Effects of Carbon Chain Length on N-Alkane Counterflow Cool Flames: A Kinetic Analysis
Abstract
1. Introduction
2. Kinetic Analysis Method
3. Results and Discussion
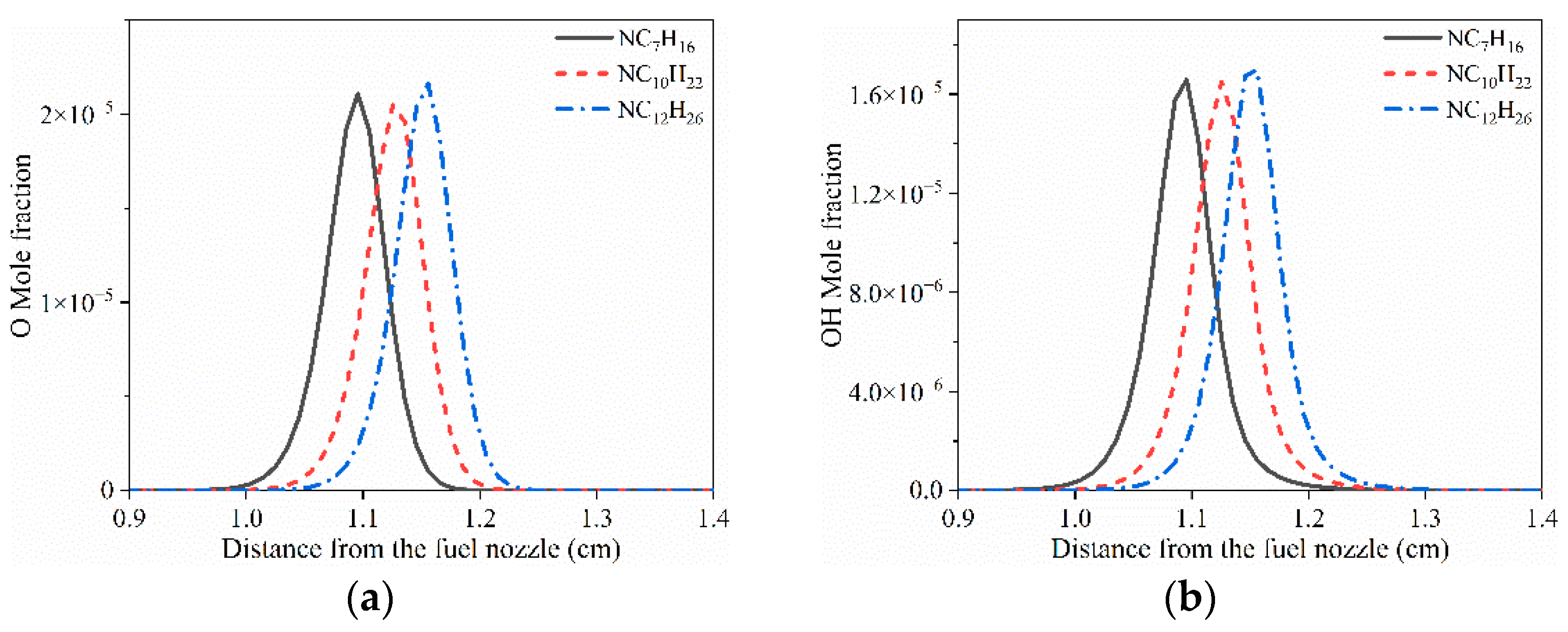
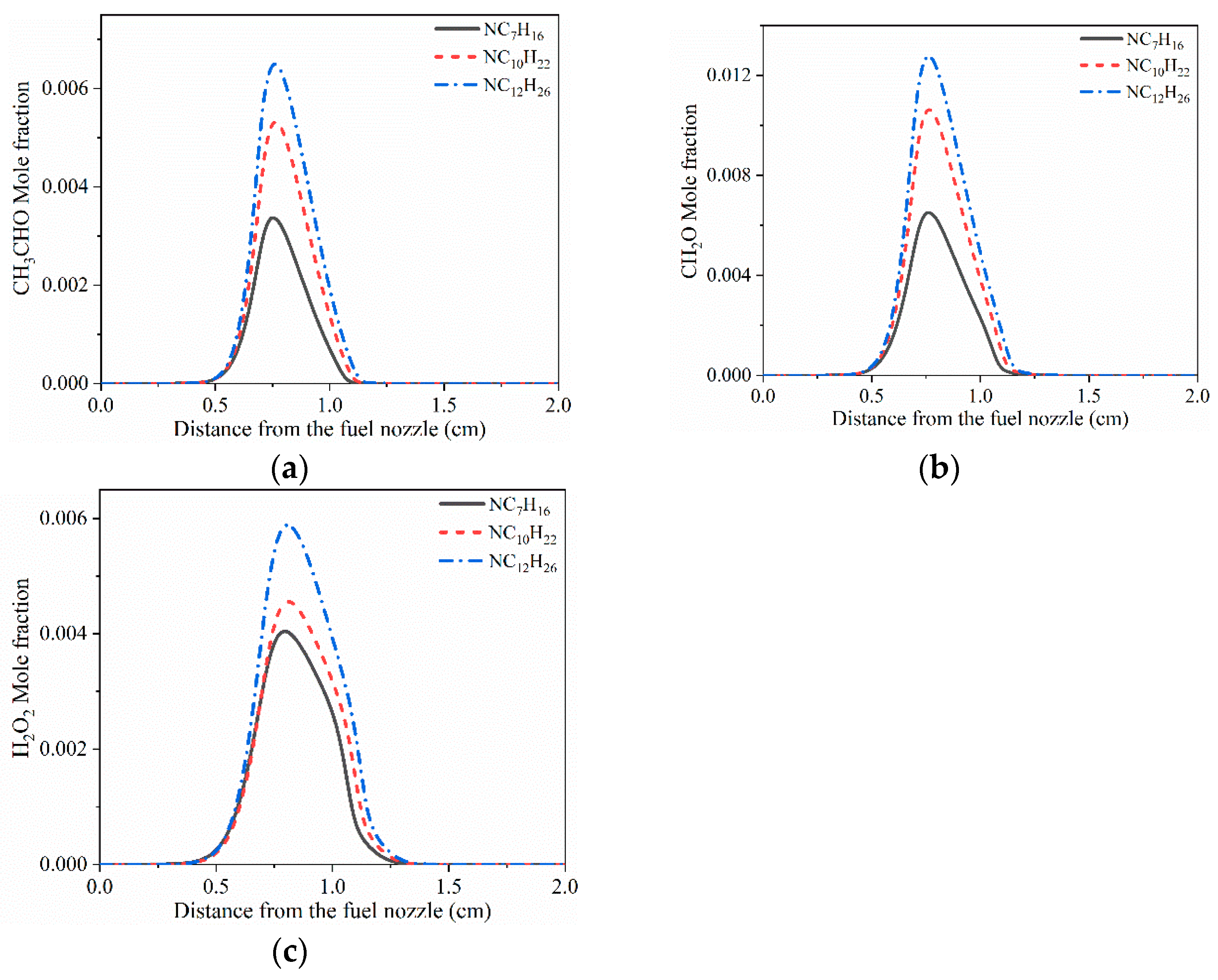
3.1. Structure of Typical Cool Diffusion Flame
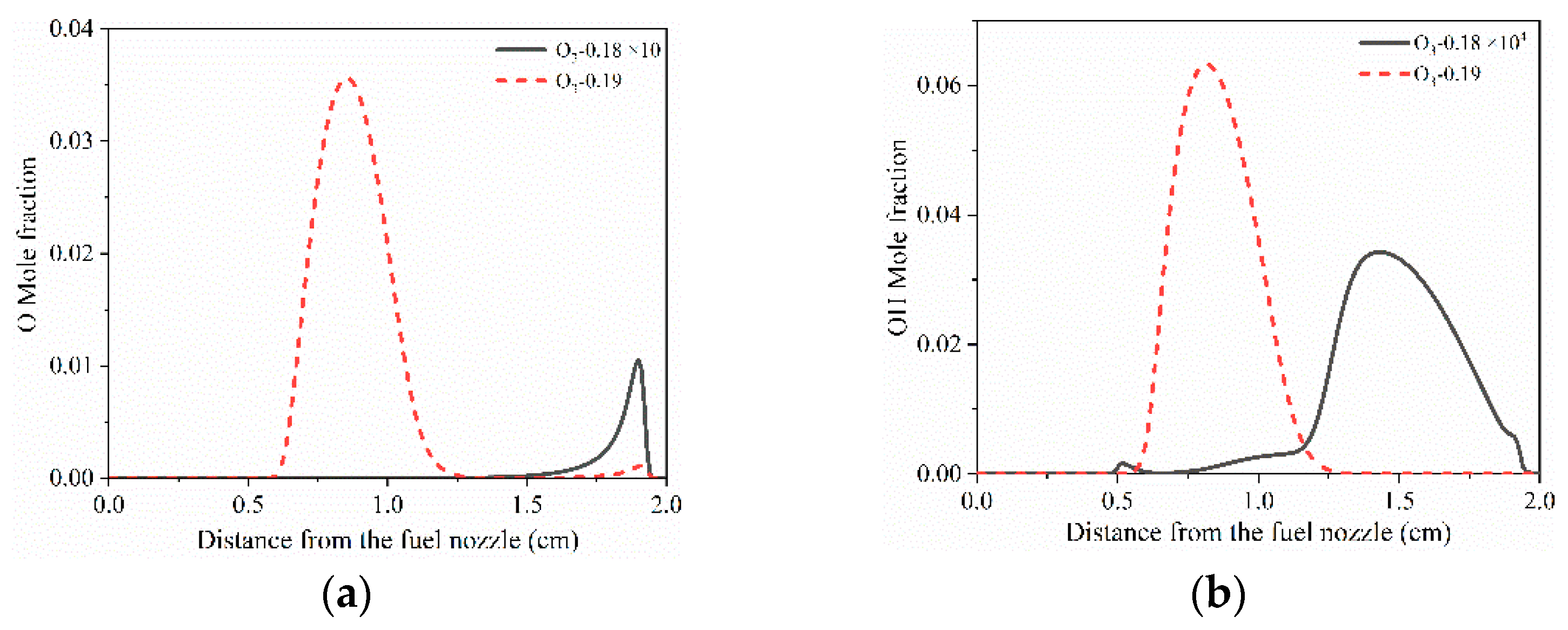
3.2. Initiation Limits of Cool Diffusion Flames
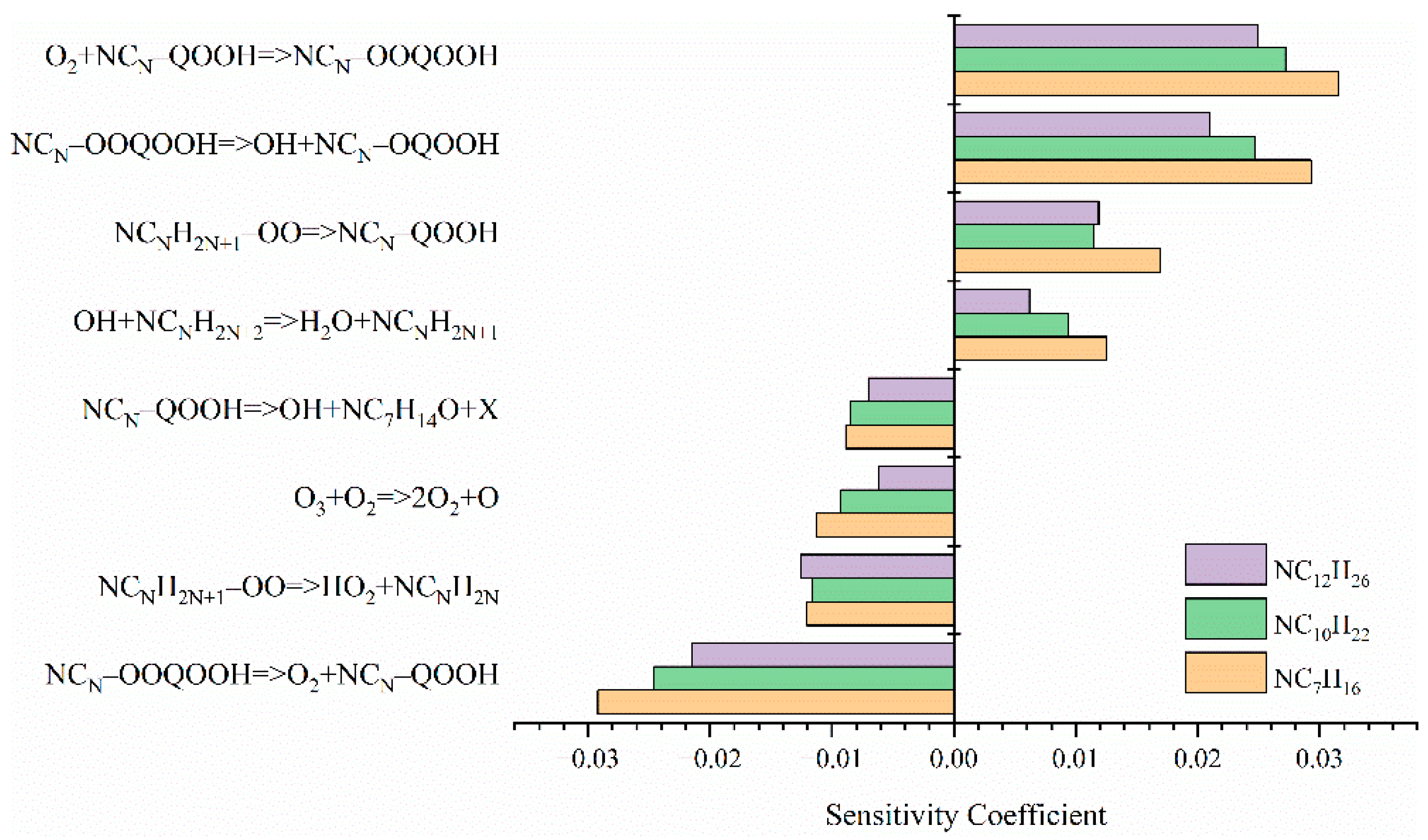
3.3. Kinetic Analysis of the Low-Temperature Reactivity of N-Alkanes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alabbad, M.; Issayev, G.; Badra, J.; Voice, A.K.; Giri, B.R.; Djebbi, K.; Ahmed, A.; Sarathy, S.M.; Farooq, A. Autoignition of straight-run naphtha: A promising fuel for advanced compression ignition engines. Combust. Flame 2018, 189, 337–346. [Google Scholar] [CrossRef]
- Shi, L.; Ji, C.; Wang, S.; Cong, X.; Su, T.; Shi, C. Impacts of dimethyl ether enrichment and various injection strategies on combustion and emissions of direct injection gasoline engines in the lean-burn condition. Fuel 2019, 254, 115636. [Google Scholar] [CrossRef]
- Aydoğan, B. Combustion characteristics, performance and emissions of an acetone/n-heptane fuelled homogenous charge compression ignition (HCCI) engine. Fuel 2020, 275, 117840. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, H. Combustion characteristics and performance of a methanol fueled homogenous charge compression ignition (HCCI) engine. J. Energy Inst. 2016, 89, 346–353. [Google Scholar] [CrossRef]
- Kaya, T.; Kutlar, O.A.; Taskiran, O.O. Evaluation of the partially premixed compression ignition combustion with diesel and biodiesel blended diesel at part load condition. Eng. Sci. Technol. Int. J. 2021, 24, 458–468. [Google Scholar] [CrossRef]
- Bahrami, S.; Poorghasemi, K.; Solmaz, H.; Calam, A.; İpci, D. Effect of nitrogen and hydrogen addition on performance and emissions in reactivity controlled compression ignition. Fuel 2021, 292, 120330. [Google Scholar] [CrossRef]
- Deng, S.; Han, D.; Law, C.K. Ignition and extinction of strained nonpremixed cool flames at elevated pressures. Combust. Flame 2017, 176, 143–150. [Google Scholar] [CrossRef]
- Zhao, P.; Liang, W.; Deng, S.; Law, C.K. Initiation and propagation of laminar premixed cool flames. Fuel 2016, 166, 477–487. [Google Scholar] [CrossRef]
- Lin, E.; Reuter, C.B.; Ju, Y. Dynamics and burning limits of near-limit hot, warm, and cool diffusion flames of dimethyl ether at elevated pressures. Proc. Combust. Inst. 2019, 37, 1791–1798. [Google Scholar] [CrossRef]
- Murakami, Y.; Reuter, C.B.; Yehia, O.R.; Ju, Y. Studies of autoignition-assisted nonpremixed cool flames. Proc. Combust. Inst. 2021, 38, 2333–2340. [Google Scholar] [CrossRef]
- Reuter, C.B.; Won, S.H.; Ju, Y. Flame structure and ignition limit of partially premixed cool flames in a counterflow burner. Proc. Combust. Inst. 2017, 36, 1513–1522. [Google Scholar] [CrossRef]
- Ju, Y.; Reuter, C.B.; Yehia, O.R.; Farouk, T.I.; Won, S.H. Dynamics of cool flames. Prog. Energy Combust. Sci. 2019, 75, 100787. [Google Scholar] [CrossRef]
- Ju, Y. Understanding cool flames and warm flames. Proc. Combust. Inst. 2021, 38, 83–119. [Google Scholar] [CrossRef]
- Alfazazi, A.; Al-Omier, A.; Secco, A.; Selim, H.; Ju, Y.; Sarathy, S.M. Cool diffusion flames of butane isomers activated by ozone in the counterflow. Combust. Flame 2018, 191, 175–186. [Google Scholar] [CrossRef]
- Gersen, S.; Mokhov, A.V.; Darmeveil, J.H.; Levinsky, H.B. Ignition properties of n-butane and iso-butane in a rapid compression machine. Combust. Flame 2010, 157, 240–245. [Google Scholar] [CrossRef]
- Healy, D.; Donato, N.S.; Aul, C.J.; Petersen, E.L.; Zinner, C.M.; Bourque, G.; Curran, H.J. n-Butane: Ignition delay measurements at high pressure and detailed chemical kinetic simulations. Combust. Flame 2010, 157, 1526–1539. [Google Scholar] [CrossRef]
- Healy, D.; Donato, N.S.; Aul, C.J.; Petersen, E.L.; Zinner, C.M.; Bourque, G.; Curran, H.J. Isobutane ignition delay time measurements at high pressure and detailed chemical kinetic simulations. Combust. Flame 2010, 157, 1540–1551. [Google Scholar] [CrossRef]
- Zhang, K.; Banyon, C.; Togbé, C.; Dagaut, P.; Bugler, J.; Curran, H.J. An experimental and kinetic modeling study of n-hexane oxidation. Combust. Flame 2015, 162, 4194–4207. [Google Scholar] [CrossRef]
- Zhang, K.; Banyon, C.; Burke, U.; Kukkadapu, G.; Wagnon, S.W.; Mehl, M.; Curran, H.J.; Westbrook, C.K.; Pitz, W.J. An experimental and kinetic modeling study of the oxidation of hexane isomers: Developing consistent reaction rate rules for alkanes. Combust. Flame 2019, 206, 123–137. [Google Scholar] [CrossRef]
- Reuter, C.B.; Won, S.H.; Ju, Y. Experimental study of the dynamics and structure of self-sustaining premixed cool flames using a counterflow burner. Combust. Flame 2016, 166, 125–132. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, P. Minimum ignition energy and propagation dynamics of laminar premixed cool flames. Proc. Combust. Inst. 2021, 38, 2315–2322. [Google Scholar] [CrossRef]
- Zhang, W.; Faqih, M.; Gou, X.; Chen, Z. Numerical study on the transient evolution of a premixed cool flame. Combust. Flame 2018, 187, 129–136. [Google Scholar] [CrossRef]
- Zhou, M.; Yehia, O.R.; Xu, W.; Reuter, C.B.; Wang, Z.; Yan, C.; Jiang, B.; Ju, Y. The radical index and the effect of oxygen concentration on non-premixed cool flame extinction of large n-alkanes. Combust. Flame 2021, 231, 111471. [Google Scholar] [CrossRef]
- Reuter, C.B.; Lee, M.; Won, S.H.; Ju, Y. Study of the low-temperature reactivity of large n-alkanes through cool diffusion flame extinction. Combust. Flame 2017, 179, 23–32. [Google Scholar] [CrossRef]
- Zhang, T.; Ju, Y. Structures and propagation speeds of autoignition-assisted premixed n-heptane/air cool and warm flames at elevated temperatures and pressures. Combust. Flame 2020, 211, 8–17. [Google Scholar] [CrossRef]
- Stagni, A.; Brignoli, D.; Cinquanta, M.; Cuoci, A.; Frassoldati, A.; Ranzi, E.; Faravelli, T. The influence of low-temperature chemistry on partially-premixed counterflow n-heptane/air flames. Combust. Flame 2018, 188, 440–452. [Google Scholar] [CrossRef]
- Brown, M.Q.; Belmont, E.L. Effects of ozone on n-heptane low temperature chemistry and premixed cool flames. Combust. Flame 2021, 225, 20–30. [Google Scholar] [CrossRef]
- Hajilou, M.; Brown, M.Q.; Brown, M.C.; Belmont, E. Investigation of the structure and propagation speeds of n-heptane cool flames. Combust. Flame 2019, 208, 99–109. [Google Scholar] [CrossRef]
- Won, S.H.; Jiang, B.; Diévart, P.; Sohn, C.H.; Ju, Y. Self-sustaining n -heptane cool diffusion flames activated by ozone. Proc. Combust. Inst. 2015, 35, 881–888. [Google Scholar] [CrossRef]
- Brown, M.C.; Belmont, E.L. Experimental characterization of ozone-enhanced n-decane cool flames and numerical investigation of equivalence ratio dependence. Combust. Flame 2021, 230, 111429. [Google Scholar] [CrossRef]
- Yehia, O.R.; Reuter, C.B.; Ju, Y. Kinetic effects of n-propylbenzene on n-dodecane counterflow nonpremixed cool flames. Combust. Flame 2019, 208, 262–272. [Google Scholar] [CrossRef]
- Zhou, M.; Yehia, O.R.; Reuter, C.B.; Burger, C.M.; Murakami, Y.; Zhao, H.; Ju, Y. Kinetic effects of NO addition on n-dodecane cool and warm diffusion flames. Proc. Combust. Inst. 2021, 38, 2351–2360. [Google Scholar] [CrossRef]
- Lee, S.; Song, S. A rapid compression machine study of hydrogen effects on the ignition delay times of n-butane at low-to-intermediate temperatures. Fuel 2020, 266, 116895. [Google Scholar] [CrossRef]
- Bugler, J.; Marks, B.; Mathieu, O.; Archuleta, R.; Camou, A.; Grégoire, C.; Heufer, K.A.; Petersen, E.L.; Curran, H.J. An ignition delay time and chemical kinetic modeling study of the pentane isomers. Combust. Flame 2016, 163, 138–156. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, M.; Yao, X.; Liu, Y.; Tang, C. Comparative studies on the ignition characteristics of diisobutylene isomers and iso-octane by using a rapid compression machine. Fuel 2020, 276, 118008. [Google Scholar] [CrossRef]
- Li, S.; Campos, A.; Davidson, D.F.; Hanson, R.K. Shock tube measurements of branched alkane ignition delay times. Fuel 2014, 118, 398–405. [Google Scholar] [CrossRef]
- Fang, R.; Kukkadapu, G.; Wang, M.; Wagnon, S.W.; Zhang, K.; Mehl, M.; Westbrook, C.K.; Pitz, W.J.; Sung, C.-J. Fuel molecular structure effect on autoignition of highly branched iso-alkanes at low-to-intermediate temperatures: Iso-octane versus iso-dodecane. Combust. Flame 2020, 214, 152–166. [Google Scholar] [CrossRef]
- Mével, R.; Chatelain, K.; Boettcher, P.A.; Dayma, G.; Shepherd, J.E. Low temperature oxidation of n-hexane in a flow reactor. Fuel 2014, 126, 282–293. [Google Scholar] [CrossRef][Green Version]
- Sudholt, A.; Cai, L.; Pitsch, H. Laminar flow reactor experiments for ignition delay time and species measurements at low temperatures: Linear alkanes and dimethyl ether. Combust. Flame 2019, 202, 347–361. [Google Scholar] [CrossRef]
- Malewicki, T.; Brezinsky, K. Experimental and modeling study on the pyrolysis and oxidation of n-decane and n-dodecane. Proc. Combust. Inst. 2013, 34, 361–368. [Google Scholar] [CrossRef]
- Wang, H.; Gong, S.; Liu, G. Atmospheric and high pressures pyrolysis study of n-decane in a flow reactor with online GC-MS/FID analysis: Experiments and modeling. J. Anal. Appl. Pyrolysis 2021, 155, 105065. [Google Scholar] [CrossRef]
- Zeng, M.; Yuan, W.; Wang, Y.; Zhou, W.; Zhang, L.; Qi, F.; Li, Y. Experimental and kinetic modeling study of pyrolysis and oxidation of n-decane. Combust. Flame 2014, 161, 1701–1715. [Google Scholar] [CrossRef]
- Lutz, A.; Kee, R.; Grcar, J.; Rupley, F. OPPDIF: A Fortran Program for Computing Opposed-Flow Diffusion Flames; Report No. 96-8243; Sandia National Laboratories: Livermore, CA, USA, 1997. [Google Scholar]
- Ranzi, E.; Frassoldati, A.; Grana, R.; Cuoci, A.; Faravelli, T.; Kelley, A.P.; Law, C.K. Hierarchical and comparative kinetic modeling of laminar flame speeds of hydrocarbon and oxygenated fuels. Prog. Energy Combust. Sci. 2012, 38, 468–501. [Google Scholar] [CrossRef]
- Ranzi, E.; Frassoldati, A.; Stagni, A.; Pelucchi, M.; Cuoci, A.; Faravelli, T. Reduced Kinetic Schemes of Complex Reaction Systems: Fossil and Biomass—Derived Transportation Fuels. Int. J. Chem. Kinet. 2014, 46, 512–542. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, X.; Ju, Y. Kinetic studies of ozone assisted low temperature oxidation of dimethyl ether in a flow reactor using molecular-beam mass spectrometry. Combust. Flame 2016, 173, 187–194. [Google Scholar] [CrossRef]
- Ombrello, T.; Won, S.H.; Ju, Y.; Williams, S. Flame propagation enhancement by plasma excitation of oxygen. Part I: Effects of O3. Combust. Flame 2010, 157, 1906–1915. [Google Scholar] [CrossRef]
- Yehia, O.R.; Reuter, C.B.; Ju, Y. On the chemical characteristics and dynamics of n-alkane low-temperature multistage diffusion flames. Proc. Combust. Inst. 2019, 37, 1717–1724. [Google Scholar] [CrossRef]
- Alfazazi, A.; Kuti, O.A.; Naser, N.; Chung, S.H.; Sarathy, S.M. Two-stage Lagrangian modeling of ignition processes in ignition quality tester and constant volume combustion chambers. Fuel 2016, 185, 589–598. [Google Scholar] [CrossRef]
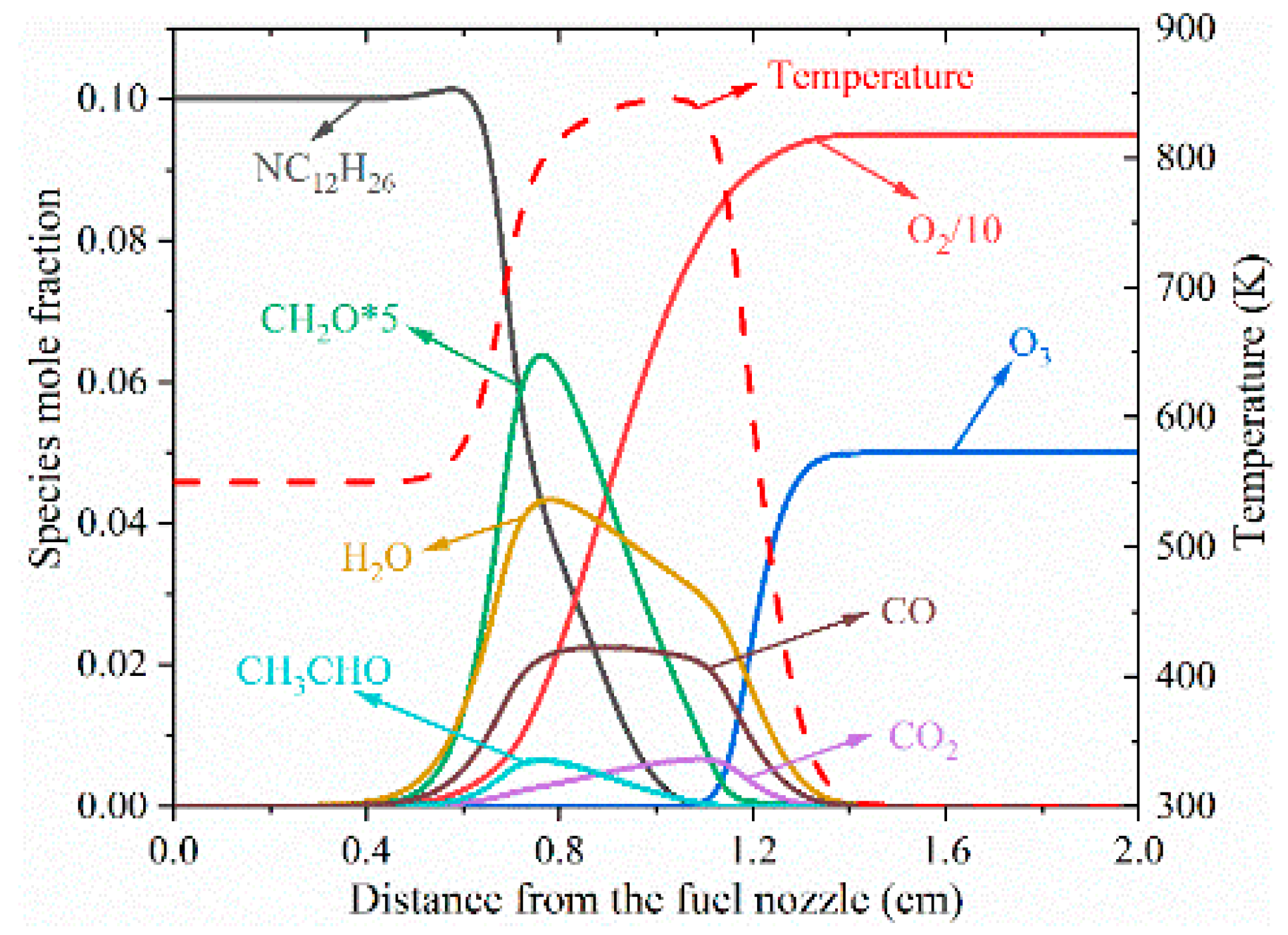
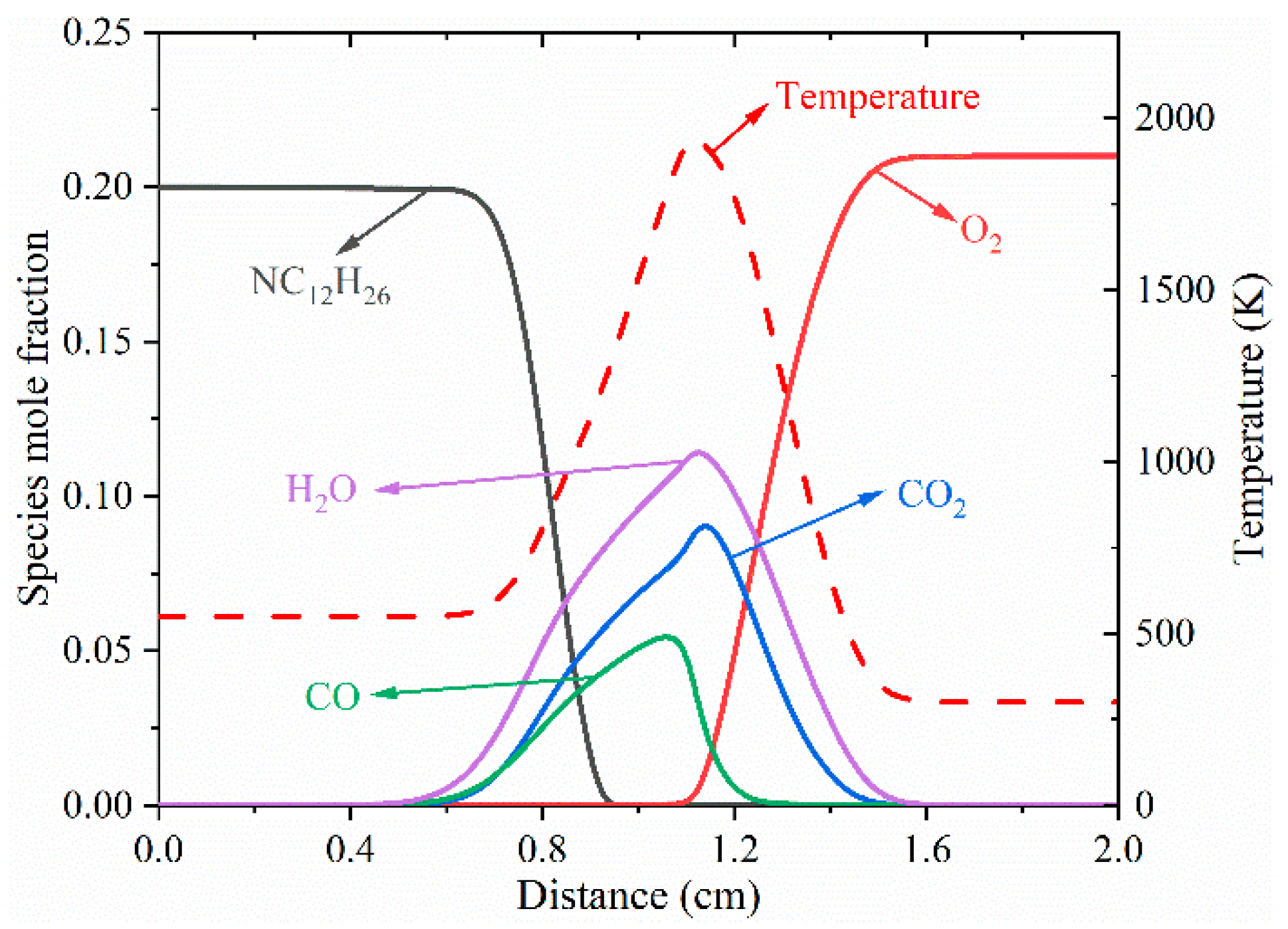

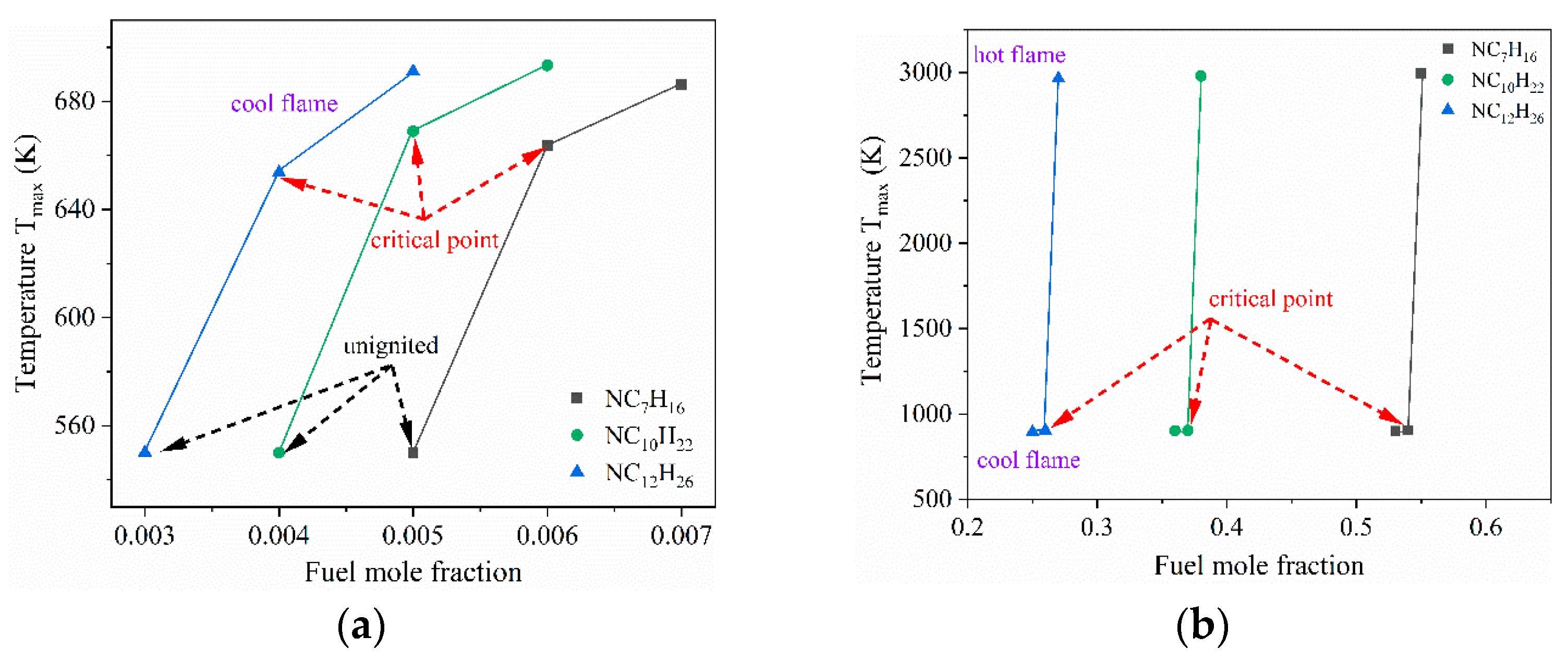
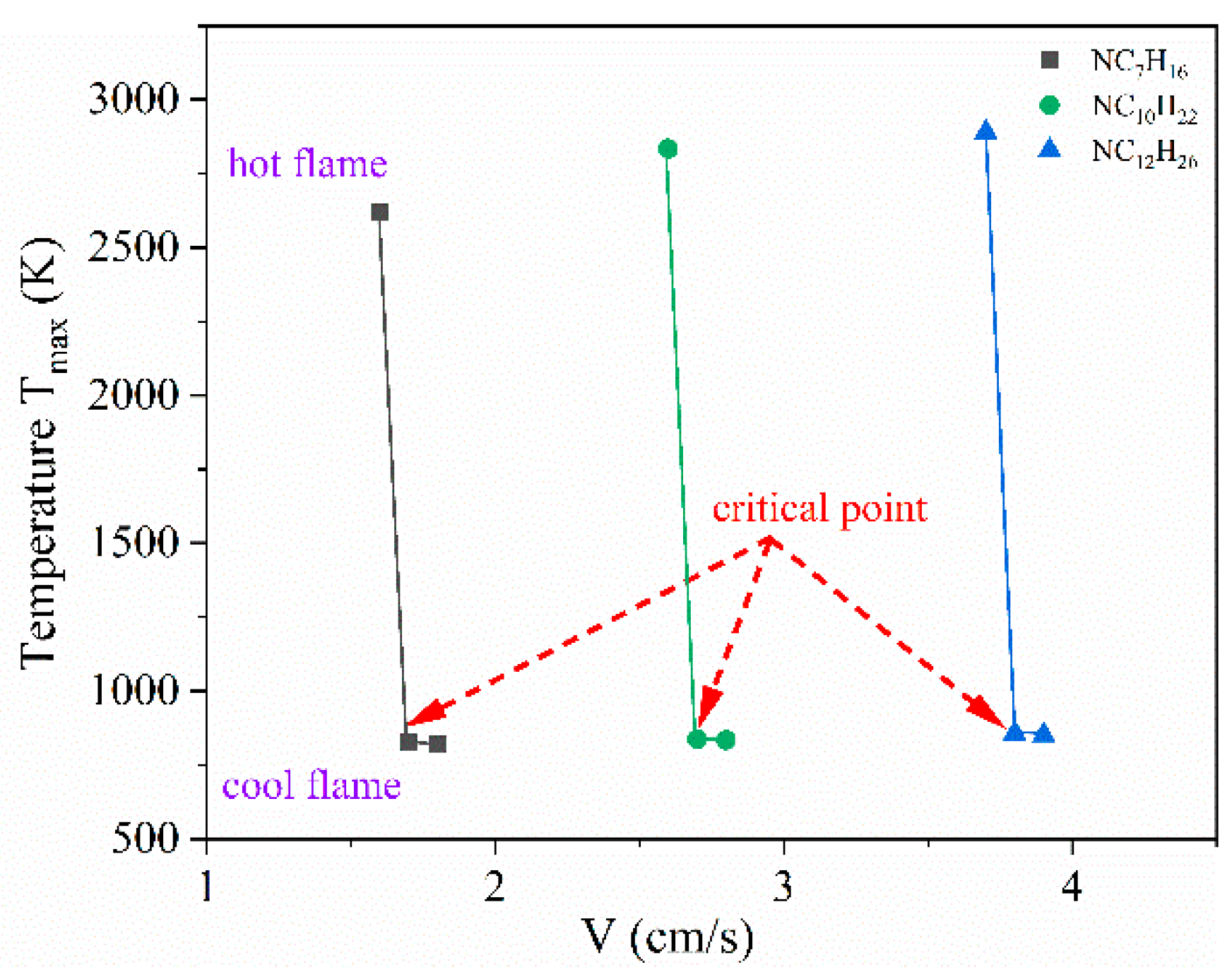


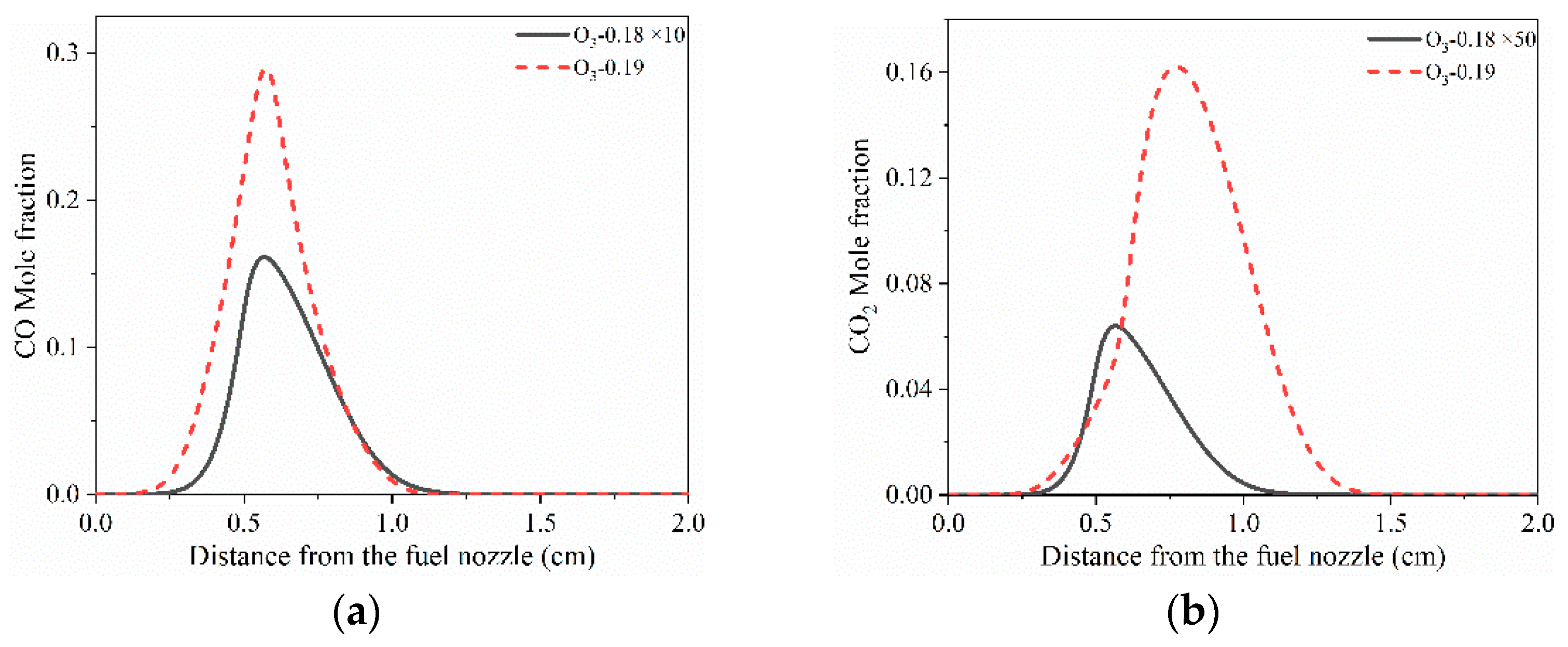
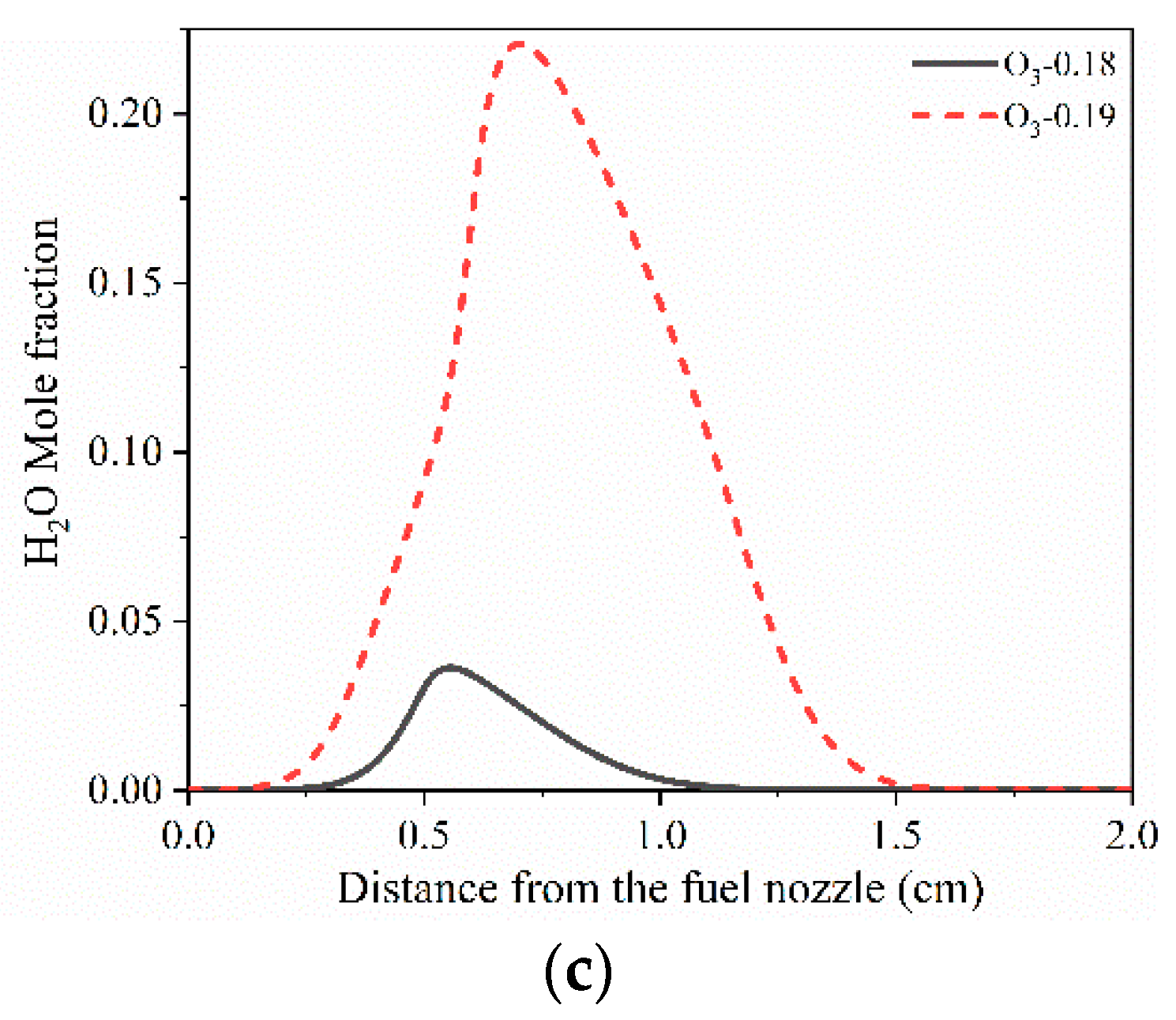
| Limit | TF (K) | VF (cm/s) | Fuel (Mole Fraction) | N2 (Mole Fraction) | TO (K) | VO (cm/s) | O3 (Mole Fraction) | O2 (Mole Fraction) |
|---|---|---|---|---|---|---|---|---|
| O3 concentration | 550 | 13 | 0.1 | 0.9 | 300 | 12 | ||
| Fuel concentration | 550 | 13 | 300 | 12 | 0.05 | 0.95 | ||
| Flow rate | 550 | 0.1 | 0.9 | 300 | 0.05 | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, D.; Xu, L.; Liu, D. Effects of Carbon Chain Length on N-Alkane Counterflow Cool Flames: A Kinetic Analysis. Fire 2022, 5, 170. https://doi.org/10.3390/fire5050170
Tian D, Xu L, Liu D. Effects of Carbon Chain Length on N-Alkane Counterflow Cool Flames: A Kinetic Analysis. Fire. 2022; 5(5):170. https://doi.org/10.3390/fire5050170
Chicago/Turabian StyleTian, Dan, Lei Xu, and Dong Liu. 2022. "Effects of Carbon Chain Length on N-Alkane Counterflow Cool Flames: A Kinetic Analysis" Fire 5, no. 5: 170. https://doi.org/10.3390/fire5050170
APA StyleTian, D., Xu, L., & Liu, D. (2022). Effects of Carbon Chain Length on N-Alkane Counterflow Cool Flames: A Kinetic Analysis. Fire, 5(5), 170. https://doi.org/10.3390/fire5050170







