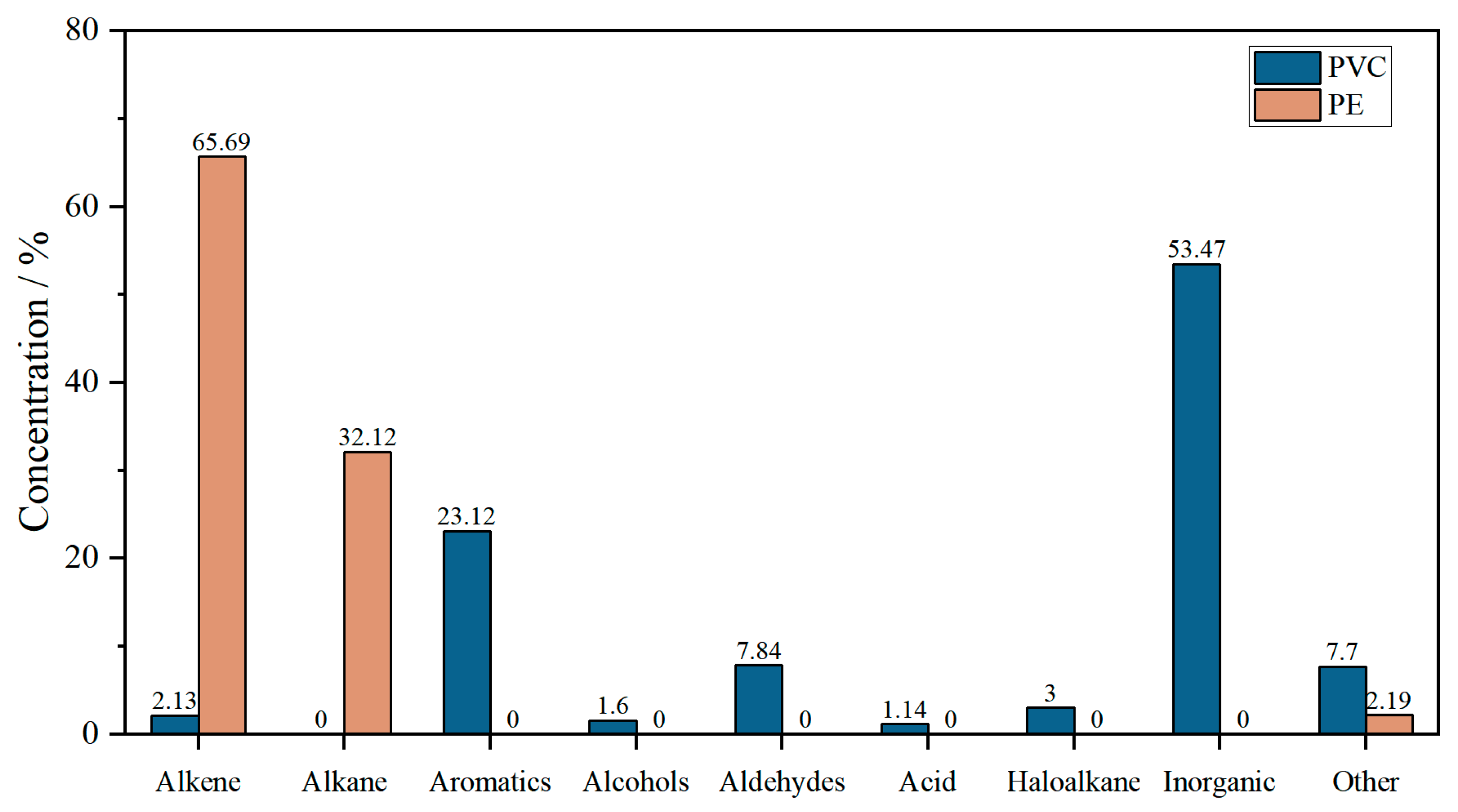
3.4.2. GC/MS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Babrauskas, V. Electrical Fire and Explosion; Fire Science Publishers: New York, NY, USA, 2021. [Google Scholar]
- Lin, Q.; Li, Y.; Man, P.; Jin, Y.; Lyu, H.; Wang, H.; Zhao, Y.; Su, W.; Deng, J. Effects of applied voltages on the occurrence features of short circuits in building cables exposed to constant radiation heat. J. Build. Eng. 2024, 98, 111038. [Google Scholar] [CrossRef]
- GB/T 19666-2019; General Rules for Flame Retardant and Fire Resistant Electric Wires and Cables or Optical Fiber Cables. State Administration for Market Regulation: Beijing, China, 2019.
- Lin, Q.-W.; Li, Y.; Deng, J.; He, F.-F.; Man, P.-R. Thermo-oxidative degradation behavior of poly(vinyl chloride) insulation for new and overloaded wires via TG-FTIR. J. Appl. Polym. Sci. 2024, 141, e55994. [Google Scholar] [CrossRef]
- Lin, Q.; Li, Y.; Deng, J. Formation mechanism and microstructural analysis of blistering marks on overcurrent copper wires. Fire Saf. J. 2024, 150, 104268. [Google Scholar] [CrossRef]
- Deng, J.; Lin, Q.; Li, Y.; Wang, C.; Wang, H.; Man, P. Effect of overload current values on the fire characteristics of polyethylene (PE) copper wires. J. Therm. Anal. Calorim. 2023, 148, 11695–11705. [Google Scholar] [CrossRef]
- Yan, L.; Wang, W.; Xu, Z. Impact of thermal aging temperatures on the combustion behavior and mechanical properties of flame-retardant electrical cables. J. Therm. Anal. Calorim. 2024, 149, 9045–9056. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef]
- Beneš, M.; Milanov, N.; Matuschek, G.; Kettrup, A.; Plaček, V.; Balek, V. Thermal degradation of PVC cable insulation studied by simultaneous TG-FTIR and TG-EGA methods. J. Therm. Anal. Calorim. 2004, 78, 621–630. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Zhang, J.; Yang, S.; Zhang, Z.; Zhao, W. Thermal degradation of flame-retarded high-voltage cable sheath and insulation via TG-FTIR. J. Anal. Appl. Pyrolysis 2018, 134, 167–175. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, N.; Lin, J.; Lu, S.; Liew, K.M. On the pyrolysis characteristic parameters of four flame-retardant classes of PVC sheathless cable insulation materials. J. Anal. Appl. Pyrolysis 2023, 170, 105901. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, G.; Wang, S.; Zhang, H.; Xu, F. TG-FTIR and Py-GC/MS study of the pyrolysis mechanism and composition of volatiles from flash pyrolysis of PVC. J. Energy Inst. 2020, 93, 2362–2370. [Google Scholar] [CrossRef]
- Liu, X.; Tian, K.; Chen, Z.; Wei, W.; Xu, B.; Ni, B.-J. Online TG-FTIR-MS analysis of the catalytic pyrolysis of polyethylene and polyvinyl chloride microplastics. J. Hazard. Mater. 2023, 441, 129881. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Lin, Q.-W.; Li, Y.; Wang, H.-B.; Wang, C.-P.; Zhao, Y.-H.; Lyu, H.-F.; Shu, C.-M. Ignition and Flame Spreading Features of Excessively Overloaded Polyvinyl Chloride Copper Wires. Fire Technol. 2023, 59, 3589–3607. [Google Scholar] [CrossRef]
- Zou, Y.; Li, Y.; Bourbigot, S.; Zhang, J.; Guo, Y.; Li, K.; He, X.; Baolati, J. Determination of solid-phase reaction mechanism and chlorine migration behavior of co-pyrolyzing PVCCaCO3 based polymer using temperature-dependent FTIR and XRD analysis. Polym. Degrad. Stab. 2021, 193, 109741. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F. Pyrolysis of synthetic polymers and plastic wastes. Kinetic study. Fuel Process. Technol. 2008, 89, 678–686. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; Yang, D.; Hao, J.; Qiao, Y.; Tian, Y. Thermal degradation of typical plastics under high heating rate conditions by TG-FTIR: Pyrolysis behaviors and kinetic analysis. Energy Convers. Manag. 2018, 171, 1106–1115. [Google Scholar] [CrossRef]
- Lin, Q.-W.; Li, X.; Li, Y.; Deng, J.; Man, P.-R.; Jia, Y.-Z. Comparative investigation on thermo-oxidative degradation and fire characteristics of flame-retardant and non-flame-retardant polyvinyl chloride wires. Therm. Sci. Eng. Prog. 2025, 57, 103210. [Google Scholar] [CrossRef]
- Sugimoto, M.; Shimada, A.; Kudoh, H.; Tamura, K.; Seguchi, T. Product analysis for polyethylene degradation by radiation and thermal ageing. Radiat. Phys. Chem. 2013, 82, 69–73. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, G.; Zhao, Y.; Zhao, M.; Liao, Z. Analysis of Internal Pyrolysis Characteristics of Crosslinked Polyethylene Cable. In Proceedings of the 2020 IEEE Electrical Insulation Conference (EIC), Knoxville, TN, USA, 22 June–3 July 2020; pp. 498–503. [Google Scholar] [CrossRef]
- Kong, J.; Zhou, K.; Ren, X.; Chen, Y.; Li, Y.; Meng, P. Insight into gaseous product distribution of cross-linked polyethylene pyrolysis using ReaxFF MD simulation and TG-MS. J. Anal. Appl. Pyrolysis 2023, 169, 105847. [Google Scholar] [CrossRef]
- Qu, H.; Wu, W.; Zheng, Y.; Xie, J.; Xu, J. Synergistic effects of inorganic tin compounds and Sb2O3 on thermal properties and flame retardancy of flexible poly(vinyl chloride). Fire Saf. J. 2011, 46, 462–467. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Málek, J. The kinetic analysis of non-isothermal data. Thermochim. Acta 1992, 200, 257–269. [Google Scholar] [CrossRef]
- Lawrence, R.L.; Wright, A. Rule-Based Classification Systems Using Classification and Regression Tree(CART) Analysis. Photogramm. Eng. Remote Sens. 2001, 67, 1137–1142. [Google Scholar]
- Wang, Y.; Kang, N.; Lin, J.; Lu, S.; Liew, K.M. Cross-heating-rate prediction of thermogravimetry of PVC and XLPE cable insulation material: A novel artificial neural network framework. J. Therm. Anal. Calorim. 2022, 147, 14467–14478. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Li, S.; Wu, Y.; Jiang, L.; Lang, J.; Yuan, J.; Liu, J. Effect of thermo-oxidative aging on thermal elongation performance of XLPE insulation for high-voltage cables. Polym. Degrad. Stab. 2023, 210, 110291. [Google Scholar] [CrossRef]
- Mo, S.-J.; Zhang, J.; Liang, D.; Chen, H.-Y. Study on Pyrolysis Characteristics of Cross-linked Polyethylene Material Cable. Procedia Eng. 2013, 52, 588–592. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, R.; Ning, X.; Xie, T.; Wang, J. Thermal degradation properties of LDPE insulation for new and aged fine wires. J. Therm. Anal. Calorim. 2019, 137, 461–471. [Google Scholar] [CrossRef]
- Xuan, W.; Yan, S.; Dong, Y. Exploration of Pyrolysis Behaviors of Waste Plastics (Polypropylene Plastic/Polyethylene Plastic/Polystyrene Plastic): Macro-Thermal Kinetics and Micro-Pyrolysis Mechanism. Processes 2023, 11, 2764. [Google Scholar] [CrossRef]
- Cho, Y.-S.; Shim, M.-J.; Kim, S.-W. Thermal degradation kinetics of PE by the Kissinger equation. Mater. Chem. Phys. 1998, 52, 94–97. [Google Scholar] [CrossRef]
- Park, J.W.; Oh, S.C.; Lee, H.P.; Kim, H.T.; Yoo, K.O. A kinetic analysis of thermal degradation of polymers using a dynamic method. Polym. Degrad. Stab. 2000, 67, 535–540. [Google Scholar] [CrossRef]
- Peterson, J.D.; Vyazovkin, S.; Wight, C.A. Kinetics of the Thermal and Thermo-Oxidative Degradation of Polystyrene, Polyethylene and Poly(propylene). Macromol. Chem. Phys. 2001, 202, 775–784. [Google Scholar] [CrossRef]
- Aboulkas, A.; El harfi, K.; El Bouadili, A. Thermal degradation behaviors of polyethylene and polypropylene. Part I: Pyrolysis kinetics and mechanisms. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Das, P.; Tiwari, P. Thermal degradation kinetics of plastics and model selection. Thermochim. Acta 2017, 654, 191–202. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, H.; Tong, L. Experimental study on the fire protection properties of PVC sheath for old and new cables. J. Hazard. Mater. 2010, 179, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xie, T.; Ning, X.; Liu, Y.; Wang, J. Thermal degradation kinetics study of polyvinyl chloride (PVC) sheath for new and aged cables. Waste Manag. 2019, 99, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, T.; Luo, X.; Han, D.; Wang, Z.; Wu, J. TG/FTIR analysis on co-pyrolysis behavior of PE, PVC and PS. Waste Manag. 2014, 34, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Li, J.; Gu, T.; Yang, R.; Fu, Z.; Yan, B.; Chen, G. Effects of torrefaction on the formation and distribution of dioxins during wood and PVC pyrolysis: An experimental and mechanistic study. J. Anal. Appl. Pyrolysis 2021, 157, 105240. [Google Scholar] [CrossRef]
- Berkowicz-Płatek, G.; Żukowski, W.; Wrona, J.; Wencel, K. Thermal decomposition of polyolefins under different oxygen content. Composition of products and thermal effects. Energy 2024, 295, 130987. [Google Scholar] [CrossRef]
- Al-Sagheer, F.; Ahmad, Z. Stabilizing poly(vinyl chloride) using its blends with poly(methyl methacarylate): Pyrolysis GC/MS studies. J. Hazard. Mater. 2014, 278, 584–591. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Striūgas, N.; Zakarauskas, K.; Praspaliauskas, M.; Abdelnaby, M.A. Pyrolysis kinetic behavior and TG-FTIR-GC–MS analysis of metallised food packaging plastics. Fuel 2020, 282, 118737. [Google Scholar] [CrossRef]
- Peller, J.R.; Mezyk, S.P.; Shidler, S.; Castleman, J.; Kaiser, S.; Horne, G.P. The Reactivity of Polyethylene Microplastics in Water under Low Oxygen Conditions Using Radiation Chemistry. Water 2021, 13, 3120. [Google Scholar] [CrossRef]
- Cheng, L.; Gu, J.; Wang, Y.; Zhang, J.; Yuan, H.; Chen, Y. Polyethylene high-pressure pyrolysis: Better product distribution and process mechanism analysis. Chem. Eng. J. 2020, 385, 123866. [Google Scholar] [CrossRef]


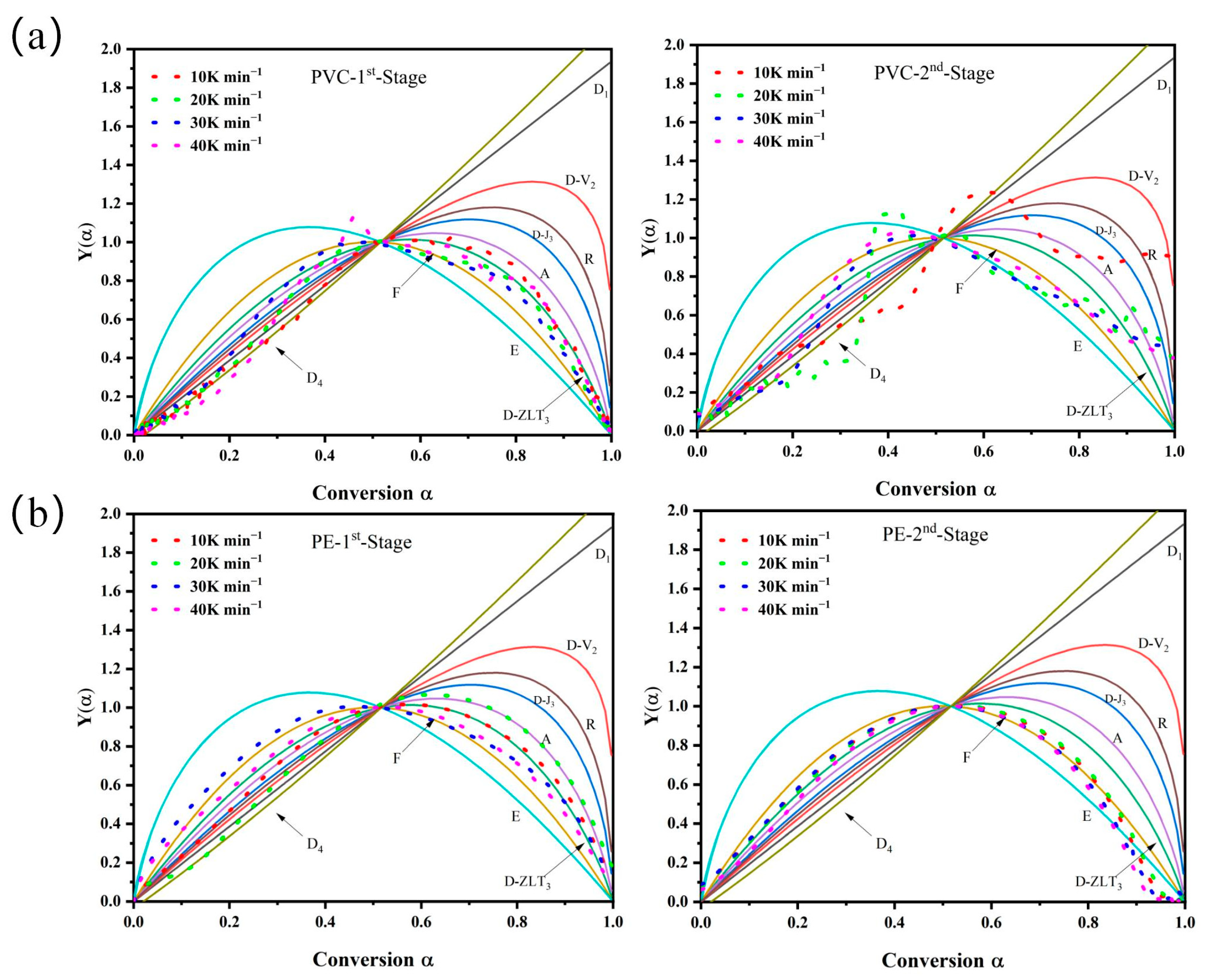

| Model | Differential Form f(α) | Integral Form g(α) |
|---|---|---|
| 1D diffusion D1 | ||
| 2D diffusion-Valensi D-V2 | ||
| 3D diffusion-Jander D-J3 | ||
| 3D Zhuravlev-Leskin-Tempelman D-ZLT3 | ||
| 3D Ginstling-Brounstein D4 | ||
| Avarami–Erofeev A2 | ||
| Avarami–Erofeev A3 | ||
| Avarami–Erofeev A4 | ||
| Second-order chemical reaction F2 | ||
| Third-order chemical reaction F3 | ||
| First-order E1 | ||
| Second-order E2 | ||
| Contracting area R2 | ||
| 3D contracting volume R3 |
| Sample | Heating Rate/K min−1 | Tonset/K | DTGpeak1/% min−1 | DTGpeak2/% min−1 | Tpeak1/K | Tpeak2/K | Massresidue/% |
|---|---|---|---|---|---|---|---|
| PVC | 10 | 480.03 | 7.22 | 2.06 | 569.18 | 710.26 | 28.05 |
| 20 | 493.72 | 16.20 | 4.10 | 586.33 | 733.34 | 28.29 | |
| 30 | 499.71 | 26.79 | 5.81 | 593.67 | 754.59 | 28.60 | |
| 40 | 502.42 | 36.95 | 6.99 | 601.78 | 760.89 | 28.84 | |
| PE | 10 | 506.69 | 6.38 | 8.21 | 566.12 | 746.20 | 39.83 |
| 20 | 508.69 | 9.43 | 16.23 | 570.69 | 756.69 | 41.07 | |
| 30 | 515.28 | 11.46 | 21.91 | 575.55 | 761.55 | 41.35 | |
| 40 | 521.56 | 19.10 | 34.80 | 592.13 | 768.13 | 42.42 |
| Sample | α | Eα (kJ/mol) | α | Eα (kJ/mol) |
|---|---|---|---|---|
| I-Stage | II-Stage | |||
| PVC | 0.1 | 230.556 | 0.1 | 365.549 |
| 0.2 | 209.058 | 0.2 | 310.331 | |
| 0.3 | 188.764 | 0.3 | 298.367 | |
| 0.4 | 181.299 | 0.4 | 292.96 | |
| 0.5 | 178.237 | 0.5 | 290.163 | |
| 0.6 | 176.106 | 0.6 | 289.179 | |
| 0.7 | 174.145 | 0.7 | 285.830 | |
| 0.8 | 172.018 | 0.8 | 282.729 | |
| 0.9 | 168.794 | 0.9 | 280.137 | |
| Mean value | 186.553 | 299.472 | ||
| PE | 0.1 | 98.123 | 0.1 | 276.985 |
| 0.2 | 96.583 | 0.2 | 323.913 | |
| 0.3 | 101.230 | 0.3 | 318.766 | |
| 0.4 | 108.764 | 0.4 | 314.861 | |
| 0.5 | 117.585 | 0.5 | 304.164 | |
| 0.6 | 124.815 | 0.6 | 298.374 | |
| 0.7 | 131.387 | 0.7 | 289.221 | |
| 0.8 | 136.885 | 0.8 | 278.504 | |
| 0.9 | 148.323 | 0.9 | 272.899 | |
| Mean value | 118.188 | 297.521 | ||
| Sample | Description | Wavenumbers (cm−1) |
|---|---|---|
| Flame-retardant PVC | H2O | 4000–3500 |
| C–H stretching | 2922 | |
| CO2 | 2400–2260 | |
| C–H aliphatic bending | 1458 | |
| C–H bending | 1240 | |
| C–Cl stretching | 660 | |
| Flame-retardant PE | H2O | 4000–3500 |
| C–H stretching | 2922, 2854 | |
| CO2 | 2400–2260 | |
| C=O & C=C stretching | 1652 | |
| CH2 scissor vibrations | 1460 | |
| C–H bending vibrations | 669 |
| Sample | Retention (min) | Component | Formula | Chemical Family |
|---|---|---|---|---|
| PVC at 569.18 K | 2.56 | Hydrogen chloride | HCl | Inorganic |
| 4.66 | Benzene | C6H6 | Aromatics | |
| 9.55 | 3-Heptene, 3-methyl | C8H10 | Aromatics Alkane Alkane | |
| 10.05 | Heptene, 3-methylene- | C8H16 | Alkene | |
| 11.82 | Arsenic trichloride | AsCl3 | Inorganic | |
| 18.09 | Benzaldehyde | C7H6O | Aldehydes | |
| 21.97 | Heptane, 3-(chloromethyl) | C8H17Cl | Haloalkane | |
| 23.61 | 1-Hexanol, 2-ehyl- | C8H18O | Alcohols | |
| 24.59 | 3-Methylphenylacethlene | C9H8 | Aromatics | |
| 38.28 | 1,2-Benzenedicarboxytic acid | C8H6O4 | Acid | |
| PE at 566.12 K | 2.94 | Propene | C3H6 | Alkene |
| 3.07 | 2-Butene, (E)- | C4H8 | Alkene | |
| 3.48 | Pentane | C5H12 | Alkane | |
| 4.29 | 1-Pentene,2-methyl- | C6H12 | Alkene | |
| 6.52 | 1-Heptene | C7H14 | Alkene | |
| 12.06 | 2,4-Dimethyl-1-heptene | C9H18 | Alkene | |
| 17.63 | 1-Decene | C10H20 | Alkene | |
| 17.93 | Decane | C10H22 | Alkane | |
| 21.06 | 4-Docame, 4-methyl- | C12H24 | Alkene | |
| 21.26 | 1-Octene, 3,7-dimethyl | C10H20 | Alkene | |
| 21.60 | 1-Tetradecene | C14H28 | Alkene | |
| 22.01 | Undecane | C11H24 | Alkane | |
| 25.89 | Tetradecane | C14H30 | Alkane | |
| 28.06 | 3-Eicosene.(E)- | C20H40 | Alkene | |
| 28.31 | 1-Heptadecene | C17H34 | Alkene |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, W.-W.; Li, Y.; Man, P.-R.; Sheng, Y.-W.; Wang, J. Pyrolysis Kinetics and Gas Evolution of Flame-Retardant PVC and PE: A TG-FTIR-GC/MS Study. Fire 2025, 8, 262. https://doi.org/10.3390/fire8070262
Su W-W, Li Y, Man P-R, Sheng Y-W, Wang J. Pyrolysis Kinetics and Gas Evolution of Flame-Retardant PVC and PE: A TG-FTIR-GC/MS Study. Fire. 2025; 8(7):262. https://doi.org/10.3390/fire8070262
Chicago/Turabian StyleSu, Wen-Wei, Yang Li, Peng-Rui Man, Ya-Wen Sheng, and Jian Wang. 2025. "Pyrolysis Kinetics and Gas Evolution of Flame-Retardant PVC and PE: A TG-FTIR-GC/MS Study" Fire 8, no. 7: 262. https://doi.org/10.3390/fire8070262
APA StyleSu, W.-W., Li, Y., Man, P.-R., Sheng, Y.-W., & Wang, J. (2025). Pyrolysis Kinetics and Gas Evolution of Flame-Retardant PVC and PE: A TG-FTIR-GC/MS Study. Fire, 8(7), 262. https://doi.org/10.3390/fire8070262






