1. Introduction
Aluminosilicate glasses are of paramount importance for contemporary technology, with current and potential applications in fibers for structural composites, glass-to-metal sealing materials for glass-to-metal systems and solid oxide fuel cells, optical and photochromic constituents, high-power laser gain media, flat-panel display substrates and bioactive materials [
1,
2]. Alkali and alkali earth aluminosilicate glasses are also useful simple model systems for naturally occurring complex magmas since silica and alumina are the main constituents of the Earth’s crust [
3].
Many authors have studied the structure of these aluminosilicate glasses, and the general view is that both SiO
4 and AlO
4 tetrahedra are joined at their corners by bridging oxygens (BOs) to form frameworks with varying degrees of polymerization [
1,
2,
3,
4,
5,
6,
7,
8]. The Al
(3+)O
4 tetrahedron has a charge deficit compared with a Si
(4+)O
4 unit, and this can be charge compensated by, for example, a positively charged alkali ion such as Na
+. If Na/Al = 1, then all (AlO
4)
− tetrahedra are compensated by the Na
+ ions, and the network is fully connected with no non-bridging oxygens (NBOs). Network connectivity can be probed using magic-angle spinning (MAS)–nuclear magnetic resonance (NMR) spectroscopy. The chemical shift of
29Si allows the degree of connectivity of the SiO
4 tetrahedra to be ascertained [
1]. This is represented by the symbol Q
n, where n is the number of BO on a tetrahedron. As the number of BO decreases, the chemical shift is displaced to higher values. When Al substitutes for Si, an additional modification of the chemical shift occurs depending on the number m of Al atoms as second neighbors. The moieties for Si and Al are denoted by {Q
nSi(mAl)} and {Q
nAl(mSi)}, indicating m Si–O–Al or Al–O–Si bonds [
2,
9]. Several studies have shown that, for Al/(Al + Na) ratios ≤ 0.5, all Al
3+ ions are found in AlO
4 tetrahedra, and Na ions are linked through NBOs to Q
3 SiO
4 tetrahedra. When Al/(Al + Na) ratio > 0.5, then Al
3+ ions are found to be in higher coordination states, i.e.,
[5]Al or
[6]Al, and the environment of Na
+ ions will change [
3,
4,
5]. However, it has been reported that these higher coordination states (mainly
[5]Al) have also been observed in tectosilicate compositions (Na/Al = 1) [
6,
7,
8,
9].
Rare earth (RE) aluminosilicate glasses [
2,
4,
10,
11] exhibit several favorable chemical, mechanical, and other properties, such as high hardness, refractive index, elastic modulus, and glass transition temperatures (T
g). In addition to the applications mentioned above, these glasses are being considered for long-term immobilization of radioactive wastes, and, effectively, they can be model systems for long-half-life actinides [
1,
2,
10]. In particular, lanthanum and yttrium have been selected to simulate actinides because the ternary RE
2O
3-Al
2O
3-SiO
2 (RE = La or Y) phase diagrams are well known, and their physicochemical properties have been investigated [
2,
10]. These rare earth ions are more suited to structural studies using MAS NMR, as they avoid the problems of paramagnetism observed with other rare earth cations.
Investigations have shown that, for glasses with RE/Al < 3, there will be insufficient numbers of RE
3+ cations to charge-compensate the (AlO
4)
− tetrahedra, and high levels of
[5]Al or
[6]Al are observed [
8,
10]. For glasses with RE/Al > 3, then effectively all AlO
4 tetrahedra should be charge-compensated, and RE cations act as modifiers, depolymerizing the network with Q
nSi(mAl)→Q
n−1Si(mAl) conversions and formation of NBOs preferentially localized on SiO
4 tetrahedra [
2,
7,
8,
9]. Even so, there is evidence for small amounts of
[5]Al and/or
[6]Al in these compositions [
12].
It should also be noted that the generalized structural models assume that Loewenstein’s rule [
13], also known as the Al-avoidance principle, always pertains. It excludes
[4]Al-O-
[4]Al constellations due to the excess negative charge of AlO
4 tetrahedra relative to SiO
4 and thereby maximizes the number of Si-O-Al bonds at each Qn unit. However, there are many exceptions to this rule; that is, the presence of
[4]Al-O-
[4]Al linkages rather than
[4]Si-O-
[4]Al or
[4]Si-O-
[4]Si in aluminosilicate glasses have been reported [
14,
15]. MAS NMR of a series of La
2O
3–Al
2O
3–SiO
2 glasses [
16] reveals Al speciations dominated by AlO
4 groups, with minor fractions of AlO
5 (5–10%) and AlO
6 (≲3%) polyhedra present; the amounts of
[5]Al and
[6]Al coordinations increase as the molar fraction of Si decreases. The glass structures exhibit a pronounced Al/Si disorder, including significant amounts of AlO
4–AlO
4 contacts [
16,
17].
While the structure and properties of SiO
2–Al
2O
3–La
2O
3 and SiO
2–Al
2O
3–Na
2O glasses have been studied extensively [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12], it is clear from our in-depth review of the glass science literature that the structure of SiO
2–Al
2O
3–La
2O
3–Na
2O glasses has not been studied previously, and thus this investigation is the first to assess the structural changes occurring when La
2O
3 is substituted by Na
2O.
Structural characterization by MAS NMR of lanthanum sodium aluminoborosilicate glasses with compositions (mol.%): 55SiO
2–(25–x)Al
2O
3–xB
2O
3–15Na
2O–5La
2O
3 have been reported previously [
18,
19], in which lanthanum is intended to simulate other lanthanides, as well as minor actinides contained in nuclear waste.
These studies highlighted the narrow range of composition within which peraluminous vitreous matrices exhibit homogeneity after quenching, extending from 0% to 5% of B2O3. Infrared spectroscopy provided comprehensive characterization of the silicate and borate networks, emphasizing the presence of Qn m(Al,B) units rather than Qn m(Al) units alone in aluminoborosilicate glasses. NMR spectroscopy reveals that boron predominantly exists in triangular BO3 units, with a small percentage in tetrahedral BO4 units. Al predominantly occurs in 4-fold coordination, with some AlO52− and AlO63− species. The structural role of Na shifts towards primarily (AlO4)− charge compensation with increasing Al/B substitution.
The structure of these glasses is rather complex due to the mixing of the network-forming cations (Si, B, and Al), with the appearance of nanoscale phase separation for glasses with more than 5% B2O3 and the formation of a La-rich phase. Therefore, the present study was undertaken to investigate the structure of a lanthanum aluminosilicate glass with similar composition (but excluding boron), that is, in mol.%: 55SiO2–25Al2O3–20La2O3. The effect of partially and wholly substituting Na2O for La2O3 is investigated in order to understand the environment of these elements and bring more clarity to results from the previous aluminoborosilicate glasses. The primary objective is to elucidate how sodium oxide substitution for lanthanum oxide influences the glass transition temperature and structure of these specific glass compositions.
It is envisaged that lanthanum aluminosilicates without boron can also be model systems for actinides. As these glasses have high melting points, the use of sodium oxide as a modifying oxide to decrease the liquidus temperature and viscosity will be necessary and this will have an effect on the lanthanum aluminosilicate glass network structure. A
27Al MAS NMR and Fourier-Transform Infrared (FTIR) study [
20] on rare earth aluminosilicate glasses suggested that these glasses have a wide distribution of Q
n units. Thus, the compositions in the present study were chosen with the same contents of SiO
2 and Al
2O
3 and the same SiO
2:Al
2O
3 ratio as in the previous studies of aluminoborosilicate glasses [
18,
19], varying only the La
2O
3:Na
2O ratios. While the number of samples is limited, it will provide a baseline study for further work on the Na-La-aluminoborosilicate series of glasses in the future.
Therefore, the present study focuses on structural and coordination relationships combining information from FTIR and MAS NMR spectroscopy to provide direct information on Qn and changes in the local environments of Si and Al: {QnSi(mAl)} and {QnAl(mSi)} as Na is substituted for La in these aluminosilicate glasses.
3. Results and Discussion
3.1. Compositional Effects on Glass Formation and Homogeneity
The quenched glasses were transparent and macroscopically homogenous. As shown in
Figure 1, no crystalline phase was observed in the XRD patterns for the three glasses (G1 = 20La
2O
3; G2 = 5La
2O
3+15Na
2O; G3 = 20Na
2O), only the broad peak at low values of 2θ, showing that the samples are amorphous.
The analyzed compositions for the three glasses are compared with the starting compositions in
Table 1. Some slight loss of the modifying oxides occurred during melting, but no substantial differences were observed between the analyzed and starting compositions.
From the bright-field TEM observations, no crystallization or phase separation was observed for all three glasses (see
Figure 2), and the samples were completely homogeneous.
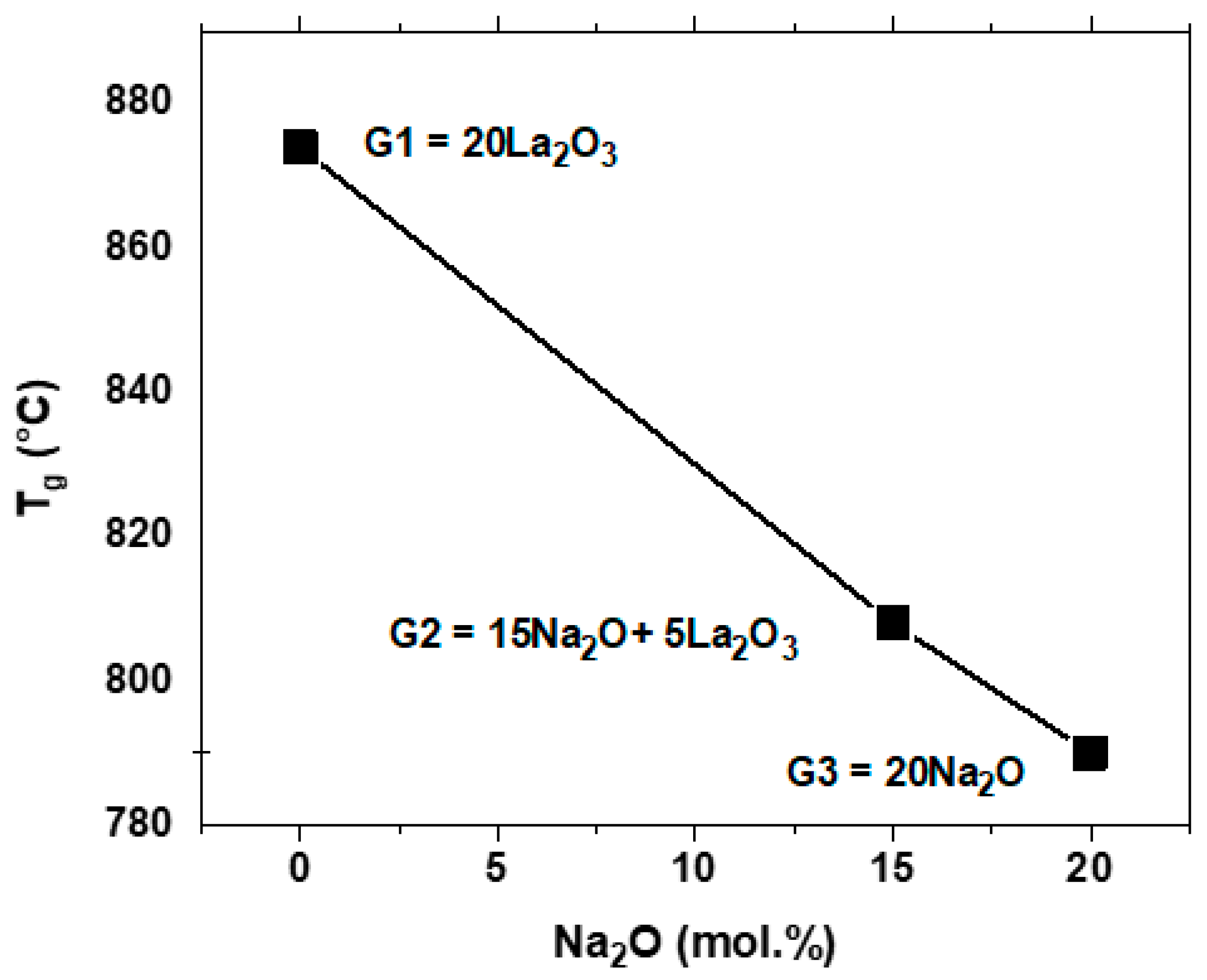
DSC analyses of the glass series revealed a strong effect of the modifying oxide compositions on the glass transition temperature, T
g.
Figure 3 shows a decrease in T
g from 874 °C for the G1 = 20La
2O
3 glass to 790 °C for the G3 = 20Na
2O glass.
The cationic field strength (CFS) is often used to provide some general insight into the relative strengths of the various M-O bonds (M = modifying cation) within a glass [
24,
25,
26,
27].
where z
c is the charge on the modifying cation, and r
c is the ionic radius. For glasses containing mixed modifying oxides, an effective CFS [
27] can be calculated based on the proportions of the modifying cations present.
Figure 4 shows that T
g increases linearly as a function of effective cation field strength, as La
2O
3 is substituted for Na
2O within the glass composition.
The value of T
g for the 20 mol.% La
2O
3 glass (874 °C) is within the range of 840–900 °C, reported on similar glasses [
2,
28]. T
g decreases significantly when 15 mol.% of the La
2O
3 is substituted by Na
2O since Na has a much lower cation field strength than La. T
g is lowered again when all of the La
2O
3 is replaced by Na
2O. Higher T
g is expected from stronger bonding of structural units by La
3+ in the glass, thus making segmental mobility more difficult.
Sodium oxide substitution for lanthanum oxide in aluminosilicate glasses significantly reduces the glass transition temperature (T
g), and this is illustrated very clearly when T
g is plotted against effective cation field strength in
Figure 4. Increasing Na content has implications for the presence of non-bridging oxygens and the local environments of Si and Al atoms.
In waste-immobilization applications, it is crucial to understand these effects: a lower Tg facilitates processing at lower temperatures, while understanding and controlling Si and Al environments ensures optimal chemical stability and resistance to leaching, essential for safely encapsulating radioactive waste materials within the glass matrix and ensuring long-term environmental safety.
3.2. Compositional Effects on Structure of Glasses
The only apparent variable compositional parameter is La2O3 to Na2O, with the SiO2 and Al2O3 concentrations remaining constant in all three glasses. As the concentration of Al2O3 is 25 mol.%, then, assuming that all the Al adopts 4-fold coordination as (AlO4)− units, 50 positive charges are required to compensate for the deficient negative charges, i.e., 16.67 La3+ or 50 Na+. The values of 16.67 La3+ and 50 Na+ represent the number of cations required to balance the charge of the deficient negative charges resulting from the assumed 4-fold coordination of Al as (AlO4)− units. In the case of La3+, this value is calculated by dividing the total positive-charge requirement (50) by the valence of La3+. Therefore, it is necessary to round this value to a practical amount, resulting in 16.67 La3+ ions. Similarly, for Na+, the entire positive-charge requirement can be provided by 50 Na+ ions due to their monovalent nature.
Clearly, for G1 with 20 mol.% La2O3, there is an excess of La3+ cations needed to balance all the (AlO4)− units, and, thus, the formation of non-bridging oxygen (NBO) atoms is expected. For the G2 glass with 5 mol.% La2O3 and 15 mol.% Na2O, there is still an excess of La3+ and Na+ cations needed to provide charge compensation for (AlO4)− units, meaning that some of the La and Na cations are acting as modifiers, and some NBOs will be formed.
However, as 20 mol.% La
2O
3 (G1) is fully substituted by 20 mol.% Na
2O (G3), the number of positive charges available for compensation of (AlO
4)
− units decreases considerably. Previous studies [
3,
4,
5] have shown that, for Al/(Al + Na) ratios ≤ 0.5, all Al
3+ ions are found in AlO
4 tetrahedra, and Na ions are linked through NBOs to Q
3 SiO
4 tetrahedra. For G3 with 20 mol.% Na
2O, there are insufficient positive charges to compensate all (AlO
4)
− units, and, therefore, some Al
3+ will be acting as modifier cations with a higher coordination than 4. Thus, changes will be expected in the FTIR and MAS NMR spectra with changes in cation composition.
3.3. Fourier-Transform Infrared Spectroscopy
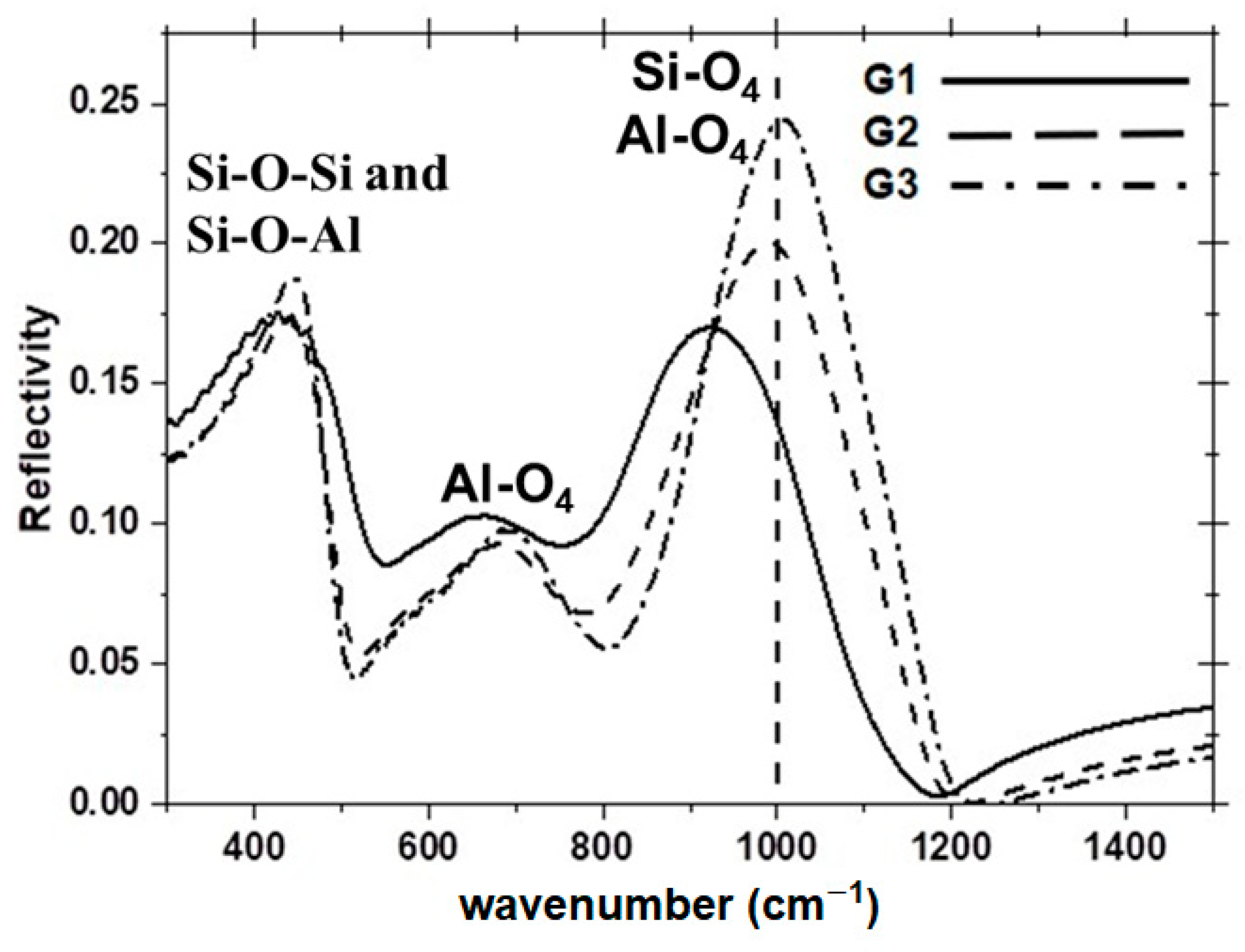
Figure 5 shows the reflectivity spectra of the three glasses, with each spectrum exhibiting three broad bands across the range of 350–1200 cm
−1, and with the most intense bands in the range 800–1200 cm
−1, followed by moderately intense bands across the 350–500 cm
−1 range and weaker bands across the 500–800 cm
−1 region. These broad bands are clear indicators of the general disorder of the silicate network [
29].
It is noted that the strong band for glass G1 (20 mol.% La
2O
3) peaks at ~900 cm
−1 [
30], whereas the Na-containing glasses show maxima at ~1000 cm
−1. These intense broad bands may be attributed to stretching vibrations of (i) SiO
4 tetrahedra with specific numbers, n, of bridging oxygen (BO) atoms to
m second-neighbor Al atoms (Q
n(
mAl)) and (ii) AlO
4 tetrahedra. The bands in the region of 350–500 cm
−1 are caused by the bending vibrations of Si-O-Si and Si-O-Al linkages [
31]. In the case of G1 and G2 glasses, the excess of positive charges from La
3+ and Na
+ ions means that Al is present mainly as (AlO
4)
− units. This is confirmed by the reflectivity bands in the region 500–800 cm
−1 (maximum at ~700 cm
−1) that are related to the Al-O bond-stretching vibrations with 4-fold coordination (
[4]Al) [
20,
32], suggesting that (AlO
4)
− units are dominant features in all three glasses.
Figure 6 shows the experimental reflectivity spectrum of the G2 glass (5La
2O
3 + 15Na
2O) and the data reproduced with the FOCUS fitting software [
21], using the Fresnel formula and a dielectric function model based on causal Gaussian components [
32].
Five Gaussian components are assigned to simulate the high-frequency stretching mode region between 800 and 1200 cm
−1. The silicate lattice has five modes. The first,
ν1, is the vibration mode, which reflects the general disorder. The other four contributions (Q
n(
mAl)) (see
Table 2 for all three glasses) are as follows:
ν2, Q
4(0A1);
ν3, Q
4(3Al);
ν4, Q
4(4Al); and
ν5, Q
3(3Al).
All glasses show the contribution of Q4(3Al), Q4(4Al), and Q3(3Al) structural features.
The Si-O-Si bond symmetric and antisymmetric stretching modes are well known to be IR-active in the 800–1300 cm
−1 region [
29,
30,
31,
32], particularly related to Q
4 and Q
3 units.
The major species (68%) in G1 glass (20La2O3) is Q3(3Al), i.e., SiO4 tetrahedra with three Si-O-Al linkages. As there is an excess of La3+ cations over those needed to charge-balance all the (AlO4)− units, the formation of non-bridging oxygen (NBO) atoms to form Q3 tetrahedra occurs linked to La3+ modifier cations. The remaining features, namely Q4(4Al) (26%) and Q4(3Al) (6%), are SiO4 tetrahedra with no NBOs and four or three Si-O-Al linkages. G2 glass (5La2O3+15Na2O) has also Q4(4Al) (43%) and Q4(3Al) (27%) features, as well as some Q3(3Al) (18%) but also some Q4(0Al) units, i.e., SiO4 tetrahedra with no Si-O-Al linkages. In this case, 10 La3+ + 30 Na+ provide a total of 60 positive charges, which can compensate fully for the 50 (AlO4)− units, but the excess positively charged cations which act as modifiers are less than for G1. Some of the La and Na (total charge of 10+) cations act as modifiers, and this would explain the presence of Q3(3Al) features with one NBO.
In the case of G3, the predominant species are, once again, Q4(4Al) (60%) and Q4(3Al) (30%), with a smaller amount of Q3(3Al) (10%), and their associated NBOs. With no La, 40 Na+ can provide charge compensation for 40 (AlO4)− units, meaning that 10 Al3+ may be acting as modifier cations (i.e., up to 20% of the Al may be in [5]Al or [6]Al coordination). The levels of Q4(4Al) and Q4(3Al), i.e., SiO4 tetrahedra with four or three Si-O-Al linkages, appear high but may be an indication of a certain ordering of Si and Al atoms. However, these are only qualitative observations, and, although we have tried to relate these results to specific structural features expected from the actual compositional variations, in order to clarify these findings, especially for the G3 glass, it is necessary to compare the data with results from MAS NMR.
3.4. MAS NMR Study
3.4.1. 29Si MAS NMR
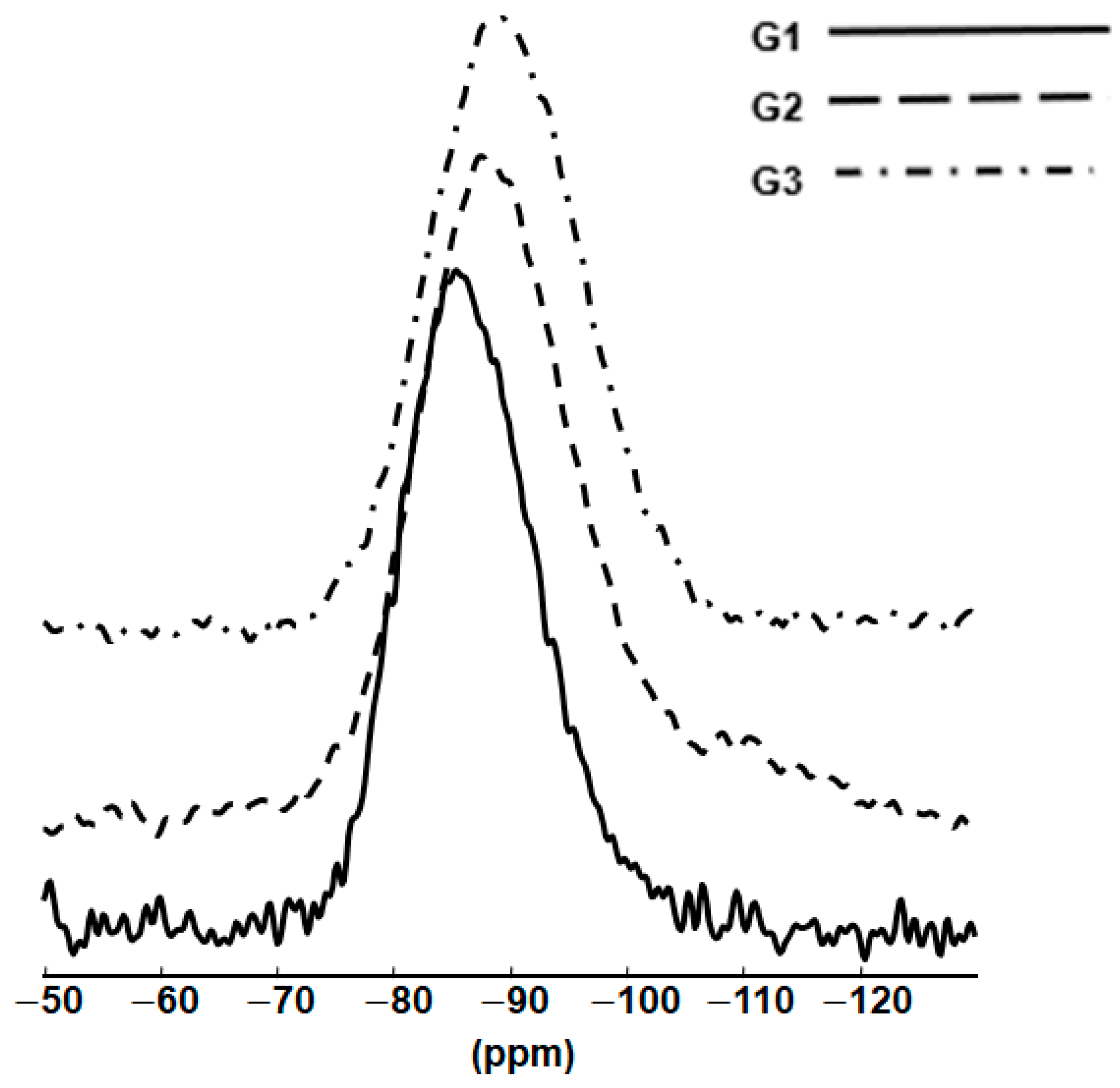
The
29Si MAS NMR spectra for the three glasses—G1 = 20La
2O
3, G2 = 5La
2O
3 + 15Na
2O, and G3 = 20Na
2O—are shown in
Figure 7. G1 shows a broad and symmetric signal extending between −75 and −105 ppm, with a peak maximum (δ
max) at −86 ppm. As Na substitutes for La, the peak maxima are displaced toward slightly more negative chemical shifts.
The chemical shift of the
29Si spectra is sensitive to the degree of polymerization of the glass network [
33], i.e., the nature of the SiO
4 Q
n units. Given the overlap of the chemical-shift domains between the different Q
n(mAl) species and the wide distribution of possible environments in a glass network, the attributions of the
29Si MAS NMR signals are difficult. Usually, the spectra of
29Si give indications only of the most representative species, as well as qualitative data concerning almost fully polymerized environments, taking into account the width of the peaks. However, indicative signal assignments have been proposed in the literature [
17,
34,
35], based on spectrum calculations and component assignments, in accordance with published chemical-shift domains for Q
n(mAl) species.
The
29Si MAS NMR spectra of the three glasses were qualitatively simulated using three components. The number of Gaussian components introduced to deconvolute the spectra is defined by the number of minimum components with similar FWHM (Full Width at Half Maximum) values, set at 11 ± 0.5 ppm for all components. The results showing Q
n(mAl) and (δ
max) are listed in
Table 3, based on previous assignments from Diallo et al. [
36].
In the La-aluminosilicate glass (G1), the main species present are Q3(2Al) and SiO4 tetrahedra with one NBO and linked to two AlO4 tetrahedra, consistent with the excess of La3+ cations over those needed to charge-balance all the (AlO4)− units. Thus, the formation of non-bridging oxygen (NBO) atoms to form Q3 tetrahedra will occur. The other main species are Q4(4Al) with no NBOs and four Si-O-Al linkages with a small fraction of Q4(2Al) features, i.e., SiO4 tetrahedra with no NBOs linking to two (AlO4)− units.
In summary, the La-aluminosilicate glass has 34% SiO4 tetrahedra with four Si-O-Al linkages. As Na2O is substituted for 15% La 2O3, this increases to 41%, but there are also similar proportions of Q3 species with either two or zero Si-O-Al linkages. The combined La3+ and Na+ cations provide sufficient positive charges to compensate for all the (AlO4)− units, with some excess positive charges acting as modifiers, thus allowing for the formation of one NBO per tetrahedron (Q3).
The fully substituted Na-aluminosilicate glass (G3) has 64% SiO4 tetrahedra with three Si-O-Al linkages: Q4(3Al) and smaller amounts of Q4(2Al) (no NBOs linking to two (AlO4)− units) and also a minor proportion of Q2(0Al) (SiO4 tetrahedra with two NBOs and no Si-O-Al linkages). In this case, Na ions, acting as modifiers, will be in close proximity. With no La3+ ions present, the monovalent Na+ can provide charge compensation for 40 (AlO4)− units, leaving 10 Al3+ cations to act as modifiers, meaning that less of the AlO4 is taking part in the glass network, and this is consistent with the types of features found, i.e., Q4(2Al) and Q2(0Al).
Overall, the results from 29Si MAS NMR for G1 and G2 glasses are compatible with those from FTIR, but with some slight divergence in the case of G3, where it may be expected that Al may be in [5]Al or [6]Al coordination. Thus, it is important to compare results with 27Al MAS-NMR.
3.4.2. 27Al MAS NMR
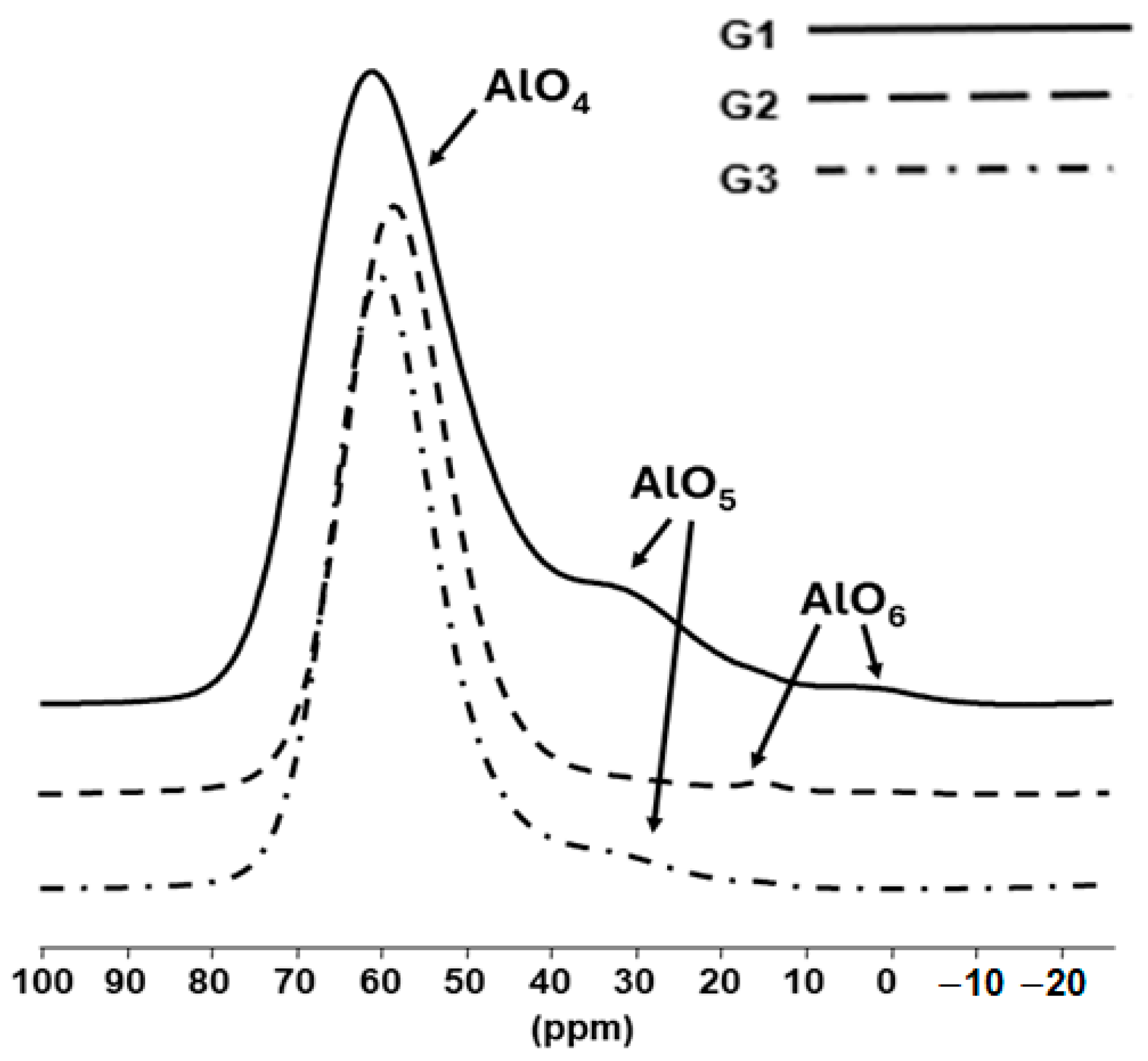
The
27Al MAS-NMR spectra for the three glasses are shown in
Figure 8. The study was performed to identify the different coordination states of Al
3+ ions:
[4]Al at ~55–60 ppm,
[5]Al in the range from 25 to 40 ppm, and
[6]Al between −15 and 20 ppm) [
37,
38].
The intense 27Al NMR resonance at ~55–60 ppm due to [4]Al is observed for all glasses. In all cases, this shows that Al primarily acts as a network-former, as implied by the results of FTIR and 29Si MAS NMR, which have confirmed significant Si-O-Al linkages.
As shown in
Table 4, for the La-aluminosilicate glass (G1), a significant amount of 4-fold coordinated Al was identified (
[4]Al), along with a minor amount of
[5]Al and an insignificant amount of 6-fold coordinated
[6]Al. It has been reported previously that higher coordinated Al is found in aluminosilicate glasses with high-field-strength cations, such as La [
39], which, in this case, are providing excess positive charges which must be charge-balanced by (
[5]AlO5)
3− units. However, a higher coordination state for La
3+ cations would negate this, leading to a higher level of
[4]Al. It has been suggested [
10] that preferential localization of La
3+ occurs near to 4-fold coordinated Al atoms, with the possible formation of oxygen triclusters or an increase in La
3+ coordination, leading to the stabilization of
[4]Al species.
As Na is substituted for La, with a lower level of positive charges to be satisfied, Al is almost wholly in 4-fold coordination with a very small amount of 5-fold [5]Al (no AlO6 units are observed). All of the (AlO4)− units should be compensated by the La3+ and Na+ ions, the excess of which creates some non-bridging oxygens leading to the formation of Q3 species, some with no Si-O-Al linkages.
For G3 with 20 mol.% Na
2O, there are insufficient positive charges to compensate all (AlO
4)
− units, and, therefore, a more significant amount of Al
3+ (13%) will be acting as modifier cations with higher coordination (
[5]Al), as found by other investigators [
5,
10,
30,
35].
The structural features of each of these glasses suggest that they are quite stable for potential use for radioactive waste containment, consisting of highly bonded networks consisting of SiO
4 tetrahedra with four or three Si-O-Al linkages. Comparing these glasses with equivalent composition glasses in the SiO
2–Al
2O
3–B
2O
3–Na
2O–La
2O
3 system studied previously [
18,
19], where Al
2O
3 was substituted by B
2O
3, we observed that phase separation and crystallization occurred at substitution levels above 5% B
2O
3, which would negate their use for radioactive waste immobilization.
4. Conclusions
A La-aluminosilicate glass was prepared with the following composition (in mol. %): 20% La2O3, 55% SiO2 and 25% Al2O3 (G1). The effects of the partial substitution of 15% La2O3 by 15% Na2O (G2) and the full substitution of all the La2O3 by 20% Na2O (G3) on the Tg and structure of the glass were investigated.
Tg decreases significantly from 874 °C for the La-aluminosilicate glass containing 20% La2O3 to 790 °C for the glass with complete substitution of Na2O for the La2O3, suggesting weaker bonding within the glass network. Higher Tg is expected from stronger bonding of structural units by La3+ in the glass, thus making segmental mobility more difficult.
Structural studies using FTIR spectroscopy, 29Si, and 27Al MAS NMR have identified the main Qn species and changes in the local environments of Si and Al: {QnSi(mAl)} and {QnAl(mSi)}. In all the glasses, (AlO4)− units are a dominant feature, showing that Al is mostly acting as a glass former within the network. A structural feature of all glasses is the presence of SiO4 tetrahedra with four Si-O-Al linkages [Q4(4Al)]. In the La-aluminosilicate glass (G1), the major species are SiO4 tetrahedra with one NBO and two Si-O-Al linkages [Q3(2Al)]. This is reduced substantially as Na2O is substituted for La2O3 (G2), with the appearance of SiO4 tetrahedra with one NBO and no Si-O-Al linkages [Q3(0Al)]. The combined La3+ and Na+ cations provide sufficient positive charges to compensate for all the (AlO4)− units, with some excess positively charged cations acting as modifiers, so allowing the formation of one NBO per tetrahedron, i.e., Q3 species with either two or zero Si-O-Al linkages. A small proportion of five-fold coordinated [5]Al is present in these glasses.
The fully substituted Na-aluminosilicate glass (G3) has substantially more SiO4 tetrahedra with three Si-O-Al linkages [64% Q4(3Al)]. In this case, Na ions as modifiers will be in close proximity. With no La3+ ions present, the monovalent Na+ can provide charge compensation for 40 (AlO4)− units, leaving 10 Al3+ cations to act as modifier cations with 5-fold coordination ([5]Al), thus meaning that less of the (AlO4)− is taking part in the glass network, and this is consistent with the types of features found, i.e., Q4(2Al) and Q2(0Al).
These findings underscore the importance of cation composition in controlling the thermal and structural properties of aluminosilicate glasses. Future research will explore the optimization of compositions, for example, glasses with SiO2:Al2O3 = 2:1, with varying Na2O/Al2O3 and La2O3/Al2O3 contents, to further elucidate the role of the two modifier cations in the structure and properties of these aluminosilicate glasses.