Thermal Properties of Energetic Materials—What Are the Sources of Discrepancies?
Abstract
1. Introduction
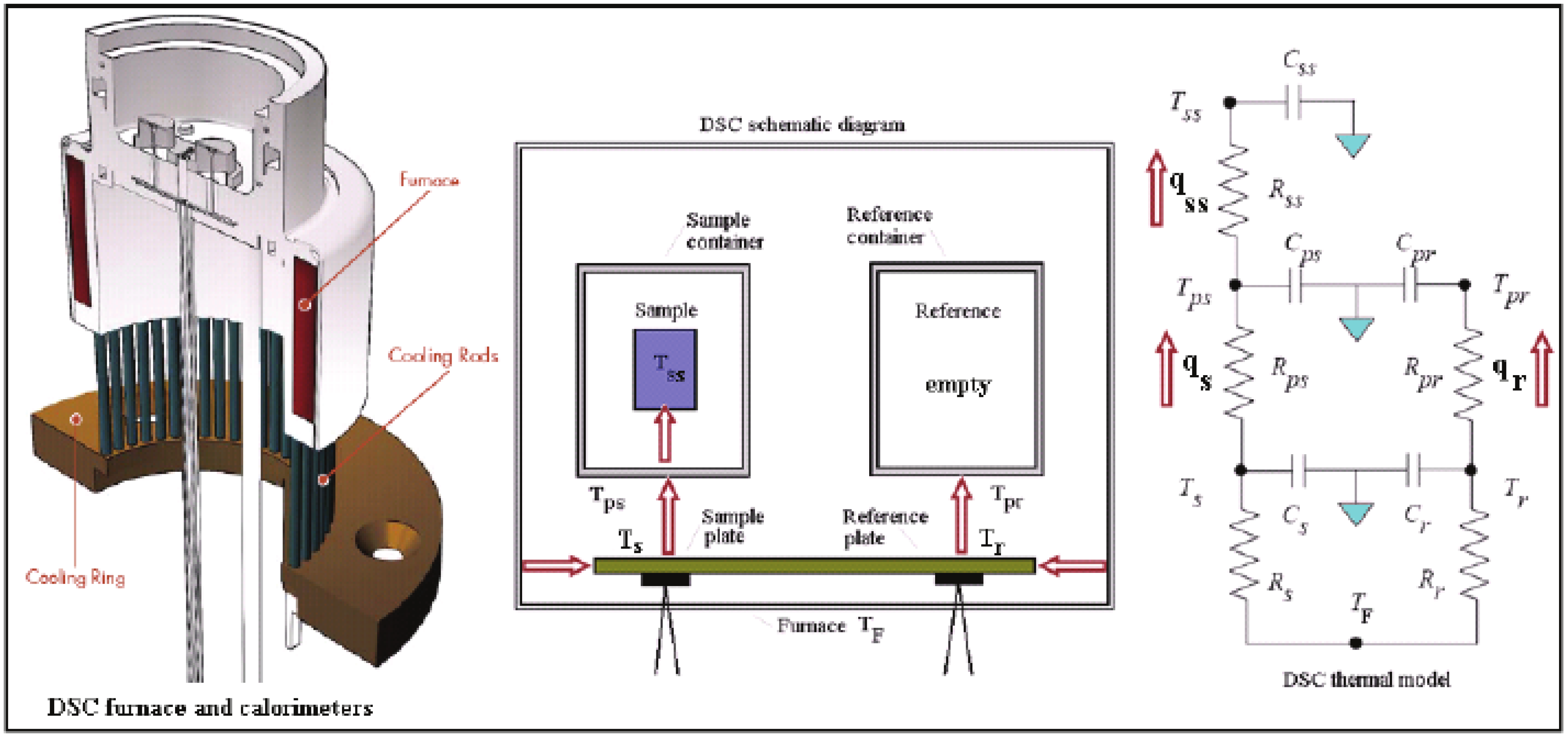
2. Methods of Investigation
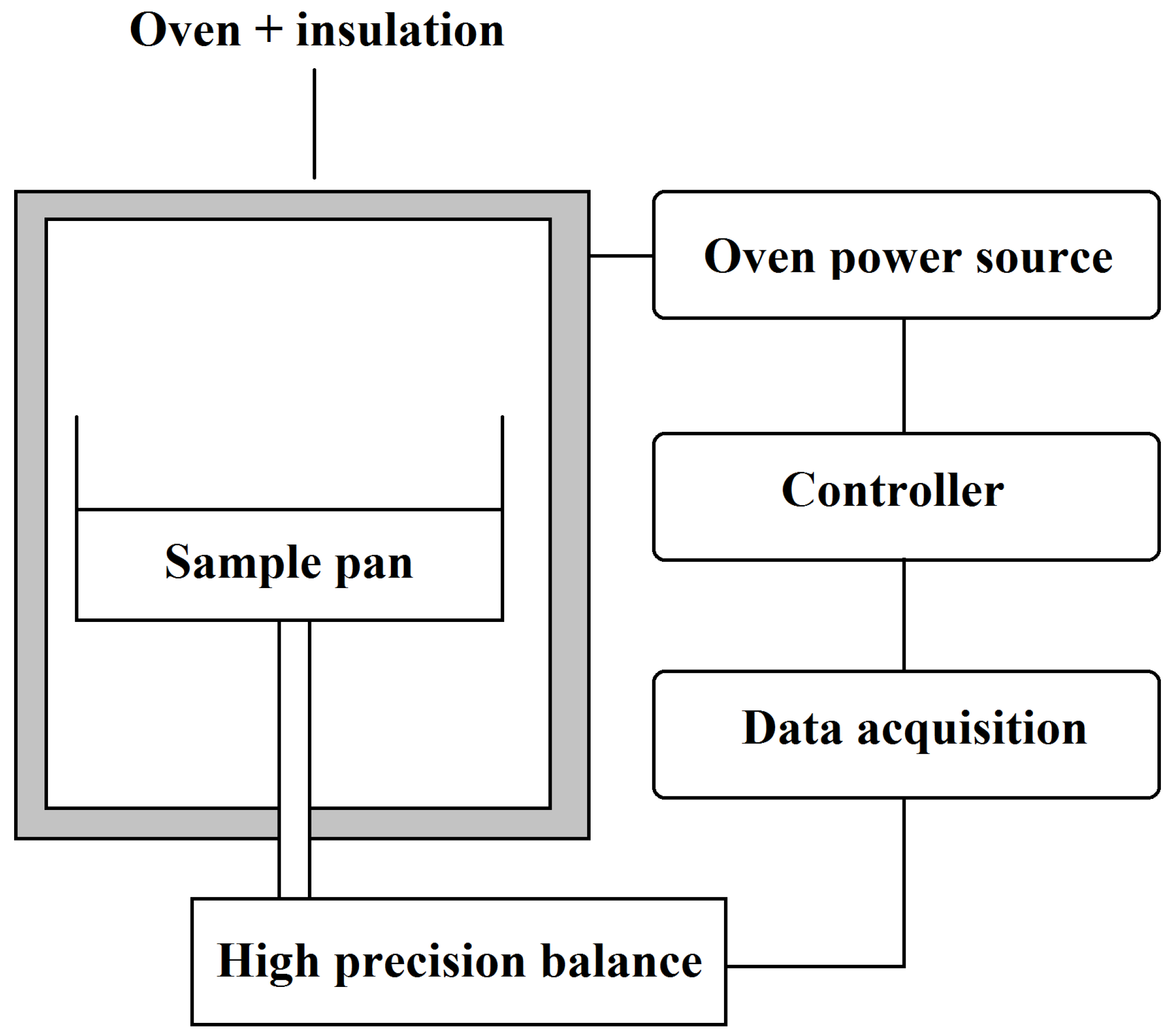
- —temperature gradient (K)
- q—eating rate (K/min)
- L—sample disc thickness (m)
- k—sample thermal diffusivity (m2/s)
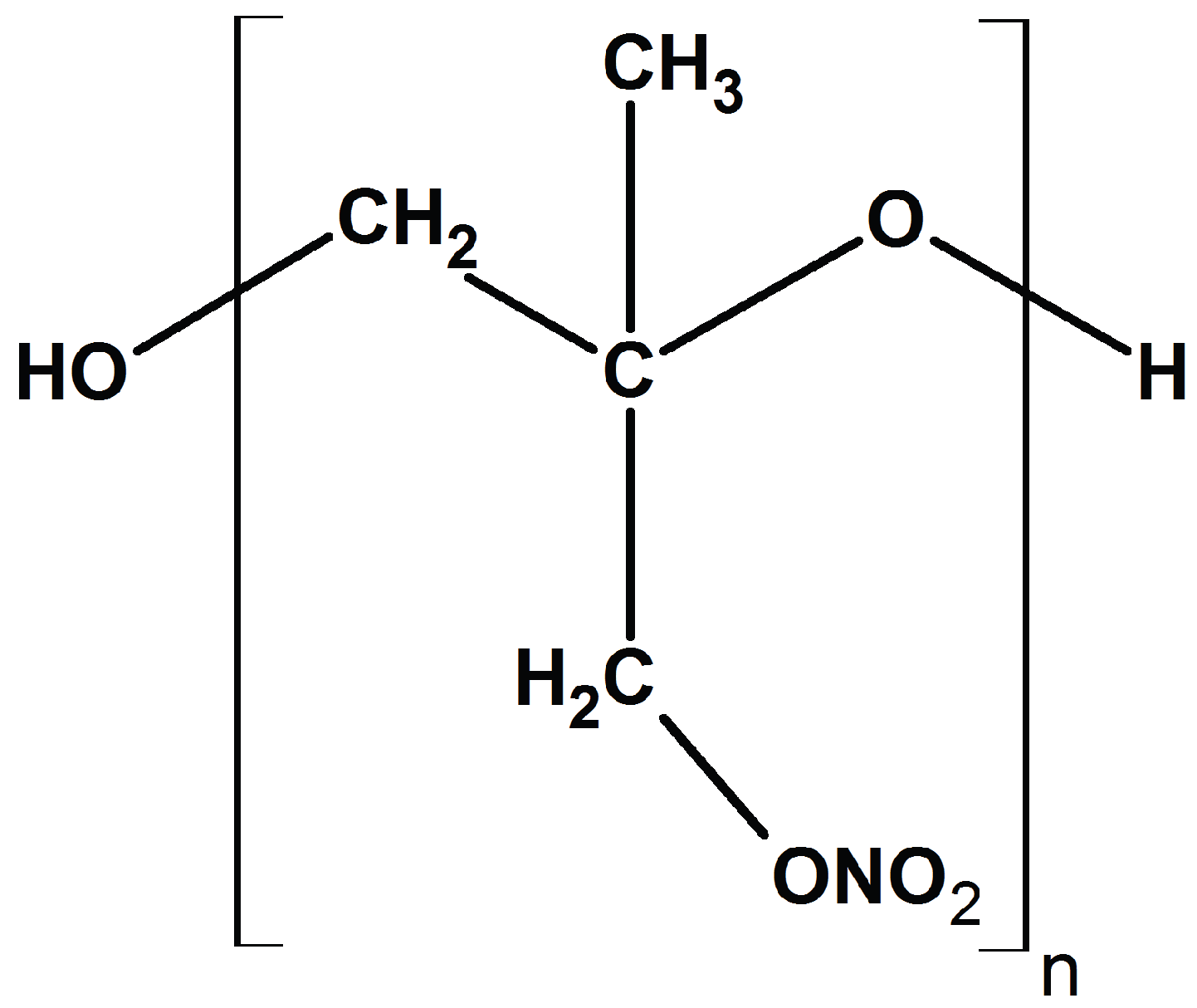
3. Case Study: Poly(3-nitratomethyl-3-methyloxetane) (PNIMMO)
4. Case Study: Assessment of the Thermal Compatibility of EM Formulation Components
5. Case Study: Use of Regression Methods
- —heating rate (K/min)
- —peak maximum (K)
- A—pre-exponential constant
- —activation energy (J/mol·K)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oluwoye, I.; Dlugogorski, B.Z.; Gore, J.; Oskierski, H.C.; Altarawneh, M. Atmospheric emission of NOx from mining explosives: A critical review. Atmos. Environ. 2017, 167, 81–96. [Google Scholar] [CrossRef]
- Pang, W.Q.; Yetter, R.A.; DeLuca, L.T.; Zarko, V.; Gany, A.; Zhang, X.H. Boron-based composite energetic materials (B-CEMs): Preparation, combustion and applications. Prog. Energy Combust. Sci. 2022, 93, 101038. [Google Scholar] [CrossRef]
- Xu, S.; Yang, X.; Zhang, F.; Liu, J. Application of a Microseismic Method of Rock Burst Risk Assessment under Blasting Mining in Ashele Copper Mine. Shock Vib. 2022, 2022, 5377528. [Google Scholar] [CrossRef]
- Seccatore, J.; Origliasso, C.; Tomi, G. Assessing a risk analysis methodology for rock blasting operations. In Proceedings of the Blasting in Mines-New Trends: Workshop Hosted by Fragblast, New Delhi, India, 24–25 November 2013. [Google Scholar]
- Alterman, D.; Stewart, M.G.; Netherton, M.D. Probabilistic assessment of airblast variability and fatality risk estimation for explosive blasts in confined building spaces. Int. J. Prot. Struct. 2019, 10, 306–329. [Google Scholar] [CrossRef]
- EN 13631-3:2005; Explosives for Civil Uses—High Explosives—Part 3: Determination of Sensitiveness to Friction of Explosives. European Standard: Pilsen, Czech Republic, 2005.
- EN 13631-4:2002; Explosives for Civil Uses. High Explosives Determination of Sensitiveness to Impact of Explosives. European Standard: Pilsen, Czech Republic, 2002.
- Skinner, D.; Olson, D.; Block-Bolten, A. Electrostatic discharge ignition of energetic materials. Propellants Explos. Pyrotech. 1998, 23, 34–42. [Google Scholar] [CrossRef]
- Ravi, P.; Badgujar, D.M.; Gore, G.M.; Tewari, S.P.; Sikder, A.K. Review on melt cast explosives. Propellants Explos. Pyrotech. 2011, 36, 393–403. [Google Scholar] [CrossRef]
- Green, S.P.; Wheelhouse, K.M.; Payne, A.D.; Hallett, J.P.; Miller, P.W.; Bull, J.A. On the use of differential scanning calorimetry for thermal hazard assessment of new chemistry: Avoiding explosive mistakes. Angew. Chem. Int. Ed. 2020, 59, 15798–15802. [Google Scholar] [CrossRef]
- Vyazovkin, S. Comments on multiple publications reporting single heating rate kinetics. Appl. Organomet. Chem. 2022, e6929. [Google Scholar] [CrossRef]
- Menczel, J.D.; Judovits, L.; Prime, R.B.; Bair, H.E.; Reading, M.; Swier, S. Differential Scanning Calorimetry (DSC). In Thermal Analysis of Polymers; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; Chapter 2; pp. 7–239. [Google Scholar]
- Rady, M.; Arquis, E. A comparative study of phase changing characteristics of granular phase change materials using DSC and T-history methods. Fluid Dyn. Mater. Process. 2010, 6, 137–152. [Google Scholar]
- Benhammada, A.; Trache, D. Thermal decomposition of energetic materials using TG-FTIR and TG-MS: A state-of-the-art review. Appl.Spectrosc. Rev. 2020, 55, 724–777. [Google Scholar] [CrossRef]
- Liu, Z. Review and prospect of thermal analysis technology applied to study thermal properties of energetic materials. FirePhysChem 2021, 1, 129–138. [Google Scholar] [CrossRef]
- Wunderlich, B. Chapter 4—Differential Thermal Analysis. In Thermal Analysis; Wunderlich, B., Ed.; Academic Press: Cambridge, MA, USA, 1990; pp. 123–218. [Google Scholar]
- Wu, Z.Q.; Dann, V.; Cheng, S.; Wunderlich, B. Fast DSC applied to the crystallization of polypropylene. J. Therm. Anal. 1988, 34, 105–114. [Google Scholar] [CrossRef]
- Yoshida, T.; Yoshizawa, F.; Ito, M.; Matsunaga, T.; Watanabe, M.; Tamura, M. Prediction of fire and explosion hazards of reactive chemicals (Part 1). Estimation of explosive properties of self-reactive chemicals from SC-DSC data. Kogyo Kayaku 1987, 48, 311–316. [Google Scholar]
- Sperry, J.B.; Azuma, M.; Stone, S. Explosive Hazard Identification in Pharmaceutical Process Development: A Novel Screening Method and Workflow for Shipping Potentially Explosive Materials. Org. Process Res. Dev. 2021, 25, 212–224. [Google Scholar] [CrossRef]
- Green, S.P.; Wheelhouse, K.M.; Payne, A.D.; Hallett, J.P.; Miller, P.W.; Bull, J.A. Thermal stability and explosive hazard assessment of diazo compounds and diazo transfer reagents. Org. Process Res. Dev. 2019, 24, 67–84. [Google Scholar] [CrossRef]
- Agrawal, J.P. Some new high energy materials and their formulations for specialized applications. Propellants, Explos. Pyrotech. 2005, 30, 316–328. [Google Scholar] [CrossRef]
- Yuan, X.; Guo, Q.; Zhang, S.; Gou, R.; Huang, Y. Comprehensive Study on Thermal Decomposition Mechanism and Interaction of 3-Nitro-1, 2, 4-Triazol-5-One/Poly-3-nitromethyl-3-methyloxetane Plastic Bonded Explosives. J. Anal. Appl. Pyrolysis 2022, 168, 105753. [Google Scholar] [CrossRef]
- Lin-quan, L.I.A.O.; Zheng, Y.; Ji-zhen, L.I. Review on synthesis, properties and applications of NIMMO and its polymer. Chin. J. Energ. Mater. 2011, 19, 113–118. [Google Scholar]
- Dong, Q.; Li, H.; Liu, X.; Huang, C. Thermal and rheological properties of PGN, PNIMMO and P (GN/NIMMO) synthesized via mesylate precursors. Propellants Explos. Pyrotech. 2018, 43, 294–299. [Google Scholar] [CrossRef]
- Bunyan, P. An investigation of the thermal decomposition of poly (3-nitratomethyl-3-methyloxetane). Thermochim. Acta 1992, 207, 147–159. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, H.; Shuai, J.; Gao, Z.; Dong, Q.; Wang, G.; Xiong, Y.; Zhu, Q.; Li, H. Synthesis and characterization of a novel fluorine-containing copolymer P (FPO/NIMMO) as a potential energetic binder. J. Fluor. Chem. 2021, 249, 109861. [Google Scholar] [CrossRef]
- Standardization Agreement (STANAG) 4147; Chemical Compatibility of Ammunition Components with Explosives (Non-Nuclear Applications). NATO Military Agency for Standardization: Brussels, Belgium, 2001.
- Kempa, T.J.; Barton, Z.M.; Cunliffe, A.V. Mechanism of the thermal degradation of prepolymeric poly (3-nitratomethyl-3-methyloxetane). Polymer 1999, 40, 65–93. [Google Scholar] [CrossRef]
- Gołofit, T.; Zyśk, K. Thermal decomposition properties and compatibility of CL-20 with binders HTPB, PBAN, GAP and polyNIMMO. J. Therm. Anal. Calorim. 2015, 119, 1931–1939. [Google Scholar] [CrossRef]
- Liao, L.Q.; Wei, H.J.; Li, J.Z.; Fan, X.Z.; Zheng, Y.; Ji, Y.P.; Fu, X.L.; Zhang, Y.J.; Liu, F.L. Compatibility of PNIMMO with some energetic materials. J. Therm. Anal. Calorim. 2012, 109, 1571–1576. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetic analysis of derivative curves in thermal analysis. J. Therm. Anal. 1970, 2, 301–324. [Google Scholar] [CrossRef]
- Jaw, K.S.; Lee, J.S. Thermal behaviors of PETN base polymer bonded explosives. J. Therm. Anal. Calorim. 2008, 93, 953–957. [Google Scholar] [CrossRef]
- Long, G.T.; Brems, B.A.; Wight, C.A. Autocatalytic thermal decomposition kinetics of TNT. Thermochim. Acta 2002, 388, 175–181. [Google Scholar] [CrossRef]
- Long, G.T.; Wight, C.A. Thermal decomposition of a melt-castable high explosive: Isoconversional analysis of TNAZ. J. Phys. Chem. B 2002, 106, 2791–2795. [Google Scholar] [CrossRef]
- Bonett, D.G.; Wright, T.A. Sample size requirements for estimating Pearson, Kendall and Spearman correlations. Psychometrika 2000, 65, 23–28. [Google Scholar] [CrossRef]
- De Winter, J.C. Using the Student’s t-test with extremely small sample sizes. Pract. Assess. Res. Eval. 2013, 18, 10. [Google Scholar]

| PNIMMO | Molecular Weight (Mn) | Carrier Gas/Heating Rate | Decomposition Temperature | T10 [°C] 1 | Tg [°C] | Ref. |
|---|---|---|---|---|---|---|
| [g/mol] | [K/min] | [°C] | ||||
| PNIMMO-A | - | N2/10 | 202.7 | - | −36 | [23] |
| PNIMMO-B | 5867 | N2/10 | 207.1 | 80 | −30.2 | [24] |
| PNIMMO-C | - | N2/10 | 218 | 77 | - | [25] |
| PNIMMO-D | 2610 | N2/10 | 218.9 | 120–200 | −35.6 | [26] |
| PNIMMO | 1600–2000 | N2/10 | - | - | - | [28] |
| PNIMMO | 2500 | N2/2 and 10 | 200 and 213 | - | - | [29] |
| PNIMMO | 3400–3500 | N2/10 | 218.2 | - | - | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stolarczyk, A.; Jarosz, T. Thermal Properties of Energetic Materials—What Are the Sources of Discrepancies? Fire 2022, 5, 206. https://doi.org/10.3390/fire5060206
Stolarczyk A, Jarosz T. Thermal Properties of Energetic Materials—What Are the Sources of Discrepancies? Fire. 2022; 5(6):206. https://doi.org/10.3390/fire5060206
Chicago/Turabian StyleStolarczyk, Agnieszka, and Tomasz Jarosz. 2022. "Thermal Properties of Energetic Materials—What Are the Sources of Discrepancies?" Fire 5, no. 6: 206. https://doi.org/10.3390/fire5060206
APA StyleStolarczyk, A., & Jarosz, T. (2022). Thermal Properties of Energetic Materials—What Are the Sources of Discrepancies? Fire, 5(6), 206. https://doi.org/10.3390/fire5060206






