Mullite 3D Printed Humidity Sensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Slurry Preparation
2.3. Digital Light Processing (DLP) and Post-Processing
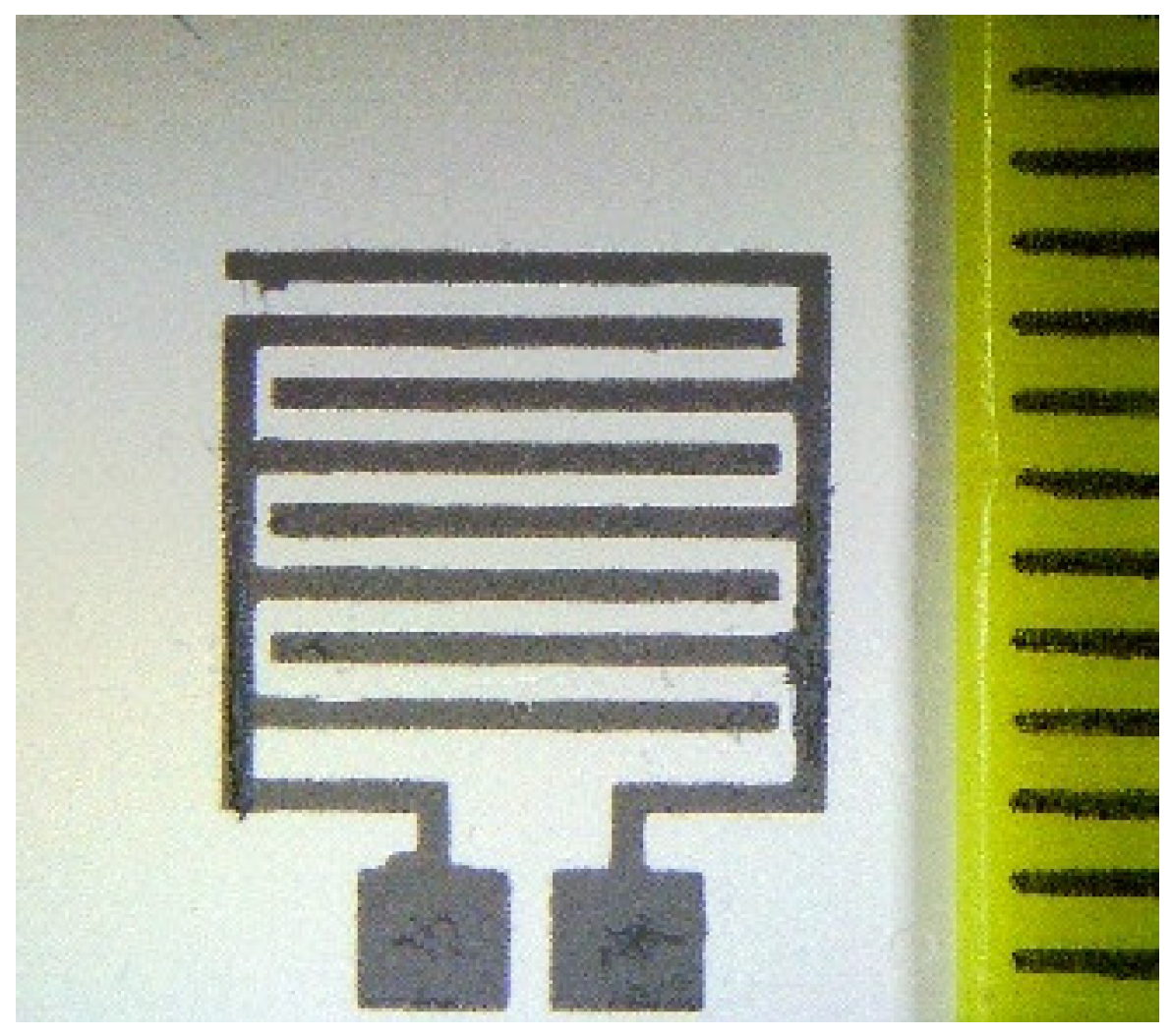
2.4. Fabrication and Measurement of Gas Sensors
3. Results and Discussion
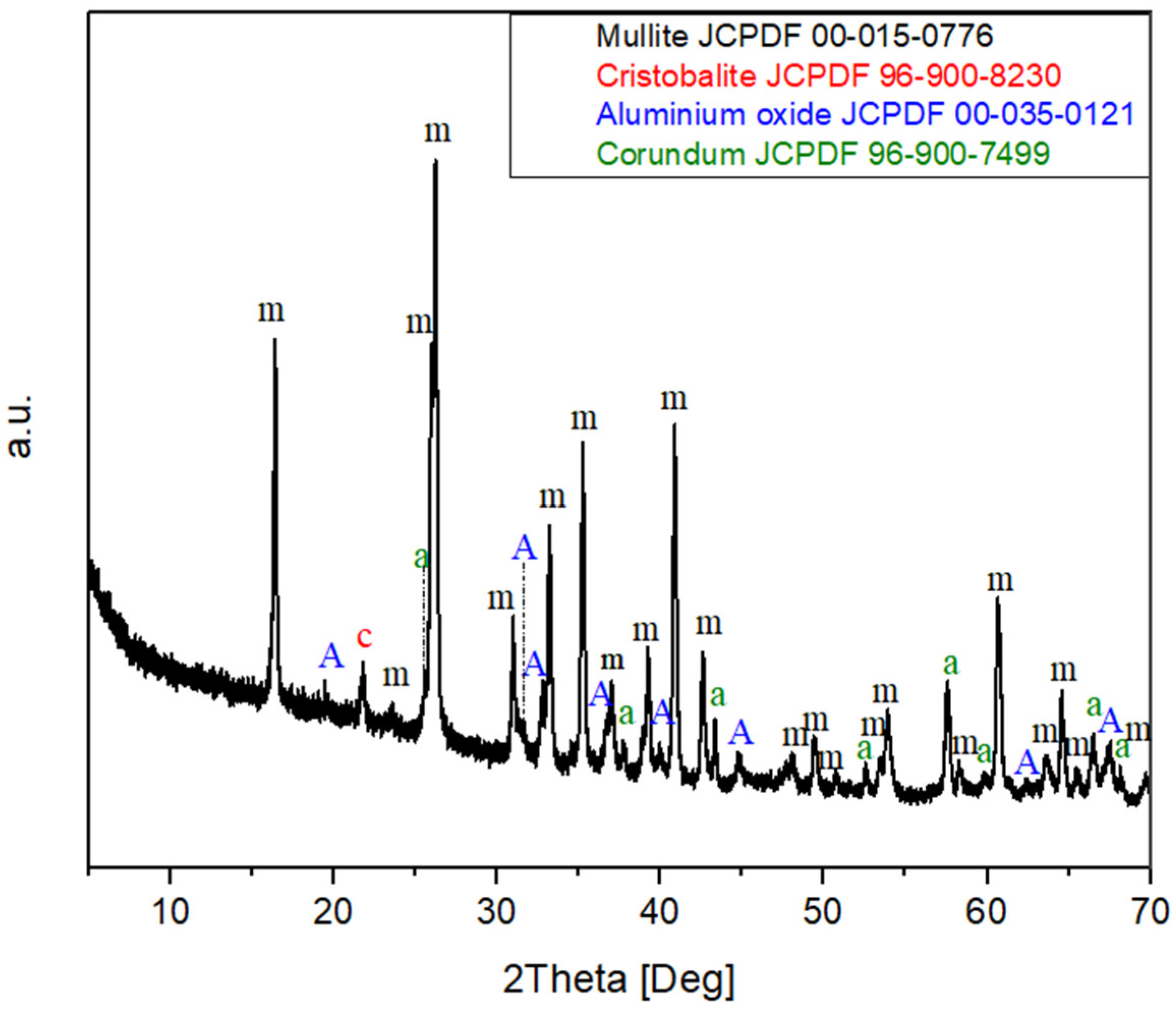
3.1. Powder Characterization
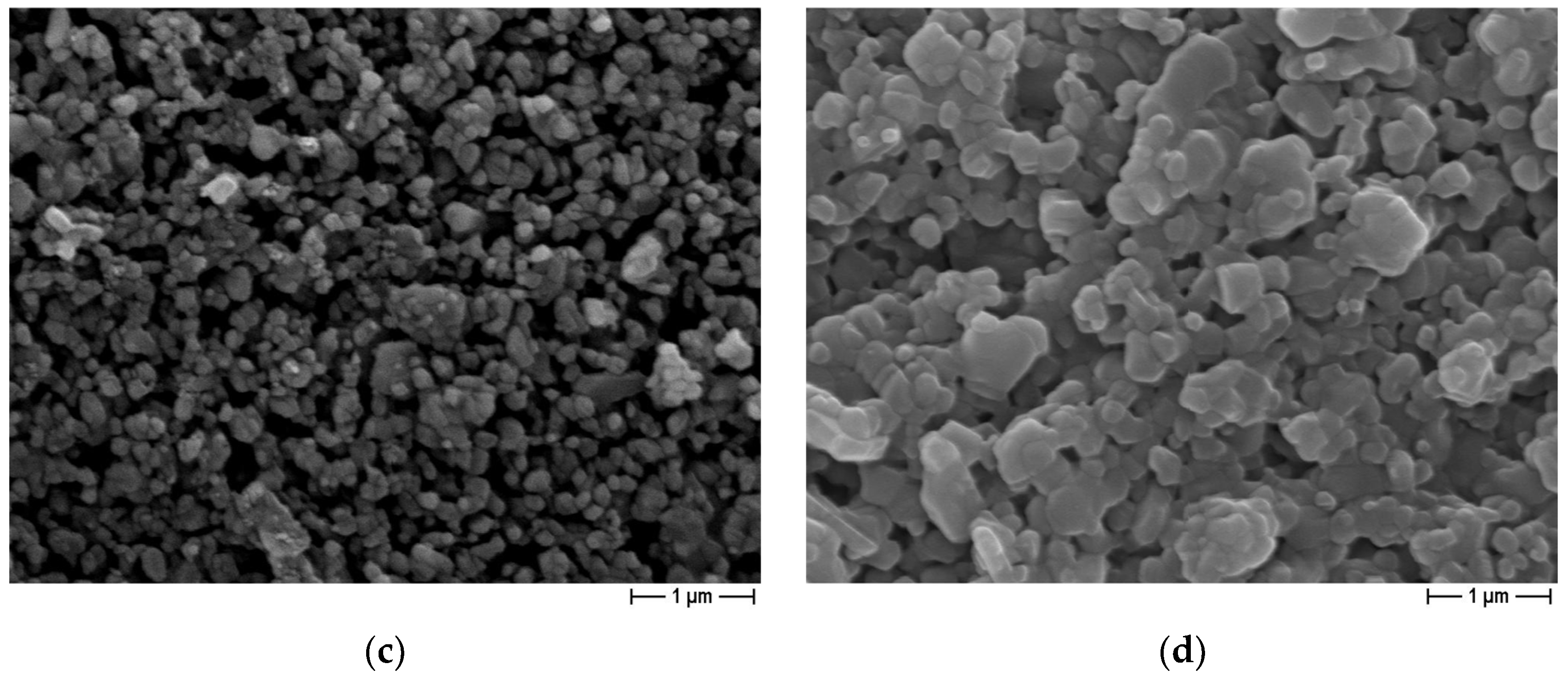
3.2. Sensor-Based Microstructural Characterization
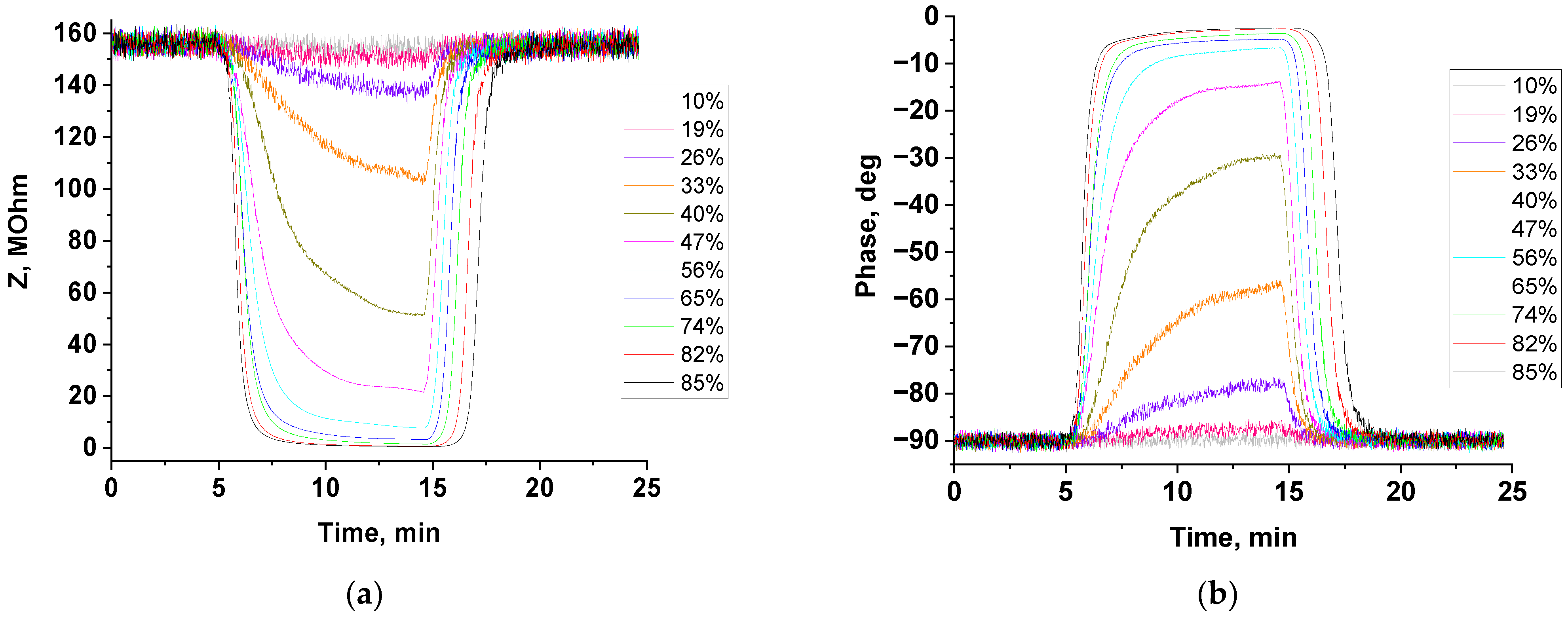
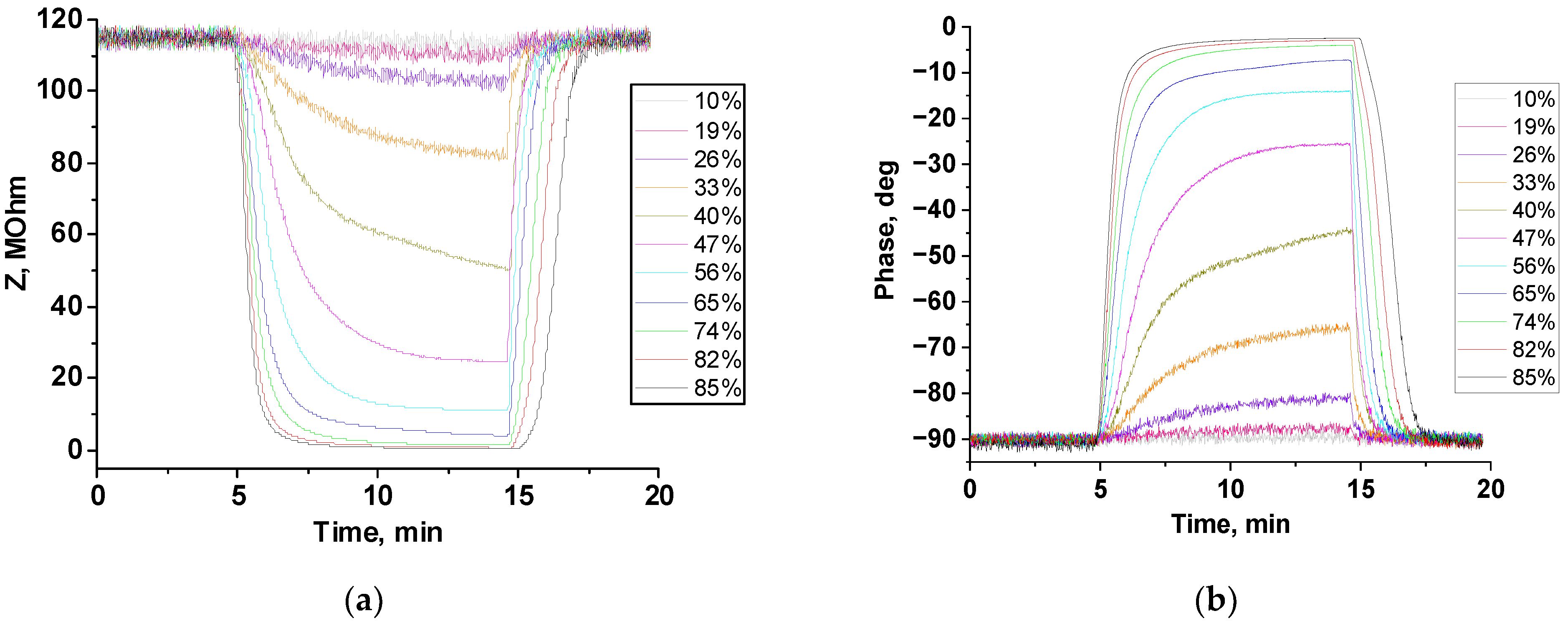
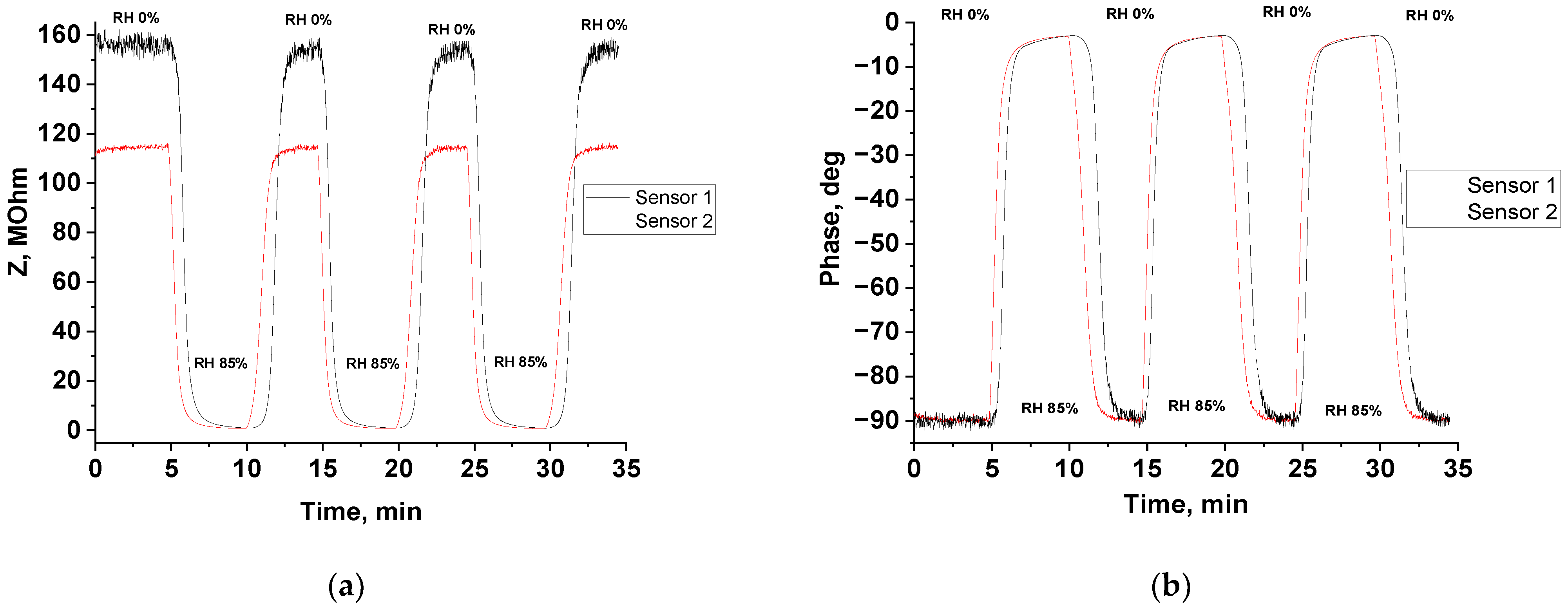
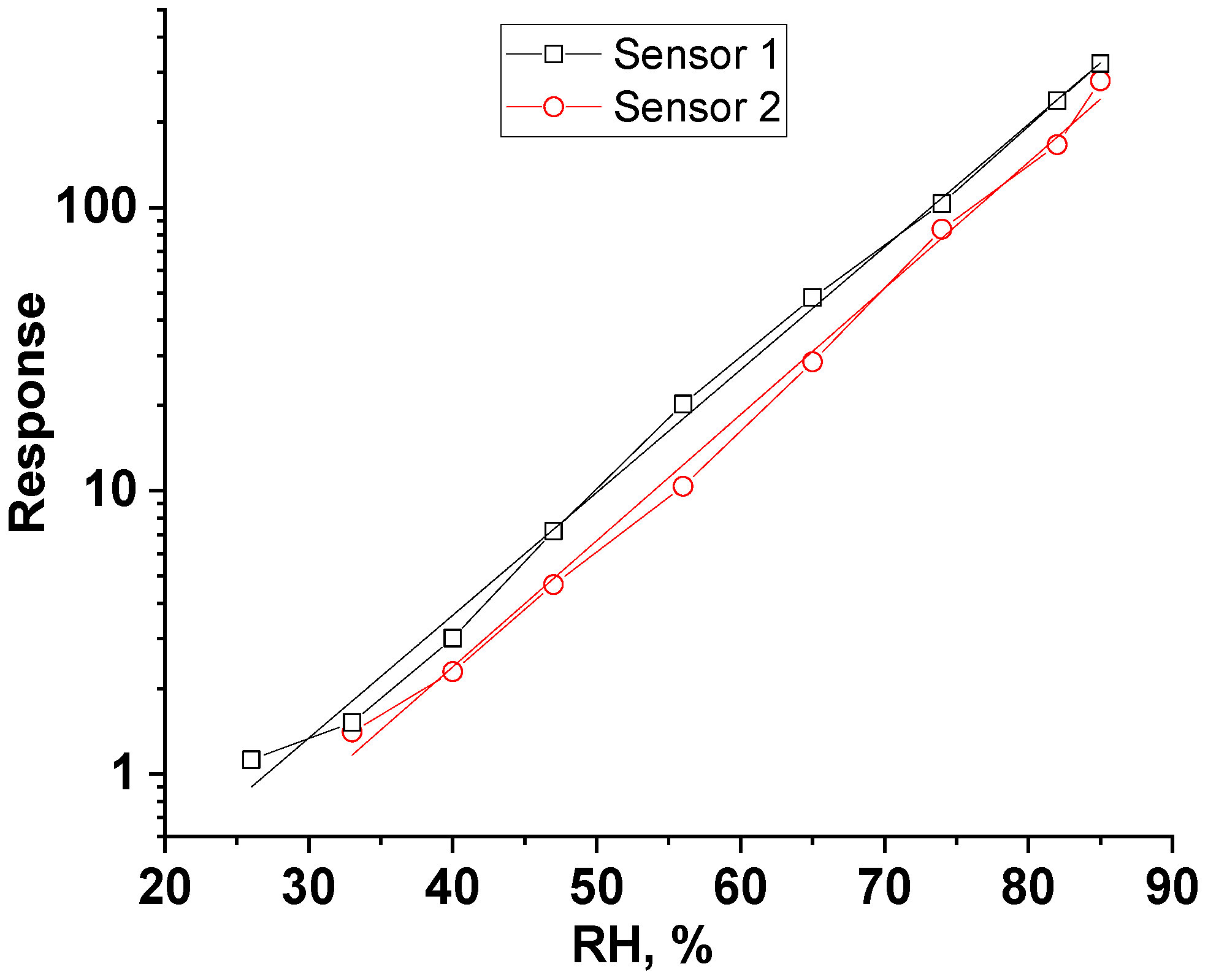
3.3. Humidity-Sensing Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barmpakos, D.; Kaltsas, G.A. Review on Humidity, Temperature and Strain Printed Sensors—Current Trends and Future Perspectives. Sensors 2021, 21, 739. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Cubillo, A.; Tulliani, J.-M.; Pecharromán, C.; Moya, J.S. Iron-oxide nanoparticles supported on sepiolite as a novel humidity sensor. J. Eur. Ceram. Soc. 2007, 27, 1983–1989. [Google Scholar] [CrossRef]
- Céline Laville, C.P.; Deletage, J.-Y. Humidity sensors for a pulmonary function diagnostic microsystem. Sens. Actuators B Chem. 2001, 76, 304–309. [Google Scholar] [CrossRef]
- Tulliani, J.-M.; Baroni, C.; Zavattaro, L.; Grignani, C. Strontium-Doped Hematite as a Possible Humidity Sensing Material for Soil Water Content Determination. Sensors 2013, 13, 12070–12092. [Google Scholar] [CrossRef] [PubMed]
- Duraia, E.M.; Beall, G.W. Humidity sensing properties of reduced humic acid. Sens. Actuators B 2015, 220, 22–26. [Google Scholar] [CrossRef]
- Islam, T.; Nimal, A.T.; Mittal, U.; Sharma, M.U. A micro interdigitated thin film metal oxide capacitive sensor for measuring moisture in the range of 175–625 ppm. Sens. Actuators B 2015, 221, 357–364. [Google Scholar] [CrossRef]
- Nanto, H.; Minami, T.; Takata, S. Zinc-oxide thin-film ammonia gas sensors with high sensitivity and excellent selectivity. J. Appl. Phys. 1986, 60, 482–484. [Google Scholar] [CrossRef]
- Kim, Y.; Jung, B.; Lee, H.; Kim, H.; Lee, K.; Park, H. Capacitive humidity sensor design based on anodic aluminum oxide. Sens. Actuators B Chem. 2009, 141, 441–446. [Google Scholar] [CrossRef]
- Balde, M.; Vena, A.; Sorli, B. Fabrication of porous anodic aluminium oxide layers on paper for humidity sensors. Sens. Actuators B 2015, 220, 829–839. [Google Scholar] [CrossRef]
- Feng, X.; Chen, W.; Yan, L. Sens. and Actuators B: Chemical Free-standing dried foam films of graphene oxide for humidity sensing. Sens. Actuators B Chem. 2015, 215, 316–322. [Google Scholar] [CrossRef]
- Fernández-Ramos, M.D.; Ordóñez, Y.F.; Capitán-Vallvey, L.F.; De Vargas-Sansalvador, I.M.P.; Ballesta-Claver, J. Optical humidity sensor using methylene blue immobilized on a hydrophilic polymer. Sens. Actuators B Chem. 2015, 220, 528–533. [Google Scholar] [CrossRef]
- Jung, D.Y.; Yang, S.Y.; Park, H.; Shin, W.C.; Oh, J.G.; Cho, B.J.; Choi, S.Y. Interface engineering for high performance graphene electronic devices. Nano Converg. 2015, 2, 11. [Google Scholar] [CrossRef]
- Phan, D.-T.; Chung, G.-S. Effects of rapid thermal annealing on humidity sensor based on graphene oxide thin films. Sens. Actuators B Chem. 2015, 220, 1050–1055. [Google Scholar] [CrossRef]
- Su, P.-G.; Shiu, W.-L.; Tsai, M.-S. Flexible humidity sensor based on Au nanoparticles/graphene oxide/thiolated silica sol–gel film. Sens. Actuators B Chem. 2015, 216, 467–475. [Google Scholar] [CrossRef]
- Su, P.-G.; Wang, C.S. Novel flexible resistive-type humidity sensor. Sens. Actuators B Chem. 2007, 123, 1071–1076. [Google Scholar] [CrossRef]
- Traversa, E. Ceramic sensors for humidity detection: The state-of-the-art and future developments. Sens. Actuators B Chem. 1995, B23, 135–156. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, C. Humidity Sensors: A Review of Materials and Mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef]
- Lukaszewicz, J.P. Carbon-film-based humidity sensor containing sodium or potassium. Recovery effect. Sens. Actuators B Chem. 1999, 60, 184–190. [Google Scholar] [CrossRef]
- Varghese, O.K.; Kichambre, P.D.; Gong, D.; Ong, K.G.; Dickey, E.C.; Grimes, C.A. Gas sensing characteristics of multi-wall carbon nanotubes. Sens. Actuators B Chem. 2001, 81, 32–41. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, X.; Guo, H.; Wu, Z. Graphene oxide thin film coated quartz crystal microbalance for humidity detection. Appl. Surf. Sci. 2011, 257, 7778–7782. [Google Scholar] [CrossRef]
- Chu, J.; Peng, X.; Feng, P.; Sheng, V.; Zhang, J. Study of humidity sensors based on nanostructured carbon films produced by physical vapor deposition. Sens. Actuators B Chem. 2013, 178, 508–513. [Google Scholar] [CrossRef]
- Tulliani, J.-M.; Inserra, B.; Ziegler, D. Carbon-Based Materials for Humidity Sensing: A Short Review. Micromachines 2019, 10, 232. [Google Scholar] [CrossRef] [PubMed]
- Afify, A.S.; Ahmad, S.; Khushnood, R.A.; Jagdale, P.; Tulliani, J.-M. Elaboration and characterization of novel humidity sensor based on micro-carbonized bamboo particles. Sens. Actuators B Chem. 2017, 239, 1251–1256. [Google Scholar] [CrossRef]
- Ziegler, D.; Palmero, P.; Giorcelli, M.; Tagliaferro, A.; Tulliani, J.-M.; Ziegler, D.; Palmero, P.; Giorcelli, M.; Tagliaferro, A.; Tulliani, J.-M. Biochars as Innovative Humidity Sensing Materials. Chemosensors 2017, 5, 35. [Google Scholar] [CrossRef]
- Konta, J. Clay and man: Clay raw materials in the service of man. Appl. Clay Sci. 1995, 4, 269–335. [Google Scholar] [CrossRef]
- Murray, H.H. Traditional and new applications for kaolin, smectite, and palygorskite: A general overview. Appl. Clay Sci. 2000, 17, 207–221. [Google Scholar] [CrossRef]
- Choy, J.-H.; Choi, S.-J.; Oh, J.-M.; Park, T. Clay minerals and layered double hydroxides for novel biological applications. Appl. Clay Sci. 2007, 36, 122–132. [Google Scholar] [CrossRef]
- Andersson, H.; Manuilskiy, A.; Unander, T.; Lidenmark, C.; Forsberg, S.; Nilsson, H.-E. Inkjet printed silver nanoparticle humidity sensor with memory effect on paper. IEEE Sens. J. 2012, 12, 1901–1905. [Google Scholar] [CrossRef]
- Burman, D.; Santra, S.; Pramank, P.; Guha, P. Pt decorated MoS2 nanoflakes for ultrasensitive resistive humidity sensor. Nanotechnology 2018, 29, 115504. [Google Scholar] [CrossRef]
- Nikulicheva, T.B.; Zakhvalinskii, V.S.; Pilyuk, E.A.; Nikulin, I.S.; Vyazmin, V.V.; Mishunin, M.V. New humidity sensor material (CaSO4⋅2H2O)0.975-(CuSO4⋅5H2O)0.025. Materialia 2023, 27, 101662. [Google Scholar] [CrossRef]
- Wu, K.; Fei, T.; Zhang, T. Humidity Sensors Based on Metal–Organic Frameworks. Nanomaterials 2022, 12, 4208. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.-A.; Chung, C.-K. Advances in Humidity Nanosensors and Their Application: Review. Sensors 2023, 23, 2328. [Google Scholar] [CrossRef] [PubMed]
- Hotza, D.; Greil, P. Review: Aqueous tape casting of ceramic powders. Mater. Sci. Eng. 1995, A202, 206–217. [Google Scholar] [CrossRef]
- Somiya, S.; Hirata, Y. Mullite Powder Technology and Applications in Japan. Bull. Am. Ceram. Soc. 1991, 70, 1624–1632. [Google Scholar]
- Montanaro, L.; Tulliani, J.M.; Perrot, C.; Negro, A. Sintering of industrial mullites. J. Eur. Ceram. Soc. 1997, 17, 14, 1715–1723. [Google Scholar] [CrossRef]
- Dortmund Data Bank (DDB). Available online: http://ddbonline.ddbst.com/antoinecalculation/antoinecalculationcgi.exe (accessed on 29 May 2024).
- Firehouse. Available online: https://www.firehouse.com/rescue/article/10574732/hazmat-math-calculating-vapor-concentrations (accessed on 29 May 2024).
- Ziegler, D.; Boschetto, F.; Marin, E.; Palmero, P.; Pezzotti, G.; Tulliani, J.M. Rice husk ash as a new humidity sensing material and its aging behavior. Sens. Actuators B Chem. 2021, 328, 129049. [Google Scholar] [CrossRef]
- Srivastava, R.; Yadav, B. Nanostructured ZnO. ZnO-TiO2 and ZnO-Nb2O5 as solid state humidity sensor. Adv. Mater. Lett. 2012, 3, 197–203. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Zhang, Y.; Cheng, X.; Feng, C.; Chen, L.; Zhou, J.; Ruan, S. A novel humidity sensor based on NaTaO3 nanocrystalline. Sens. Actuators B Chem. 2012, 174, 485–489. [Google Scholar] [CrossRef]
- Georgieva, B.; Nenova, Z.; Podolesheva, I.; Pirov, J.; Nenov, T. Investigation of humidity sensors based on Sn-O-Te films by impedance spectroscopy. Bulg. Chem. Commun. 2013, 45, 63–67. [Google Scholar]
- Qi, G.; Weng, Y.; Chen, J. Preparation of porous SnO2-based ceramics with lattice structure by DLP. Ceram. Int. 2022, 48, 14568–14577. [Google Scholar] [CrossRef]
- Burman, D.; Choudhary, D.S.; Guha, P.K. ZnO/MoS2-based enhanced humidity sensor prototype with android app interface for mobile platform. IEEE Sens. J. 2019, 19, 3993–3999. [Google Scholar] [CrossRef]
- Kundu, S.; Majumder, R.; Ghosh, R.; Pal Chowdhury, M. Superior positive relative humidity sensing properties of porous nanostructured Al:ZnO thin films deposited by jet-atomizer spray pyrolysis technique. J. Mater. Sci Mater. Electron. 2019, 30, 4618–4625. [Google Scholar] [CrossRef]
- Babu Reddy, L.P.; Megha, R.; Raj Prakash, H.G.; Ravikiran, Y.T.; Ramana, C.H.V.V.; Vijaya Kumari, S.C.; Kim, D. Copper ferrite-yttrium oxide (CFYO) nanocomposite as remarkable humidity sensor. Inorg. Chem. Commun. 2019, 99, 180–188. [Google Scholar] [CrossRef]
- Manut, A.; Zoolfakar, A.S.; Mamat, M.H.; Ab Ghani, N.S.; Zolkapli, M. Characterization of Titanium Dioxide (TiO2) Nanotubes for Resistive-type Humidity Sensor. In Proceedings of the IEEE International Conference on Semiconductor Electronics (ICSE), Kuala Lumpur, Malaysia, 28–29 July 2020; pp. 104–107. [Google Scholar]
- Pi, C.; Chen, W.; Zhou, W.; Yan, S.; Liu, Z.; Wang, C.; Guo, Q.; Qiu, J.; Yu, X.; Liu, B.; et al. Highly stable humidity sensor based on lead-free Cs3Bi2Br9 perovskite for breath monitoring. J. Mater. Chem. C 2021, 9, 11299–11305. [Google Scholar] [CrossRef]
- Duy, L.T.; Baek, J.Y.; Mun, Y.J.; Seo, H. Patternable production of SrTiO3 nanoparticles using 1-W laser directly on flexible humidity sensor platform based on ITO/SrTiO3/CNT. J. Mater. Sci. Technol. 2021, 71, 186–194. [Google Scholar] [CrossRef]
- Doroftei, C.; Leontie, L. Porous nanostructured gadolinium aluminate for high-sensitivity humidity sensors. Materials 2021, 14, 7102. [Google Scholar] [CrossRef] [PubMed]
- El-Denglawey, A.; Manjunatha, K.; Vijay Sekhar, E.; Chethan, B.; Zhuang, J.; Jagadeesha Angadi, V. Rapid response in recovery time, humidity sensing behavior and magnetic properties of rare earth(Dy & Ho) doped Mn–Zn ceramics. Ceram. Int. 2021, 47, 28614–28622. [Google Scholar]
- Shah, Z.; Shaheen, K.; Arshad, T.; Ahmad, B.; Khan, S.B. Al doped Sr and Cd metal oxide nanomaterials for resistive response of humidity sensing. Mater. Chem. Phys. 2022, 290, 126632. [Google Scholar] [CrossRef]
- Subki, A.S.R.A.; Mamat, M.H.; Musa, M.Z.; Abdullah, M.H.; Shameem Banu, I.B.; Vasimalai, N.; Ahmad, M.K.; Nafarizal, N.; Suriani, A.B.; Mohamad, A.; et al. Effects of varying the amount of reduced graphene oxide loading on the humidity sensing performance of zinc oxide/reduced graphene oxide nanocomposites on cellulose filter paper. J. Alloys Compd. 2022, 926, 166728. [Google Scholar] [CrossRef]
- Dubey, R.S.; Srilali, S.; Ravikiran, Y.T.; Babu, G.S.; Katta, K.V. Synthesis and characterization of Znx-1Al2O4(TiO2)x nanocomposite ceramics and their humidity sensing properties. J. Mater. Sci. 2022, 57, 2636–2649. [Google Scholar] [CrossRef]
- Yasin, E.; Javed, Y.; Imran, Z.; Anwar, H.; Shahid, M. Exploration of dielectric and humidity sensing properties of dysprosium oxide nanorods. Eur. Phys. J. Plus 2023, 138, 1050. [Google Scholar] [CrossRef]
- Mohamed Zahidi, M.; Mamat, M.H.; Subki, A.S.R.A.; Abdullah, M.H.; Hassan, H.; Ahmad, M.K.; Bakar, S.A.; Mohamed, A.; Ohtani, B. Formation of a nanorod-assembled TiO2 actinomorphic-flower-like microsphere film via Ta doping using a facile solution immersion method for humidity sensing. Nanomaterials 2023, 13, 256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, F.; Zheng, Y. Highly sensitive resistive humidity sensor based on strontium-doped lanthanum ferrite nanofibers. Sens. Actuators A Phys. 2023, 358, 114435. [Google Scholar] [CrossRef]
- Ravichandran, R.; Quine, S.D.; Arularasu, M.V. Humidity sensing performance of nitrogen doped reduced graphene oxide-WO3 composite. BioNanoSci 2023, 13, 2205–2214. [Google Scholar] [CrossRef]
- Dhariwal, N.; Yadav, P.; Kumari, M.; Jain, P.; Sanger, A.; Kumar, V.; Thakur, O.P. Iron oxide-based nanoparticles for fast-response humidity sensing, real-time respiration monitoring, and noncontact sensing. IEEE Sens. J. 2023, 23, 22217–22224. [Google Scholar] [CrossRef]
- Kumar, A.; Kumari, P.; Kumar, M.S.; Gupta, G.; Shivagan, D.D.; Bapna, K. SnO2 nanostructured thin film as humidity sensor and its application in breath monitoring. Ceram. Int. 2023, 49, 24911–24921. [Google Scholar] [CrossRef]
- Malik, P.; Duhan, S.; Malik, R. A high-performance humidity sensor based on 3D porous SnO2-encapsulated MCM-48 for real-time breath monitoring and contactless gesture detection. Mater. Adv. 2024, 5, 2510–2525. [Google Scholar] [CrossRef]
- Jiang, W.; Su, M.; Zheng, Y.; Fei, T. Efficient electron transfer through interfacial water molecules across two-dimensional MoO3 for humidity sensing. ACS Appl. Mater. Interfaces 2024, 16, 7406–7414. [Google Scholar] [CrossRef]
- Huang, S.; Shan, H.; Zhao, Y.; Ren, Y.; Gu, X. Preparation of humidity sensors based on CsPbBr3 quantum dots for applications in microcrack detection. Chin. J. Inorg. Chem. 2024, 40, 383–393. [Google Scholar]



| ∅10 (µm) | ∅50 (µm) | ∅90 (µm) | |
|---|---|---|---|
| As received | 0.90 | 28.70 | 106.00 |
| 5 min sonication | 0.58 | 1.76 | 4.36 |
| Humidity | Sensor Response (R = Zo/Zg) | Response Time, s | Recovery Time, s | |||
|---|---|---|---|---|---|---|
| Sensor 1 | Sensor 2 | Sensor 1 | Sensor 2 | Sensor 1 | Sensor 2 | |
| 19% | 1.0 | 1.0 | ||||
| 26% | 1.1 | 1.1 | ||||
| 33% | 1.5 | 1.4 | 406 | 372 | 67 | 35 |
| 40% | 3.0 | 2.3 | 350 | 382 | 66 | 36 |
| 47% | 7.2 | 4.7 | 255 | 238 | 71 | 46 |
| 56% | 20.2 | 10.4 | 174 | 174 | 87 | 56 |
| 65% | 48.1 | 28.5 | 130 | 122 | 113 | 71 |
| 74% | 103.3 | 83.9 | 117 | 97 | 122 | 81 |
| 82% | 238.5 | 166.9 | 93 | 70 | 149 | 104 |
| 85% | 322.9 | 280.5 | 91 | 64 | 167 | 119 |
| Material | Sensor Response, R = Zo/Zg | Response Time, s | Recovery Time, s | Reference |
|---|---|---|---|---|
| Pt decorated MoS2 nanoflakes | ~4000 at 85% RH | 92 | 154 | [29] |
| ZnO/MoS2 | ~301 at 85% RH | 138 | 166 | [43] |
| Porous aluminum-doped ZnO | 733% at 90% RH | ~238 | ~202 | [44] |
| Copper ferrite-yttrium oxide nanocomposite | 4895 at 97% RH | 9 | 23 | [45] |
| Titanium dioxide nanotubes | 58.5 at 90% RH | NA | NA | [46] |
| Cs3Bi2Br9 perovskite | 987 at 90% RH | 5.56 | 6.24 | [47] |
| SrTiO3 nanoparticles | 1.12 at 85% RH | 100 | 300 | [48] |
| GdAlO3 | 8000 at 97% RH | 45 | 60 | [49] |
| Mn0.5Zn0.5DyxHoyFe2−xO4 (x = 0.005 to 0.03) nanoparticles | 99% at 97% RH | 90 | 18 | [50] |
| Al–Sr and Al–Cd nano-materials | 2.87 at 95% RH 3.19 at 95% RH | 60 44 | 29 45 | [51] |
| Reduced graphene oxide/zinc oxide nanostructured powder | 172 at 90% RH | NA | NA | [52] |
| Znx−1Al2O4(TiO2)x | 265 at 97% RH | 195 | 28 | [53] |
| Dy2O3 nanorods | 15 at 97% RH | 2 | 5 | [54] |
| Ta-doped TiO2/reduced graphene oxide | 232% at 90% RH | 4.2 | 3.3 | [55] |
| Sr-doped LaFeO3 nanofibers | 60,597 at 90% RH | NA | NA | [56] |
| N-doped graphene oxide-WO3 | 3427 at 98% RH | 24 | 53 | [57] |
| Nanosized α-Fe2O3 nanoparticles | 48,569 at 95% RH | 9 | 4 | [58] |
| (CaSO4·2H2O)0.975-(CuSO4·5H2O)0.025 | 6.75 at 90% RH | 5 | 3 | [30] |
| SnO2 thin film | 3.1 at 95% RH | 84 | 576 | [59] |
| Porous SnO2/MCM-48 | 105 at 98% RH | 9 | 12 | [60] |
| 2D MoO3 | 4024 at 75% RH | 8 | 40 | [61] |
| Perovskite CsPbBr3-Fe quantum dots | 1.1 at 70% RH | 38 | 38 | [62] |
| Mullite | 322.9 at 85% RH | 91 | 167 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milovanov, Y.; Bertero, A.; Coppola, B.; Palmero, P.; Tulliani, J.-M. Mullite 3D Printed Humidity Sensors. Ceramics 2024, 7, 807-820. https://doi.org/10.3390/ceramics7020053
Milovanov Y, Bertero A, Coppola B, Palmero P, Tulliani J-M. Mullite 3D Printed Humidity Sensors. Ceramics. 2024; 7(2):807-820. https://doi.org/10.3390/ceramics7020053
Chicago/Turabian StyleMilovanov, Yurii, Arianna Bertero, Bartolomeo Coppola, Paola Palmero, and Jean-Marc Tulliani. 2024. "Mullite 3D Printed Humidity Sensors" Ceramics 7, no. 2: 807-820. https://doi.org/10.3390/ceramics7020053
APA StyleMilovanov, Y., Bertero, A., Coppola, B., Palmero, P., & Tulliani, J.-M. (2024). Mullite 3D Printed Humidity Sensors. Ceramics, 7(2), 807-820. https://doi.org/10.3390/ceramics7020053









