Prescribed Fire Smoke: A Review of Composition, Measurement Methods, and Analysis
Abstract
1. Introduction
2. Prescribed Fire—An Evolving Strategy for Land Management
3. Smoke Composition—Prescribed Fire vs. Wildfire
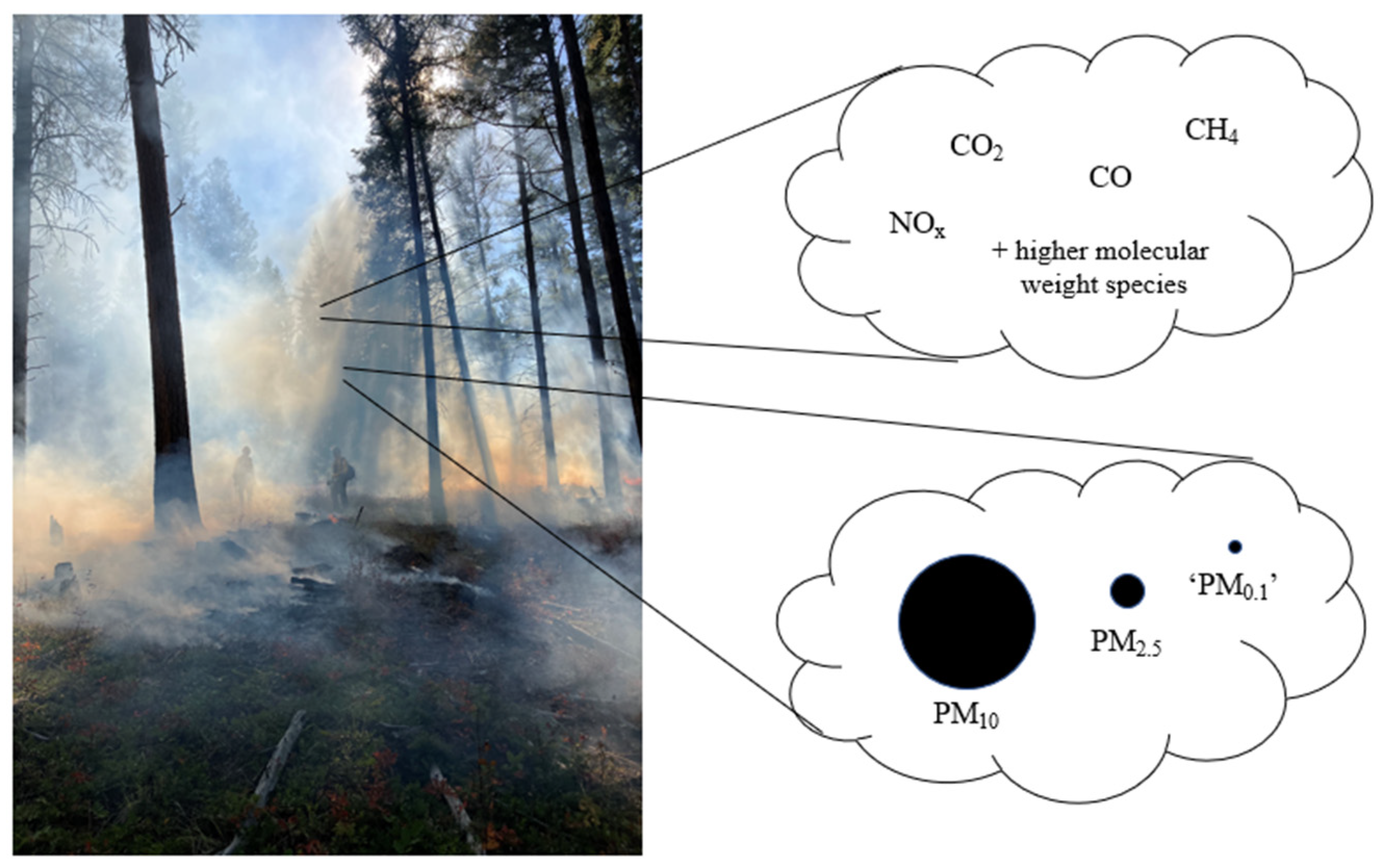
3.1. Chemical Composition
3.2. Particulate Composition
- Variations in Composition by Air Quality Index AQI Classification
- Green and Yellow: Low PM concentrations, dominated by coarse particles (PM10) with minimal organic carbon and elemental carbon.
- Orange and Red: Increased PM2.5 levels, higher organic carbon/elemental carbon ratios, and greater secondary aerosol formation.
- Purple and Maroon: Severe pollution events with elevated black carbon, toxic metals, and harmful gaseous co-pollutants.
4. Measuring and Monitoring Prescribed Fire and Wildfire Smoke Composition
4.1. Measuring Molecular Constituents
4.2. Measuring Particulate Matter
5. Conclusions and Future Opportunities
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ritter, S.; Morici, K.; Stevens-Rumann, C. Efficacy of prescribed fire as a fuel reduction treatment in the Colorado Front Range. Can. J. For. Res. 2023, 53, 455–462. [Google Scholar] [CrossRef]
- Morgan, G.; Tolhurst, K.G.; Poynter, M.; Cooper, N.; McGuffog, T.; Ryan, R.; Wouters, M.; Stephens, N.; Black, P.; Sheehan, D. Prescribed burning in south-eastern Australia: History and future directions. Aust. For. 2020, 83, 4–28. [Google Scholar] [CrossRef]
- Arrogante-Funes, F.; Aguado, I.; Chuvieco, E. Global assessment and mapping of ecological vulnerability to wildfires. Nat. Hazards Earth Syst. Sci. 2022, 22, 2981–3003. [Google Scholar] [CrossRef]
- Roy, D.P.; De Lemos, H.; Huang, H.; Giglio, L.; Houborg, R.; Miura, T. Multi-resolution monitoring of the 2023 maui wildfires, implications and needs for satellite-based wildfire disaster monitoring. Sci. Remote Sens. 2024, 10, 100142. [Google Scholar] [CrossRef]
- Qiu, M.; Chen, D.; Kelp, M.; Li, J.; Huang, G.; Yazdi, M.D. The rising threats of wildland-urban interface fires in the era of climate change: The Los Angeles 2025 fires. Innovation 2025, 6, 100835. [Google Scholar] [CrossRef]
- Garofalo, L.A.; Pothier, M.A.; Levin, E.J.T.; Campos, T.; Kreidenweis, S.M.; Farmer, D.K. Emission and Evolution of Submicron Organic Aerosol in Smoke from Wildfires in the Western United States. ACS Earth Space Chem. 2019, 3, 1237–1247. [Google Scholar] [CrossRef]
- Rezaie, N.; Pallozzi, E.; Ciccioli, P.; Calfapietra, C.; Fares, S. Temperature dependence of emission of volatile organic compounds (VOC) from litters collected in two Mediterranean ecosystems determined before the flaming phase of biomass burning. Environ. Pollut. 2023, 338, 122703. [Google Scholar] [CrossRef]
- Marthoenis. The January 2025 Southern California Wildfires: Public Mental Health Impacts and a Call to Action. Asian J. Public Health Nurs. 2025, 1, 1–3. [Google Scholar] [CrossRef]
- Hung, W.-T.; Lu, C.-H.; Shrestha, B.; Lin, H.-C.; Lin, C.-A.; Grogan, D.; Hong, J.; Ahmadov, R.; James, E.; Joseph, E. The impacts of transported wildfire smoke aerosols on surface air quality in New York State: A case study in summer 2018. Atmos. Environ. 2020, 227, 117415. [Google Scholar] [CrossRef]
- Alba, C.; Skálová, H.; McGregor, K.F.; D’Antonio, C.; Pyšek, P. Native and exotic plant species respond differently to wildfire and prescribed fire as revealed by meta-analysis. J. Veg. Sci. 2015, 26, 102–113. [Google Scholar] [CrossRef]
- McMillan, M. Five Burning Questions About Tree Planting and Wildfire; Courtesy of the U.S. Forest Service: Washington, DC, USA, 2023.
- Saide, P.; Krishna, M.; Ye, X.; Thapa, L.; Turney, F.; Howes, C.; Schmidt, C. Estimating fire radiative power using weather radar products for wildfires. Geophys. Res. Lett. 2023, 50, e2023GL104824. [Google Scholar] [CrossRef]
- Freeborn, P.H.; Jolly, W.M.; Cochrane, M.A.; Roberts, G. Large wildfire driven increases in nighttime fire activity observed across CONUS from 2003–2020. Remote Sens. Environ. 2022, 268, 112777. [Google Scholar] [CrossRef]
- Li, T.; Jeřábek, J.; Winkler, J.; Vaverková, M.D.; Zumr, D. Effects of prescribed fire on topsoil properties: A small-scale straw burning experiment. J. Hydrol. Hydromech. 2022, 70, 450–461. [Google Scholar] [CrossRef]
- Lyon, Z.D.; Morgan, P.; Stevens-Rumann, C.S.; Sparks, A.M.; Keefe, R.F.; Smith, A.M.S. Fire behaviour in masticated forest fuels: Lab and prescribed fire experiments. Int. J. Wildland Fire 2018, 27, 280. [Google Scholar] [CrossRef]
- Jones, B.A.; McDermott, S.; Champ, P.A.; Berrens, R.P. More smoke today for less smoke tomorrow? We need to better understand the public health benefits and costs of prescribed fire. Int. J. Wildland Fire 2022, 31, 918–926. [Google Scholar] [CrossRef]
- Jiang, K.; Xing, R.; Luo, Z.; Huang, W.; Yi, F.; Men, Y.; Zhao, N.; Chang, Z.; Zhao, J.; Pan, B.; et al. Pollutant emissions from biomass burning: A review on emission characteristics, environmental impacts, and research perspectives. Particuology 2024, 85, 296–309. [Google Scholar] [CrossRef]
- Agbor, E.; Zhang, X.; Kumar, A. A review of biomass co-firing in North America. Renew. Sustain. Energy Rev. 2014, 40, 930–943. [Google Scholar] [CrossRef]
- Xu, Q.; Westerling, A.L.; Notohamiprodjo, A.; Wiedinmyer, C.; Picotte, J.J.; Parks, S.A.; Hurteau, M.D.; Marlier, M.E.; Kolden, C.A.; Sam, J.A.; et al. Wildfire burn severity and emissions inventory: An example implementation over California. Environ. Res. Lett. 2022, 17, 085008. [Google Scholar] [CrossRef]
- Heal, M.R.; Kumar, P.; Harrison, R.M. Particles, air quality, policy and health. Chem. Soc. Rev. 2012, 41, 6606. [Google Scholar] [CrossRef]
- Altshuler, S.L.; Zhang, Q.; Kleinman, M.T.; Garcia-Menendez, F.; Moore, C.T.; Hough, M.L.; Stevenson, E.D.; Chow, J.C.; Jaffe, D.A.; Watson, J.G. Wildfire and prescribed burning impacts on air quality in the United States. J. Air Waste Manag. Assoc. 2020, 70, 961–970. [Google Scholar] [CrossRef]
- Bone, C.; Shultz, C.; Huber-Stearns, H.; Kelley, J.; Cunnin, E. Evaluating the potential role of federal air quality standards in constraining applications of prescribed fire in the western United States. Appl. Geogr. 2023, 157, 102996. [Google Scholar] [CrossRef]
- Kwon, H.-S.; Ryu, M.H.; Carlsten, C. Ultrafine particles: Unique physicochemical properties relevant to health and disease. Exp. Amp; Mol. Med. 2020, 52, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Graw, R.L.; Anderson, B.A. Strategies to reduce wildfire smoke in frequently impacted communities in south-western Oregon. Int. J. Wildland Fire 2022, 31, 1155–1166. [Google Scholar] [CrossRef]
- Maji, K.J.; Li, Z.; Vaidyanathan, A.; Hu, Y.; Stowell, J.D.; Milando, C.; Wellenius, G.; Kinney, P.L.; Russell, A.G.; Odman, M.T. Estimated impacts of prescribed fires on air quality and premature deaths in Georgia and surrounding areas in the US, 2015–2020. Environ. Sci. Technol. 2024, 58, 12343–12355. [Google Scholar] [CrossRef]
- Keeley, J.E. Fire intensity, fire severity and burn severity: A brief review and suggested usage. Int. J. Wildland Fire 2009, 18, 116–126. [Google Scholar] [CrossRef]
- Parks, S.A.; Miller, C.; Nelson, C.R.; Holden, Z.A. Previous Fires Moderate Burn Severity of Subsequent Wildland Fires in Two Large Western US Wilderness Areas. Ecosystems 2014, 17, 29–42. [Google Scholar] [CrossRef]
- Turney, F.A.; Saide, P.E.; Jimenez Munoz, P.A.; Muñoz-Esparza, D.; Hyer, E.J.; Peterson, D.A.; Frediani, M.E.; Juliano, T.W.; Decastro, A.L.; Kosović, B.; et al. Sensitivity of Burned Area and Fire Radiative Power Predictions to Containment Efforts, Fuel Density, and Fuel Moisture Using WRF-Fire. J. Geophys. Res. Atmos. 2023, 128, e2023JD038873. [Google Scholar] [CrossRef]
- Warneke, C.; Schwarz, J.P.; Dibb, J.; Kalashnikova, O.; Frost, G.; Al-Saad, J.; Brown, S.S.; Brewer, W.A.; Soja, A.; Seidel, F.C.; et al. Fire Influence on Regional to Global Environments and Air Quality (FIREX-AQ). J. Geophys. Res. Atmos. 2023, 128, e2022JD037758. [Google Scholar] [CrossRef]
- Nowacki, G.J.; Maccleery, D.W.; Lake, F.K. Native Americans, Ecosystem Development, and Historical Range of Variation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 76–91. [Google Scholar] [CrossRef]
- Spetich, M.A.; Perry, R.W.; Harper, C.A.; Clark, S.L. Fire in Eastern Hardwood Forests Through 14,000 Years; Springer: Dordrecht, The Netherlands, 2011; pp. 41–58. [Google Scholar] [CrossRef]
- Ryan, K.C.; Knapp, E.E.; Varner, J.M. Prescribed fire in North American forests and woodlands: History, current practice, and challenges. Front. Ecol. Environ. 2013, 11, e15–e24. [Google Scholar] [CrossRef]
- Pyne, S.J. Fire in America: A Cultural History of Wildland and Rural Fire; University of Washington Press: Seattle, WA, USA, 2017. [Google Scholar]
- Pyne, S.J.; Andrews, P.L.; Laven, R.D. Introduction to Wildland Fire; John Wiley and Sons: New York, NY, USA, 1996. [Google Scholar]
- Williams, G.W. The USDA Forest Service: The First Century; USDA Forest Service: Washington, DC, USA, 2000; Volume 650.
- Matlack, G.R. Reassessment of the Use of Fire as a Management Tool in Deciduous Forests of Eastern North America. Conserv. Biol. 2013, 27, 916–926. [Google Scholar] [CrossRef]
- Abella, S.R.; Fornwalt, P.J. Ten years of vegetation assembly after a North American mega fire. Glob. Change Biol. 2015, 21, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Nowacki, G.J.; Abrams, M.D. The demise of fire and “mesophication” of forests in the eastern United States. BioScience 2008, 58, 123–138. [Google Scholar] [CrossRef]
- Stephens, S.L.; Martin, R.E.; Clinton, N.E. Prehistoric fire area and emissions from California’s forests, woodlands, shrublands, and grasslands. For. Ecol. Manag. 2007, 251, 205–216. [Google Scholar] [CrossRef]
- Bergeron, Y.; Flannigan, M.; Gauthier, S.; Leduc, A.; Lefort, P. Past, current and future fire frequency in the Canadian boreal forest: Implications for sustainable forest management. AMBIO: A J. Hum. Environ. 2004, 33, 356–360. [Google Scholar] [CrossRef]
- Domínguez, R.M.; Trejo, D.A.R. Forest Fires in Mexico and Central América. In Proceedings of the Second International Symposium on Fire Economics, Planning, and Policy: A Global View, Córdoba, Spain, 19–22 April 2004; pp. 19–22. [Google Scholar]
- Jaffe, D.A.; O’Neill, S.M.; Larkin, N.K.; Holder, A.L.; Peterson, D.L.; Halofsky, J.E.; Rappold, A.G. Wildfire and prescribed burning impacts on air quality in the United States. J. Air Waste Manag. Assoc. 2020, 70, 583–615. [Google Scholar] [CrossRef]
- Weise, D.R.; Johnson, T.J.; Reardon, J. Particulate and trace gas emissions from prescribed burns in southeastern U.S. fuel types: Summary of a 5-year project. Fire Saf. J. 2015, 74, 71–81. [Google Scholar] [CrossRef]
- Li, Z.; Maji, K.J.; Hu, Y.; Vaidyanathan, A.; O’Neill, S.M.; Odman, M.T.; Russell, A.G. An Analysis of Prescribed Fire Activities and Emissions in the Southeastern United States from 2013 to 2020. Remote Sens. 2023, 15, 2725. [Google Scholar] [CrossRef]
- Yokelson, R.J.; Goode, J.G.; Ward, D.E.; Susott, R.A.; Babbitt, R.E.; Wade, D.D.; Bertschi, I.; Griffith, D.W.; Hao, W.M. Emissions of formaldehyde, acetic acid, methanol, and other trace gases from biomass fires in North Carolina measured by airborne Fourier transform infrared spectroscopy. J. Geophys. Res. Atmos. 1999, 104, 30109–30125. [Google Scholar] [CrossRef]
- Hoshiko, S.; Buckman, J.R.; Jones, C.G.; Yeomans, K.R.; Mello, A.; Thilakaratne, R.; Sergienko, E.; Allen, K.; Bello, L.; Rappold, A.G. Responses to Wildfire and Prescribed Fire Smoke: A Survey of a Medically Vulnerable Adult Population in the Wildland-Urban Interface, Mariposa County, California. Int. J. Environ. Res. Public Health 2023, 20, 1210. [Google Scholar] [CrossRef]
- McCaffrey, S.M.; Olsen, C.S. Research Perspectives on the Public and Fire Management: A Synthesis of Current Social Science on Eight Essential Questions; NRS-GTR-104; U.S. Department of Agriculture, Forest Service, Northern Research Station: Newton Square, PA, USA, 2012.
- USDA Forest Service. National Prescribed Fire Resource Mobilization Strategy Release; USDA Forest Service: Washington, DC, USA, 2023.
- Li, N.; Georas, S.; Alexis, N.; Fritz, P.; Xia, T.; Williams, M.A.; Horner, E.; Nel, A. A work group report on ultrafine particles (AAAAI) why ambient ultrafine and engineered nanoparticles should receive special attention for possible adverse health outcomes in humans. J. Allergy Clin. Immunol. 2016, 138, 386. [Google Scholar] [CrossRef]
- Akagi, S.K.; Burling, I.R.; Mendoza, A.; Johnson, T.J.; Cameron, M.; Griffith, D.W.T.; Paton-Walsh, C.; Weise, D.R.; Reardon, J.; Yokelson, R.J. Field measurements of trace gases emitted by prescribed fires in southeastern US pine forests using an open-path FTIR system. Atmos. Chem. Phys. 2014, 14, 199–215. [Google Scholar] [CrossRef]
- El Asmar, R.; Li, Z.; Tanner, D.J.; Hu, Y.; O’Neill, S.; Huey, L.G.; Odman, M.T.; Weber, R.J. A multi-site passive approach to studying the emissions and evolution of smoke from prescribed fires. Atmos. Chem. Phys. 2024, 24, 12749–12773. [Google Scholar] [CrossRef]
- Sun, M.; Cui, J.n.; Zhao, X.; Zhang, J. Impacts of precursors on peroxyacetyl nitrate (PAN) and relative formation of PAN to ozone in a southwestern megacity of China. Atmos. Environ. 2020, 231, 117542. [Google Scholar] [CrossRef]
- Liu, T.; Hong, Y.; Li, M.; Xu, L.; Chen, J.; Bian, Y.; Yang, C.; Dan, Y.; Zhang, Y.; Xue, L.; et al. Atmospheric oxidation capacity and ozone pollution mechanism in a coastal city of southeastern China: Analysis of a typical photochemical episode by an observation-based model. Atmos. Chem. Phys. 2022, 22, 2173–2190. [Google Scholar] [CrossRef]
- Raza, M.; Chen, Y.; Trapp, J.; Sun, H.; Huang, X.; Ren, W. Smoldering peat fire detection by time-resolved measurements of transient CO2 and CH4 emissions using a novel dual-gas optical sensor. Fuel 2023, 334, 126750. [Google Scholar] [CrossRef]
- Levine, J.S.; Cofer, W.R., III; Sebacher, D.I.; Rhinehart, R.P.; Winstead, E.L.; Sebacher, S.; Hinkle, C.R.; Schmalzer, P.A.; Koller, A.M., Jr. The effects of fire on biogenic emissions of methane and nitric oxide from wetlands. J. Geophys. Res. Atmos. 1990, 95, 1853–1864. [Google Scholar] [CrossRef]
- May, A.; McMeeking, G.; Lee, T.; Taylor, J.; Craven, J.; Burling, I.; Sullivan, A.P.; Akagi, S.; Collett Jr, J.; Flynn, M. Aerosol emissions from prescribed fires in the United States: A synthesis of laboratory and aircraft measurements. J. Geophys. Res. Atmos. 2014, 119, 11826–811849. [Google Scholar] [CrossRef]
- Travis, K.R.; Crawford, J.H.; Soja, A.J.; Gargulinski, E.M.; Moore, R.H.; Wiggins, E.B.; Diskin, G.S.; DiGangi, J.P.; Nowak, J.B.; Halliday, H. Emission Factors for Crop Residue and Prescribed Fires in the Eastern US During FIREX-AQ. J. Geophys. Res. Atmos. 2023, 128, e2023JD039309. [Google Scholar] [CrossRef]
- Permar, W.; Wielgasz, C.; Jin, L.; Chen, X.; Coggon, M.M.; Garofalo, L.A.; Gkatzelis, G.I.; Ketcherside, D.; Millet, D.B.; Palm, B.B. Assessing formic and acetic acid emissions and chemistry in western US wildfire smoke: Implications for atmospheric modeling. Environ. Sci. Atmos. 2023, 3, 1620–1641. [Google Scholar] [CrossRef]
- Hodgson, A.K.; Morgan, W.T.; O’Shea, S.; Bauguitte, S.; Allan, J.D.; Darbyshire, E.; Flynn, M.J.; Liu, D.; Lee, J.; Johnson, B. Near-field emission profiling of tropical forest and Cerrado fires in Brazil during SAMBBA 2012. Atmos. Chem. Phys. 2018, 18, 5619–5638. [Google Scholar] [CrossRef]
- Urbanski, S.P. Combustion efficiency and emission factors for wildfire-season fires in mixed conifer forests of the northern Rocky Mountains, US. Atmos. Chem. Phys. 2013, 13, 7241–7262. [Google Scholar] [CrossRef]
- Harper, A.R.; Doerr, S.H.; Santin, C.; Froyd, C.A.; Sinnadurai, P. Prescribed fire and its impacts on ecosystem services in the UK. Sci. Total Environ. 2018, 624, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Yaro, V.S.O.; Bondé, L.; Bougma, P.-T.C.; Sedgo, I.; Guuroh, R.T.; Gebremichael, A.W.; Neya, T.; Linstädter, A.; Ouédraogo, O. Greenhouse gas emission from prescribed fires is influenced by vegetation types in West African Savannas. Sci. Rep. 2024, 14, 23754. [Google Scholar] [CrossRef] [PubMed]
- García-Carmona, M.; Santín, C.; Cawson, J.; Chafer, C.J.; Duff, T.; Knowles, L.; McCaw, W.L.; Doerr, S.H. Pyrogenic carbon production in eucalypt forests under low to moderate fire severities. For. Ecol. Manag. 2025, 585, 122590. [Google Scholar] [CrossRef]
- Wright, J.; Delamater, D.; Simha, A.; Ury, E.; Ficken, C. Changes in Prescribed Fire Frequency Alter Ecosystem Carbon Dynamics. Ecosystems 2021, 24, 640–651. [Google Scholar] [CrossRef]
- Paton-Walsh, C.; Smith, T.E.L.; Young, E.L.; Griffith, D.W.T.; Guérette, É. New emission factors for Australian vegetation fires measured using open-path Fourier transform infrared spectroscopy—Part 1: Methods and Australian temperate forest fires. Atmos. Chem. Phys. 2014, 14, 11313–11333. [Google Scholar] [CrossRef]
- Andreae, M.O. Emission of trace gases and aerosols from biomass burning–an updated assessment. Atmos. Chem. Phys. 2019, 19, 8523–8546. [Google Scholar] [CrossRef]
- Akagi, S.K.; Yokelson, R.J.; Wiedinmyer, C.; Alvarado, M.J.; Reid, J.S.; Karl, T.; Crounse, J.D.; Wennberg, P.O. Emission factors for open and domestic biomass burning for use in atmospheric models. Atmos. Chem. Phys. 2011, 11, 4039–4072. [Google Scholar] [CrossRef]
- Roberts, J.M.; Stockwell, C.E.; Yokelson, R.J.; de Gouw, J.; Liu, Y.; Selimovic, V.; Koss, A.R.; Sekimoto, K.; Coggon, M.M.; Yuan, B.; et al. The nitrogen budget of laboratory-simulated western US wildfires during the FIREX 2016 Fire Lab study. Atmos. Chem. Phys. 2020, 20, 8807–8826. [Google Scholar] [CrossRef]
- Gao, H.L.; Jaffe, D.A. Comparison of ultraviolet absorbance and NO-chemiluminescence for ozone measurement in wildfire plumes at the Mount Bachelor Observatory. Atmos. Environ. 2017, 166, 224–233. [Google Scholar] [CrossRef]
- Navarro, K. Working in Smoke. Clin. Chest Med. 2020, 41, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Wei, Y.; Li, N.; Guo, A.; Zhang, E.; Li, C.; Peng, Y.; Wei, J.; Zang, Z. Development of an early warning fire detection system based on a laser spectroscopic carbon monoxide sensor using a 32-bit system-on-chip. Infrared Phys. Technol. 2019, 96, 44–51. [Google Scholar] [CrossRef]
- Cristofanelli, P.; Trisolino, P.; Calzolari, F.; Busetto, M.; Calidonna, C.R.; Amendola, S.; Arduini, J.; Fratticioli, C.; Hundal, R.A.; Maione, M.; et al. Influence of wildfire emissions to carbon dioxide (CO2) observed at the Mt. Cimone station (Italy, 2165 m asl): A multi-year investigation. Atmos. Environ. 2024, 330, 120577. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on Toxicology. Emergency and Continuous Exposure Limits for Selected Airborne Contaminants; National Academies Press (US): Washington, DC, USA, 1984. [Google Scholar] [CrossRef]
- Dickinson, G.N.; Miller, D.D.; Bajracharya, A.; Bruchard, W.; Durbin, T.A.; McGarry, J.K.P.; Moser, E.P.; Nuñez, L.A.; Pukkila, E.J.; Scott, P.S.; et al. Health Risk Implications of Volatile Organic Compounds in Wildfire Smoke During the 2019 FIREX-AQ Campaign and Beyond. GeoHealth 2022, 6, e2021GH000546. [Google Scholar] [CrossRef]
- Magro, C.; Gonçalves, O.C.; Nunes, L.; Perry, S.H.; Rego, F.C.; Vieira, P. Remote sensing of volatile organic compounds release during prescribed fires in pine forests using open-path Fourier transform infra-red spectroscopy. Int. J. Wildland Fire 2024, 33, WF23019. [Google Scholar]
- U.S. Environmental Protection Agency. Nitrogen Oxides (NOx), Why and How They Are Controlled; EPA: Washington, DC, USA, 1999.
- Bishop, S. Air Quality Measurements Series: Nitrogen Dioxide. 2021. Available online: https://www.clarity.io/blog/air-quality-measurements-series-nitrogen-dioxide (accessed on 17 April 2025).
- Tomsche, L.; Piel, F.; Mikoviny, T.; Nielsen, C.J.; Guo, H.; Campuzano-Jost, P.; Nault, B.A.; Schueneman, M.K.; Jimenez, J.L.; Halliday, H.; et al. Measurement report: Emission factors of NH3 and NHx for wildfires and agricultural fires in the United States. Atmos. Chem. Phys. 2023, 23, 2331–2343. [Google Scholar] [CrossRef]
- Weisel, C.P. Benzene exposure: An overview of monitoring methods and their findings. Chem. -Biol. Interact. 2010, 184, 58–66. [Google Scholar] [CrossRef]
- Huang, G.; Gao, L.; Duncan, J.; Harper, J.D.; Sanders, N.L.; Ouyang, Z.; Cooks, R.G. Direct detection of benzene, toluene, and ethylbenzene at trace levels in ambient air by atmospheric pressure chemical ionization using a handheld mass spectrometer. J. Am. Soc. Mass Spectrom. 2010, 21, 132–135. [Google Scholar] [CrossRef]
- Yi, Z.; Kun-Lin, Y. Quantitative detection of phenol in wastewater using square wave voltammetry with pre-concentration. Anal. Chim. Acta 2021, 1178, 338788. [Google Scholar] [CrossRef]
- Burling, I.; Yokelson, R.; Akagi, S.; Johnson, T.; Griffith, D.; Urbanski, S.; Taylor, J.; Craven, J.; McMeeking, G.; Roberts, J. First results from a large, multi-platform study of trace gas and particle emissions from biomass burning. In Proceedings of the AGU Fall Meeting Abstracts, California, CA, USA, 13–17 December 2010; Volume 2010, p. A23C-03. [Google Scholar]
- Goode, J.G.; Yokelson, R.J.; Susott, R.A.; Ward, D.E. Trace gas emissions from laboratory biomass fires measured by open-path Fourier transform infrared spectroscopy: Fires in grass and surface fuels. J. Geophys. Res. Atmos. 1999, 104, 21237–21245. [Google Scholar] [CrossRef]
- Yokelson, R.J.; Burling, I.; Gilman, J.; Warneke, C.; Stockwell, C.; De Gouw, J.; Akagi, S.; Urbanski, S.; Veres, P.; Roberts, J. Coupling field and laboratory measurements to estimate the emission factors of identified and unidentified trace gases for prescribed fires. Atmos. Chem. Phys. 2013, 13, 89–116. [Google Scholar] [CrossRef]
- Keene, W.C.; Lobert, J.M.; Crutzen, P.J.; Maben, J.R.; Scharffe, D.H.; Landmann, T.; Hély, C.; Brain, C. Emissions of major gaseous and particulate species during experimental burns of southern African biomass. J. Geophys. Res. Atmos. 2006, 111, e2005JD006319. [Google Scholar] [CrossRef]
- Horn, S.A.; Dasgupta, P.K. The Air Quality Index (AQI) in historical and analytical perspective a tutorial review. Talanta 2024, 267, 125260. [Google Scholar] [CrossRef] [PubMed]
- Sablan, O.; Ford, B.; Gargulinski, E.; Hammer, M.S.; Henery, G.; Kondragunta, S.; Martin, R.V.; Rosen, Z.; Slater, K.; van Donkelaar, A. Quantifying prescribed-fire smoke exposure using low-cost sensors and satellites: Springtime burning in Eastern Kansas. GeoHealth 2024, 8, e2023GH000982. [Google Scholar] [CrossRef]
- Scharko, N.K.; Oeck, A.M.; Tonkyn, R.G.; Baker, S.P.; Lincoln, E.N.; Chong, J.; Corcoran, B.M.; Burke, G.M.; Weise, D.R.; Myers, T.L.; et al. Identification of gas-phase pyrolysis products in a prescribed fire: First detections using infrared spectroscopy for naphthalene, methyl nitrite, allene, acrolein and acetaldehyde. Atmos. Meas. Tech. 2019, 12, 763–776. [Google Scholar] [CrossRef]
- Juncosa Calahorrano, J.F.; Lindaas, J.; O’Dell, K.; Palm, B.B.; Peng, Q.; Flocke, F.; Pollack, I.B.; Garofalo, L.A.; Farmer, D.K.; Pierce, J.R.; et al. Daytime Oxidized Reactive Nitrogen Partitioning in Western U.S. Wildfire Smoke Plumes. J. Geophys. Res. Atmos. 2021, 126, e2020JD033484. [Google Scholar] [CrossRef]
- Fisher, J.A.; Jacob, D.J.; Wang, Q.; Bahreini, R.; Carouge, C.C.; Cubison, M.J.; Dibb, J.E.; Diehl, T.; Jimenez, J.L.; Leibensperger, E.M. Sources, distribution, and acidity of sulfate–ammonium aerosol in the Arctic in winter–spring. Atmos. Environ. 2011, 45, 7301–7318. [Google Scholar] [CrossRef]
- Plaza, J.; Pujadas, M.; Gómez-Moreno, F.J.; Sánchez, M.; Artíñano, B. Mass size distributions of soluble sulfate, nitrate and ammonium in the Madrid urban aerosol. Atmos. Environ. 2011, 45, 4966–4976. [Google Scholar] [CrossRef]
- Boaggio, K.; LeDuc, S.D.; Rice, R.B.; Duffney, P.F.; Foley, K.M.; Holder, A.L.; McDow, S.; Weaver, C.P. Beyond particulate matter mass: Heightened levels of lead and other pollutants associated with destructive fire events in California. Environ. Sci. Technol. 2022, 56, 14272–14283. [Google Scholar] [CrossRef]
- Sparks, T.L.; Wagner, J. Composition of particulate matter during a wildfire smoke episode in an urban area. Aerosol Sci. Technol. 2021, 55, 734–747. [Google Scholar] [CrossRef]
- Lanzerstorfer, C. Potential of industrial de-dusting residues as a source of potassium for fertilizer production–a mini review. Resour. Conserv. Recycl. 2019, 143, 68–76. [Google Scholar] [CrossRef]
- Liu, X.; Huey, L.G.; Yokelson, R.J.; Selimovic, V.; Simpson, I.J.; Mueller, M.; Jimenez, J.L.; Campuzano-Jost, P.; Beyersdorf, A.J.; Blake, D.R.; et al. Airborne measurements of western US wildfire emissions: Comparison with prescribed burning and air quality implications. J. Geophys. Res. -Atmos. 2017, 122, 6108–6129. [Google Scholar] [CrossRef]
- Holder, A.L.; Hagler, G.S.W.; Aurell, J.; Hays, M.D.; Gullett, B.K. Particulate matter and black carbon optical properties and emission factors from prescribed fires in the southeastern United States. J. Geophys. Res. -Atmos. 2016, 121, 3465–3483. [Google Scholar] [CrossRef]
- Williamson, G.J.; Bowman, D.; Price, O.F.; Henderson, S.B.; Johnston, F.H. A transdisciplinary approach to understanding the health effects of wildfire and prescribed fire smoke regimes. Environ. Res. Lett. 2016, 11, 125009. [Google Scholar] [CrossRef]
- Robertson, K.M.; Hsieh, Y.P.; Bugna, G.C. Fire environment effects on particulate matter emission factors in southeastern US pine-grasslands. Atmos. Environ. 2014, 99, 104–111. [Google Scholar] [CrossRef]
- Heilman, W.E.; Liu, Y.; Urbanski, S.; Kovalev, V.; Mickler, R. Wildland fire emissions, carbon, and climate: Plume rise, atmospheric transport, and chemistry processes. For. Ecol. Manag. 2014, 317, 70–79. [Google Scholar] [CrossRef]
- Liu, J.C.; Peng, R.D. The impact of wildfire smoke on compositions of fine particulate matter by ecoregion in the Western US. J. Expo. Sci. Amp; Environ. Epidemiol. 2019, 29, 765–776. [Google Scholar] [CrossRef]
- Hodzic, A.; Madronich, S.; Bohn, B.; Massie, S.; Menut, L.; Wiedinmyer, C. Wildfire particulate matter in Europe during summer 2003: Meso-scale modeling of smoke emissions, transport and radiative effects. Atmos. Chem. Phys. 2007, 7, 4043–4064. [Google Scholar] [CrossRef]
- Brock, C.A.; Cozic, J.; Bahreini, R.; Froyd, K.D.; Middlebrook, A.M.; McComiskey, A.; Brioude, J.; Cooper, O.R.; Stohl, A.; Aikin, K.C.; et al. Characteristics, sources, and transport of aerosols measured in spring 2008 during the aerosol, radiation, and cloud processes affecting Arctic Climate (ARCPAC) Project. Atmos. Chem. Phys. 2011, 11, 2423–2453. [Google Scholar] [CrossRef]
- Sofiev, M.; Ermakova, T.; Vankevich, R. Evaluation of the smoke-injection height from wild-land fires using remote-sensing data. Atmos. Chem. Phys. 2012, 12, 1995–2006. [Google Scholar] [CrossRef]
- Akagi, S.K.; Craven, J.S.; Taylor, J.W.; McMeeking, G.R.; Yokelson, R.J.; Burling, I.R.; Urbanski, S.P.; Wold, C.E.; Seinfeld, J.H.; Coe, H.; et al. Evolution of trace gases and particles emitted by a chaparral fire in California. Atmos. Chem. Phys. 2012, 12, 1397–1421. [Google Scholar] [CrossRef]
- Hsiao, C.J.; Özdemir, A.; Lin, J.L.; Chen, C.H. Portable particle mass spectrometer. Analyst 2022, 147, 2644–2654. [Google Scholar] [CrossRef] [PubMed]
- Han, X.X.; Li, J.X.; Jiang, J.C.; Wu, C.X.; Chen, P.; Zhang, Z.P.; Hou, K.Y.; Li, H.Y. Rapid Detection of Trace Volatile Organic Compounds By Electric Driven Cryo-Trap Movable Mass Spectrometer. Chin. J. Anal. Chem. 2018, 46, 642–649. [Google Scholar] [CrossRef]
- Pathirana, S.L.; Van Der Veen, C.; Popa, M.E.; Röckmann, T. An analytical system for stable isotope analysis on carbon monoxide using continuous-flow isotope-ratio mass spectrometry. Atmos. Meas. Technol. 2015, 8, 5315–5324. [Google Scholar] [CrossRef]
- Hung, H.-M.; Hsu, M.-N.; Hoffmann, M.R. Quantification of SO2 Oxidation on Interfacial Surfaces of Acidic Micro-Droplets: Implication for Ambient Sulfate Formation. Environ. Sci. & Technol. 2018, 52, 9079–9086. [Google Scholar] [CrossRef]
- Diaz, J.A.; Pieri, D.; Wright, K.; Sorensen, P.; Kline-Shoder, R.; Arkin, C.R.; Fladeland, M.; Bland, G.; Buongiorno, M.F.; Ramirez, C.; et al. Unmanned Aerial Mass Spectrometer Systems for In-Situ Volcanic Plume Analysis. J. Am. Soc. Mass Spectrom. 2015, 26, 292–304. [Google Scholar] [CrossRef]
- Clemitshaw, K. A Review of Instrumentation and Measurement Techniques for Ground-Based and Airborne Field Studies of Gas-Phase Tropospheric Chemistry. Crit. Rev. Environ. Sci. Technol. 2004, 34, 1–108. [Google Scholar] [CrossRef]
- Li, D.-X.; Gan, L.; Bronja, A.; Schmitz, O.J. Gas chromatography coupled to atmospheric pressure ionization mass spectrometry (GC-API-MS): Review. Anal. Chim. Acta 2015, 891, 43–61. [Google Scholar] [CrossRef]
- Wallace, W.E.; Moorthy, A.S. NIST Mass Spectrometry Data Center standard reference libraries and software tools: Application to seized drug analysis. J. Forensic Sci. 2023, 68, 1484–1493. [Google Scholar] [CrossRef]
- Zauscher, M.D.; Wang, Y.; Moore, M.J.; Gaston, C.J.; Prather, K.A. Air quality impact and physicochemical aging of biomass burning aerosols during the 2007 San Diego wildfires. Environ. Sci. Technol. 2013, 47, 7633–7643. [Google Scholar] [CrossRef]
- Lindaas, J.; Pollack, I.B.; Calahorrano, J.J.; O’Dell, K.; Garofalo, L.A.; Pothier, M.A.; Farmer, D.K.; Kreidenweis, S.M.; Campos, T.; Flocke, F.; et al. Empirical insights into the fate of ammonia in western US wildfire smoke plumes. J. Geophys. Res. Atmos. 2021, 126, e2020JD033730. [Google Scholar] [CrossRef]
- Permar, W.; Wang, Q.; Selimovic, V.; Wielgasz, C.; Yokelson, R.J.; Hornbrook, R.S.; Hills, A.J.; Apel, E.C.; Ku, I.T.; Zhou, Y. Emissions of trace organic gases from Western US wildfires based on WE-CAN aircraft measurements. J. Geophys. Res. Atmos. 2021, 126, e2020JD033838. [Google Scholar] [CrossRef]
- Casotto, R.; Kušan, A.C.; Bhattu, D.; Cui, T.; Manousakas, M.; Frka, S.; Kroflič, A.; Grgić, I.; Ciglenečki, I.; Baltensperger, U. Chemical composition and sources of organic aerosol on the Adriatic coast in Croatia. Atmos. Environ. X 2022, 13, 100159. [Google Scholar] [CrossRef]
- Winklhofer, E.; Fuchs, H. Laser induced fluorescence and flame photography—Tools in gasoline engine combustion analysis. Opt. Lasers Eng. 1996, 25, 379–400. [Google Scholar] [CrossRef]
- Daily, J.W. Laser induced fluorescence spectroscopy in flames. Prog. Energy Combust. Sci. 1997, 23, 133–199. [Google Scholar] [CrossRef]
- Hottle, J.R.; Huisman, A.J.; Digangi, J.P.; Kammrath, A.; Galloway, M.M.; Coens, K.L.; Keutsch, F.N. A Laser Induced Fluorescence-Based Instrument for In-Situ Measurements of Atmospheric Formaldehyde. Environ. Sci. Amp; Technol. 2009, 43, 790–795. [Google Scholar] [CrossRef]
- Di Carlo, P.; Aruffo, E.; Biancofiore, F.; Busilacchio, M.; Pitari, G.; Dari-Salisburgo, C.; Tuccella, P.; Kajii, Y. Wildfires impact on surface nitrogen oxides and ozone in Central Italy. Atmos. Pollut. Res. 2015, 6, 29–35. [Google Scholar] [CrossRef]
- Rollins, A. ATom: Sulfur Dioxide by Laser Induced Fluorescence (LIF-SO2) for ATom-4 Campaign; ORNL DAAC: Oak Ridge, TN, USA, 2021.
- Papanastasiou, D.K.; Bernard, F.; Burkholder, J.B. Atmospheric fate of methyl isocyanate, CH3NCO: OH and Cl reaction kinetics and identification of formyl isocyanate, HC (O) NCO. ACS Earth Space Chem. 2020, 4, 1626–1637. [Google Scholar] [CrossRef]
- Gudem, M.; Hazra, A. Mechanism of the chemiluminescent reaction between nitric oxide and ozone. J. Phys. Chem. A 2018, 123, 715–722. [Google Scholar] [CrossRef]
- Vasil’ev, R.F. Chemiluminescence excitation mechanisms. Russ. Chem. Rev. 1970, 39, 529. [Google Scholar] [CrossRef]
- Bourgeois, I.; Peischl, J.; Neuman, J.A.; Brown, S.S.; Allen, H.M.; Campuzano-Jost, P.; Coggon, M.M.; DiGangi, J.P.; Diskin, G.S.; Gilman, J.B. Comparison of airborne measurements of NO, NO 2, HONO, NO y and CO during FIREX-AQ. Atmos. Meas. Technol. Discuss. 2022, 2022, 1–47. [Google Scholar] [CrossRef]
- Novak, G.A.; Vermeuel, M.P.; Bertram, T.H. Simultaneous detection of ozone and nitrogen dioxide by oxygen anion chemical ionization mass spectrometry: A fast-time-response sensor suitable for eddy covariance measurements. Atmos. Meas. Technol. 2020, 13, 1887–1907. [Google Scholar] [CrossRef]
- Hong, T.-K.; Roh, B.-S.; Park, S.-H. Measurements of Optical Properties of Smoke Particulates Produced from Burning Polymers and Their Implications. Energies 2020, 13, 2299. [Google Scholar] [CrossRef]
- Fonollosa, J.; Solórzano, A.; Marco, S. Chemical Sensor Systems and Associated Algorithms for Fire Detection: A Review. Sensors 2018, 18, 553. [Google Scholar] [CrossRef]
- Lux, L.; Phal, Y.; Hsieh, P.-H.; Bhargava, R. On the Limit of Detection in Infrared Spectroscopic Imaging. Appl. Spectrosc. 2022, 76, 105–117. [Google Scholar] [CrossRef]
- Selimovic, V.; Yokelson, R.J.; Warneke, C.; Roberts, J.M.; De Gouw, J.; Reardon, J.; Griffith, D.W. Aerosol optical properties and trace gas emissions by PAX and OP-FTIR for laboratory-simulated western US wildfires during FIREX. Atmos. Chem. Phys. 2018, 18, 2929–2948. [Google Scholar] [CrossRef]
- Burling, I.; Yokelson, R.J.; Griffith, D.W.; Johnson, T.J.; Veres, P.; Roberts, J.; Warneke, C.; Urbanski, S.; Reardon, J.; Weise, D. Laboratory measurements of trace gas emissions from biomass burning of fuel types from the southeastern and southwestern United States. Atmos. Chem. Phys. 2010, 10, 11115–11130. [Google Scholar] [CrossRef]
- Akagi, S.; Yokelson, R.J.; Burling, I.; Meinardi, S.; Simpson, I.; Blake, D.R.; McMeeking, G.; Sullivan, A.; Lee, T.; Kreidenweis, S. Measurements of reactive trace gases and variable O 3 formation rates in some South Carolina biomass burning plumes. Atmos. Chem. Phys. 2013, 13, 1141–1165. [Google Scholar] [CrossRef]
- Sadi, M.; Zhang, Y.; Xie, W.-F.; Hossain, F.M.A. Forest Fire Detection and Localization Using Thermal and Visual Cameras. In Proceedings of the 2021 International Conference on Unmanned Aircraft Systems (ICUAS), Athens, Greece, 15 June 2021; IEEE: Long Beach, CA, USA; pp. 744–749. [Google Scholar] [CrossRef]
- Yuan, C.; Liu, Z.; Zhang, Y. Fire Detection Using Infrared Images for UAV-Based Forest Fire Surveillance. In Proceedings of the International Conference on Unmanned Aircraft Systems (ICUAS), Miami, FL, USA, 13–16 June 2017. [Google Scholar]
- Hua, L.; Shao, G. The progress of operational forest fire monitoring with infrared remote sensing. J. For. Res. 2017, 28, 215–229. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, C.; Liu, X.; Tian, Y.; Zhang, J.; Cui, W. Forest Fire Monitoring Method Based on UAV Visual and Infrared Image Fusion. Remote Sens. 2023, 15, 3173. [Google Scholar] [CrossRef]
- Gowravaram, S.; Chao, H.; Zhao, T.; Parsons, S.; Hu, X.; Xin, M.; Flanagan, H.; Tian, P. Prescribed grass fire evolution mapping and rate of spread measurement using orthorectified thermal imagery from a fixed-wing UAS. Int. J. Remote Sens. 2022, 43, 2357–2376. [Google Scholar] [CrossRef]
- Chen, X.; Hopkins, B.; Wang, H.; O’Neill, L.; Afghah, F.; Razi, A.; Fulé, P.; Coen, J.; Rowell, E.; Watts, A. Wildland Fire Detection and Monitoring Using a Drone-Collected RGB/IR Image Dataset. IEEE Access 2022, 10, 121301–121317. [Google Scholar] [CrossRef]
- Katurji, M.; Zhang, J.; Satinsky, A.; McNair, H.; Schumacher, B.; Strand, T.; Valencia, A.; Finney, M.; Pearce, G.; Kerr, J. Turbulent thermal image velocimetry at the immediate fire and atmospheric interface. J. Geophys. Res. Atmos. 2021, 126, e2021JD035393. [Google Scholar] [CrossRef]
- Tsai, P.-F.; Liao, C.-H.; Yuan, S.-M. Using deep learning with thermal imaging for human detection in heavy smoke scenarios. Sensors 2022, 22, 5351. [Google Scholar] [CrossRef]
- Spampinato, L.; Calvari, S.; Oppenheimer, C.; Boschi, E. Volcano surveillance using infrared cameras. Earth-Sci. Rev. 2011, 106, 63–91. [Google Scholar] [CrossRef]
- Guan, S.; Wong, D.C.; Gao, Y.; Zhang, T.; Pouliot, G. Impact of wildfire on particulate matter in the southeastern United States in November 2016. Sci. Total Environ. 2020, 724, 138354. [Google Scholar] [CrossRef]
- Landguth, E.L.; Holden, Z.A.; Graham, J.; Stark, B.; Mokhtari, E.B.; Kaleczyc, E.; Anderson, S.; Urbanski, S.; Jolly, M.; Semmens, E.O.; et al. The delayed effect of wildfire season particulate matter on subsequent influenza season in a mountain west region of the USA. Environ. Int. 2020, 139, 105668. [Google Scholar] [CrossRef]
- Deflorio-Barker, S.; Crooks, J.; Reyes, J.; Rappold, A.G. Cardiopulmonary Effects of Fine Particulate Matter Exposure among Older Adults, during Wildfire and Non-Wildfire Periods, in the United States 2008–2010. Environ. Health Perspect. 2019, 127, 037006. [Google Scholar] [CrossRef]
- Mazzoleni, C.; Kuhns, H.D.; Moosmüller, H. Monitoring Automotive Particulate Matter Emissions with LiDAR: A Review. Remote Sens. 2010, 2, 1077–1119. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, T.; Xiang, Y.; Chai, W.; Liu, W. Distribution and transport characteristics of fine particulate matter in beijing with mobile lidar measurements from 2015 to 2018. J. Environ. Sci. 2022, 115, 65–75. [Google Scholar] [CrossRef]
- Alpers, M.; Eixmann, R.; Fricke-Begemann, C.; Gerding, M.; Höffner, J. Temperature lidar measurements from 1 to 105 km altitude using resonance, Rayleigh, and Rotational Raman scattering. Atmos. Chem. Phys. 2004, 4, 793–800. [Google Scholar] [CrossRef]
- Damiano, R.; Amoruso, S.; Sannino, A.; Boselli, A. Lidar Optical and Microphysical Characterization of Tropospheric and Stratospheric Fire Smoke Layers Due to Canadian Wildfires Passing over Naples (Italy). Remote Sens. 2024, 16, 538. [Google Scholar] [CrossRef]
- Warner, T.A.; Skowronski, N.S.; La Puma, I. The influence of prescribed burning and wildfire on lidar-estimated forest structure of the New Jersey Pinelands National Reserve. Int. J. Wildland Fire 2020, 29, 1100. [Google Scholar] [CrossRef]
- Ceolato, R.; Berg, M.J. Aerosol light extinction and backscattering: A review with a lidar perspective. J. Quant. Spectrosc. Radiat. Transf. 2021, 262, 107492. [Google Scholar] [CrossRef]
- Wu, Y.; Cordero, L.; Gross, B.; Moshary, F.; Ahmed, S. Smoke plume optical properties and transport observed by a multi-wavelength lidar, sunphotometer and satellite. Atmos. Environ. 2012, 63, 32–42. [Google Scholar] [CrossRef]
- Innocenti, F.; Robinson, R.; Gardiner, T.; Finlayson, A.; Connor, A. Differential absorption lidar (DIAL) measurements of landfill methane emissions. Remote Sens. 2017, 9, 953. [Google Scholar] [CrossRef]
- Ronen, A.; Agassi, E.; Yaron, O. Sensing with polarized lidar in degraded visibility conditions due to fog and low clouds. Sensors 2021, 21, 2510. [Google Scholar] [CrossRef]
- Fersch, T.; Buhmann, A.; Koelpin, A.; Weigel, R. The influence of rain on small aperture LiDAR sensors. In Proceedings of the 2016 German Microwave Conference (GeMiC), Bochum, Germany, 14–16 March 2016; pp. 84–87. [Google Scholar]
- Risbøl, O.; Gustavsen, L. LiDAR from drones employed for mapping archaeology–Potential, benefits and challenges. Archaeol. Prospect. 2018, 25, 329–338. [Google Scholar] [CrossRef]
- Mehadi, A.; Moosmüller, H.; Campbell, D.E.; Ham, W.; Schweizer, D.; Tarnay, L.; Hunter, J. Laboratory and field evaluation of real-time and near real-time PM2.5 smoke monitors. J. Air Amp; Waste Manag. Assoc. 2020, 70, 158–179. [Google Scholar] [CrossRef]
- Han, J.; Liu, X.; Chen, D.; Jiang, M. Influence of relative humidity on real-time measurements of particulate matter concentration via light scattering. J. Aerosol Sci. 2020, 139, 105462. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.; Roh, S. Effects of gas and steam humidity on particulate matter measurements obtained using light-scattering sensors. Sensors 2023, 23, 6199. [Google Scholar] [CrossRef] [PubMed]
- Atkins, J.W.; Bohrer, G.; Fahey, R.T.; Hardiman, B.S.; Morin, T.H.; Stovall, A.E.L.; Zimmerman, N.; Gough, C.M. Quantifying vegetation and canopy structural complexity from terrestrial LiDAR data using the FORESTR R package. Methods Ecol. Evol. 2018, 9, 2057–2066. [Google Scholar] [CrossRef]
- Ross, C.W.; Loudermilk, E.L.; O’Brien, J.J.; Flanagan, S.A.; McDaniel, J.; Aubrey, D.P.; Lowe, T.; Hiers, J.K.; Skowronski, N.S. Lidar-derived estimates of forest structure in response to fire frequency. Fire Ecol. 2024, 20, 44. [Google Scholar] [CrossRef]
- Carbonell-Rivera, J.P.; Moran, C.J.; Seielstad, C.A.; Parsons, R.A.; Hoff, V.; Ruiz, L.A.; Torralba, J.; Estornell, J. Relationships of Fire Rate of Spread with Spectral and Geometric Features Derived from UAV-Based Photogrammetric Point Clouds. Fire 2024, 7, 132. [Google Scholar] [CrossRef]
- Lutsch, E.; Strong, K.; Jones, D.B.A.; Blumenstock, T.; Conway, S.; Fisher, J.A.; Hannigan, J.W.; Hase, F.; Kasai, Y.; Mahieu, E.; et al. Detection and attribution of wildfire pollution in the Arctic and northern midlatitudes using a network of Fourier-transform infrared spectrometers and GEOS-Chem. Atmos. Chem. Phys. 2020, 20, 12813–12851. [Google Scholar] [CrossRef]
- Stöbener, A.; Naefken, U.; Kleber, J.; Liese, A. Determination of trace amounts with ATR FTIR spectroscopy and chemometrics: 5-(hydroxymethyl) furfural in honey. Talanta 2019, 204, 1–5. [Google Scholar] [CrossRef]
- Brilli, F.; Gioli, B.; Ciccioli, P.; Zona, D.; Loreto, F.; Janssens, I.A.; Ceulemans, R. Proton Transfer Reaction Time-of-Flight Mass Spectrometric (PTR-TOF-MS) determination of volatile organic compounds (VOCs) emitted from a biomass fire developed under stable nocturnal conditions. Atmos. Environ. 2014, 97, 54–67. [Google Scholar] [CrossRef]
- Zarrouk, E.; El Balkhi, S.; Saint-Marcoux, F. Low-Resolution or High-Resolution Mass Spectrometry for Clinical and Forensic Toxicology: Some Considerations from Two Real Cases. LC GC Int. 2023, 19, 10–13. [Google Scholar]
- Barkjohn, K.K.; Holder, A.L.; Frederick, S.G.; Clements, A.L. Correction and Accuracy of PurpleAir PM2.5 Measurements for Extreme Wildfire Smoke. Sensors 2022, 22, 9669. [Google Scholar] [CrossRef]
- Holder, A.L.; Mebust, A.K.; Maghran, L.A.; McGown, M.R.; Stewart, K.E.; Vallano, D.M.; Elleman, R.A.; Baker, K.R. Field Evaluation of Low-Cost Particulate Matter Sensors for Measuring Wildfire Smoke. Sensors 2020, 20, 4796. [Google Scholar] [CrossRef]
- Zavala, M.; Herndon, S.C.; Wood, E.C.; Jayne, J.T.; Nelson, D.D.; Trimborn, A.M.; Dunlea, E.; Knighton, W.B.; Mendoza, A.; Allen, D.T.; et al. Comparison of emissions from on-road sources using a mobile laboratory under various driving and operational sampling modes. Atmos. Chem. Phys. 2009, 9, 1–14. [Google Scholar] [CrossRef]
- Herndon, S.C.; Jayne, J.T.; Zahniser, M.S.; Worsnop, D.R.; Knighton, B.; Alwine, E.; Lamb, B.K.; Zavala, M.; Nelson, D.D.; McManus, J.B.; et al. Characterization of urban pollutant emission fluxes and ambient concentration distributions using a mobile laboratory with rapid response instrumentation. Faraday Discuss. 2005, 130, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Carroll, B.J.; Brewer, W.A.; Strobach, E.; Lareau, N.; Brown, S.S.; Valero, M.M.; Kochanski, A.; Clements, C.B.; Kahn, R.; Noyes, K.T.J.; et al. Measuring Coupled Fire-Atmosphere Dynamics. Bull. Am. Meteorol. Soc. 2024, 105, E690–E708. [Google Scholar] [CrossRef]

| Ecosystem Type | Fire Type | CO2 (g/kg) | CO (g/kg) | CH4 (g/kg) | NOX (g/kg) | PM2.5 (g/kg) | Source |
|---|---|---|---|---|---|---|---|
| Temperate Forest | Prescribed | 1590–1620 | 95–110 | 3.2–3.8 | 1.9–2.1 | 11.0–13.0 | Urbanski (2013) [60] |
| Temperate Forest | Wildfire | 1650–1700 | 125–140 | 5.8–6.5 | 2.1–2.5 | 15.5–18.0 | Andreae (2019) [66]; Akagi et al. (2011) [67] |
| Shrubland/Chaparral | Prescribed | 1600–1640 | 85–95 | 3.8–4.2 | 1.8–2.2 | 13.0–15.0 | Akagi et al. (2011) [67] |
| Shrubland/Chaparral | Wildfire | 1650–1690 | 115–125 | 4.8–5.5 | 2.3–2.7 | 15.0–17.0 | Akagi et al. (2011) [67]; Andreae (2019) [66] |
| Savanna | Prescribed | 1660–1690 | 58–65 | 1.8–2.2 | 2.4–2.6 | 5.0–6.0 | Andreae (2019) [66] |
| Savanna | Wildfire | 1680–1710 | 65–75 | 2.3–2.7 | 2.6–2.8 | 5.8–6.5 | Akagi et al. (2011) [67] |
| Gas | Regulatory Limit (ppm) | Measurable Limit (ppm) | Detection Method | Reference |
|---|---|---|---|---|
| Carbon Monoxide (CO) | 9 ppm TWA, 35 ppm/1 h (EPA) | 25 ppm | Infrared radiation adsorption, electrochemical sensors | Navarro (2020) [70], Qui et al. (2019) [71] |
| Carbon Dioxide (CO2) | 5000 (TWA), 5000/8 h | 17 ppm | Gas chromatography, optical sensors, IR spectroscopy | Raza et al. [54], Cristofanelli et al. [72] |
| Methane (CH4) | 5000 ppm/24 h TWA recommended | 1 | Gas chromatography, laser absorption spectroscopy, infrared detectors | Raza et al. (2023) [54] ENR (1984) [73] |
| Volatile Organic Compounds (VOCs) | Varies | 0.01 | Gas chromatography, mass spectroscopy | Dickinson et al. (2022) [74], Margo et al. (2024) [75] |
| Nitrogen Oxides (NOx) | ~0.05 | 0.001 | Chemiluminescence, IR spectroscopy | EPA(1999) [76], Bishop S. (2021) [77] |
| Ammonia (NH3) | 25 ppm/10 h TWA | 0.4 | Chemiluminescence, laser absorption spectroscopy | Tomsche et al. (2023) [78], Margo et al. (2024) [75] |
| Benzene (C6H6) | 1 | 0.005 | Gas chromatography | Weisel (2010) [79], Huang et al. (2010) [80] |
| Toluene (C7H8) | 200 | 0.0005 | Gas chromatography | Huang et al. (2010) [80] |
| Phenol (C6H5OH) | 5 | 0.01 | HPLC, spectroscopy | Yi, and Kun-Lin [81] |
| Detection Technique | Use | Advantages | Challenges | References |
|---|---|---|---|---|
| LIDAR | Characterize aerosols | Long-range measurement, up to 3 km | Needs a straight path | Atkins et al. (2018) [159], Ross et al. (2024) [160] |
| Thermal Camera | Fuel inventory, fire radiative power | Can measure thermal emission with high accuracy | Not capable of resolving species-specific smoke composition | Katurji et al. (2021) [139], Carbonell-Rivera et al. (2024) [161] |
| Optical instruments (FTIR, luminescence) | Molecular composition of smoke | Molecular specificity; suitable for portable deployment; high sensititivity (~10 ppb) | Requires species that have IR absorption cross-section | Lutsch et al. (2020) [162], Akagi et al. (2014) [50], Stobener (2019) [163] |
| Mass spectrometer | Molecular composition of smoke; coupled PM weight and molecular composition | Molecular specificity; high sensitivity (<3 ppb) | Destructive to sample; large instrument footprint; cannot measure reactive intermediates | Brilli et al. (2014) [164], Permar (2021) [115], Zarrouk et al. (2023) [165] |
| Light scattering techniques | PM detection, smoke plume tracking | Can track PM levels in real time | Not suitable to measure molecular composition | Barkjohn et al. (2024) [166], Holder (2020) [167] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fesomade, K.I.; Walker, R.A. Prescribed Fire Smoke: A Review of Composition, Measurement Methods, and Analysis. Fire 2025, 8, 241. https://doi.org/10.3390/fire8070241
Fesomade KI, Walker RA. Prescribed Fire Smoke: A Review of Composition, Measurement Methods, and Analysis. Fire. 2025; 8(7):241. https://doi.org/10.3390/fire8070241
Chicago/Turabian StyleFesomade, Kayode I., and Robert A. Walker. 2025. "Prescribed Fire Smoke: A Review of Composition, Measurement Methods, and Analysis" Fire 8, no. 7: 241. https://doi.org/10.3390/fire8070241
APA StyleFesomade, K. I., & Walker, R. A. (2025). Prescribed Fire Smoke: A Review of Composition, Measurement Methods, and Analysis. Fire, 8(7), 241. https://doi.org/10.3390/fire8070241






