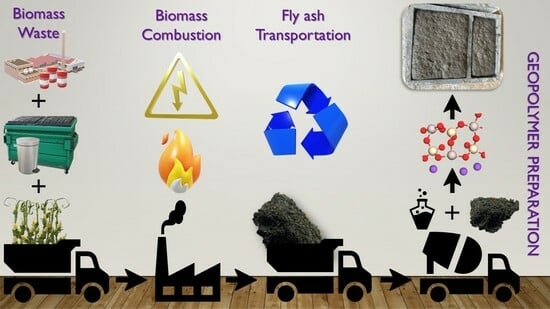
Unlocking the Potential of Biomass Fly Ash: Exploring Its Application in Geopolymeric Materials and a Comparative Case Study of BFA-Based Geopolymeric Concrete against Conventional Concrete
Abstract
1. Introduction
2. Description of Fly Ash
2.1. Coal Combustion Fly Ash
2.2. Biomass Combustion Fly Ash
2.2.1. Waste Biomass
2.2.2. Dangerous Features of Biomass Ash
2.3. Current State of Biomass Ash Management
2.3.1. Use of Biomass Fly Ash in Construction
2.3.2. Further Use of Biomass Ash
3. Biomass Fly Ash-Based Geopolymers
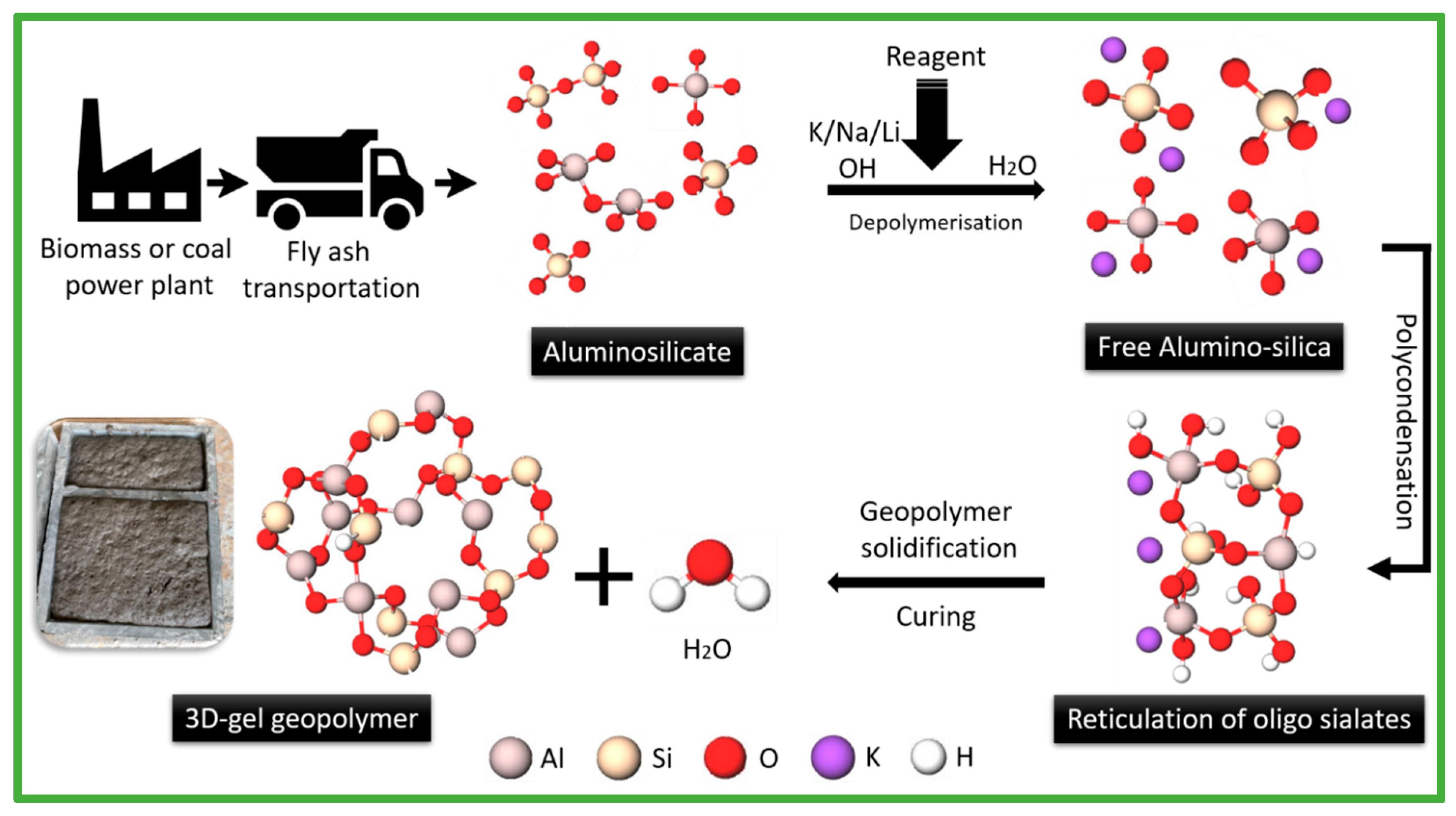
3.1. Description of Geopolymer
3.2. Biomass Fly Ash in Geopolymer Composites
3.3. Environmental Impact of Biomass Fly Ash Recovery on Geopolymer Formation
4. Comparative Case Studies
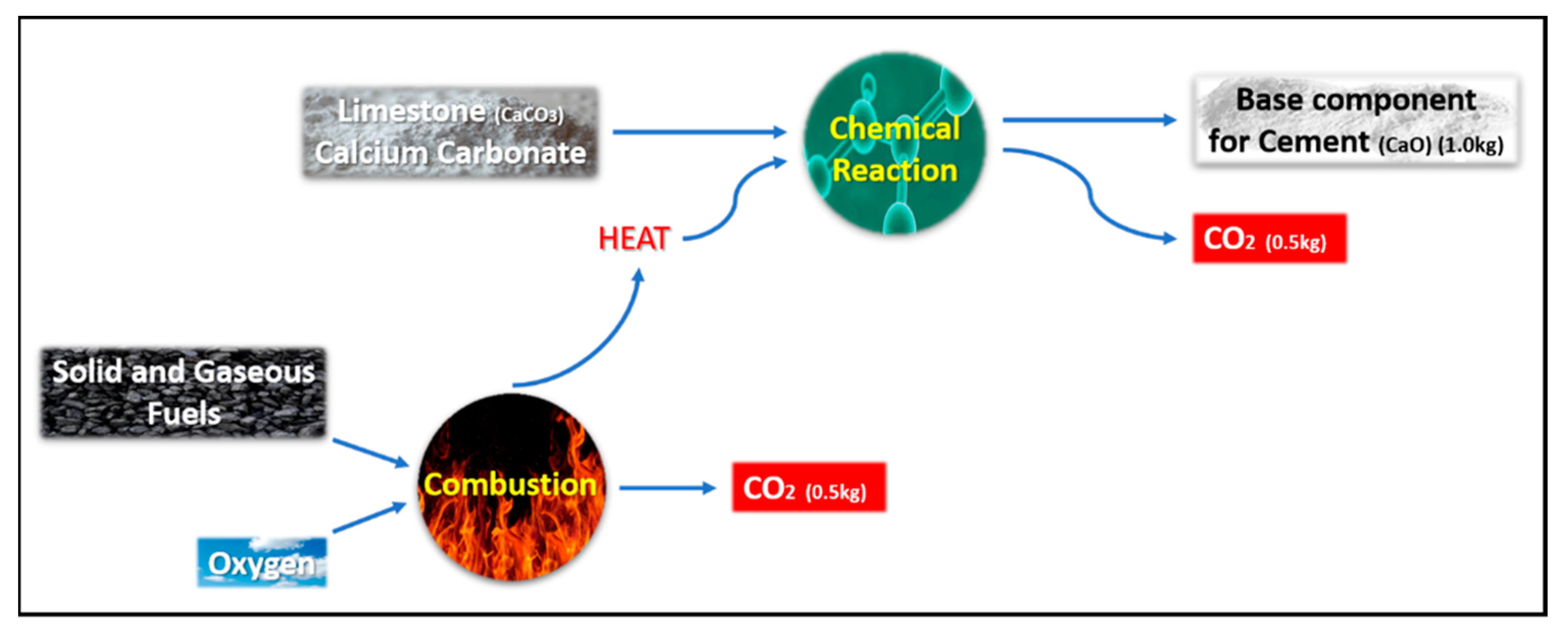
4.1. Calculation of Carbon Dioxide Emissions: Conventional Concrete Versus Geopolymer Concrete
- The direct (primary) footprint refers to the quantifiable quantity of greenhouse gases that are emitted directly as a result of a particular activity, such as electricity generation, heating, or fuel combustion.
- The indirect (or secondary) footprint refers to the quantity of greenhouse gases that are released throughout the complete life cycle of a product, encompassing its production, usage, and eventual disposal.
- The CO2 emissions associated with the transportation of primary materials from the seller to the construction site, as well as the on-site application processes such as the use of mixing machines, are equivalent for both concrete and geopolymer materials. Consequently, the significance of transportation costs for the movement of goods will be disregarded.
- The compressive strength of both the conventional and geopolymer concrete mixtures is expected to reach 75 MPa after a curing period of 28 days.
- The calculations have been conducted using a quantity of 1 ton of prepared concrete.
4.2. Calculation of Production Cost: Conventional Concrete versus Geopolymer Concrete
5. Conclusions
- The composition and quantity of inorganic matter present in biomass fly ash exhibit a higher degree of variability due to the utilization of a wide array of fuels and sources, which are influenced by the prevailing growing conditions. Hence, the chemical and physical composition of fly ash exerts an influence on the ultimate quality of geopolymers.
- Geopolymers derived from biomass fly ash demonstrate enhanced initial mechanical strength, exceptional resistance to acid and sulfate degradation, and reduced shrinkage in comparison to conventional concrete materials.
- Biomass fly ash exhibits potential applications as an adsorbent, membrane filter, Fenton catalyst, and photocatalyst.
- The fly ash should possess a maximum unburnt material content of 5%, iron oxide content of 10%, and CaO content of 10%. The concentration of reactive silicon is typically observed to range between 40% and 50%. The proportion of particles measuring less than 45 μm in size is observed to be between 80% and 90%.
- The impact of reducing carbon dioxide emissions with a geopolymer matrix is a well-established phenomenon. In our case study, we substantiated this claim by utilizing authentic and industrial concrete production recipes. We found the emission factor associated with conventional concrete to be 0.772 kg of carbon dioxide emitted per kilogram of concrete. On the other hand, the emission factor for geopolymer concrete is 0.338 kg CO2 per kg. The geopolymer concrete demonstrates a decrease in carbon dioxide emissions of 56.0%. The findings of our study are corroborated by the existing literature.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Meteorological Organization (WMO). State of the Global Climate 2021 (WMO-No. 1290); WMO: Geneva, Switzerland, 2022; ISBN 978-92-63-11290-3. [Google Scholar]
- Greenhouse Gas Emissions from Energy Data Explorer—Data Tools. Available online: https://www.iea.org/data-and-statistics/data-tools/greenhouse-gas-emissions-from-energy-data-explorer (accessed on 23 December 2022).
- Martins, F.; Felgueiras, C.; Smitkova, M.; Caetano, N. Analysis of Fossil Fuel Energy Consumption and Environmental Impacts in European Countries. Energies 2019, 12, 964. [Google Scholar] [CrossRef]
- Benhelal, E.; Shamsaei, E.; Rashid, M.I. Challenges against CO2 Abatement Strategies in Cement Industry: A Review. J. Environ. Sci. 2021, 104, 84–101. [Google Scholar] [CrossRef]
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable Cement Production—Present and Future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Barcelo, L.; Kline, J.; Walenta, G.; Gartner, E. Cement and Carbon Emissions. Mater. Struct. 2014, 47, 1055–1065. [Google Scholar] [CrossRef]
- Li, C.; Gong, X.Z.; Cui, S.P.; Wang, Z.H.; Zheng, Y.; Chi, B.C. CO2 Emissions Due to Cement Manufacture; Trans Tech Publishers: Zurich, Switzerland, 2011; Volume 685, pp. 181–187. [Google Scholar]
- Çevik, A.; Alzeebaree, R.; Humur, G.; Niş, A.; Gülşan, M.E. Effect of Nano-Silica on the Chemical Durability and Mechanical Performance of Fly Ash Based Geopolymer Concrete. Ceram. Int. 2018, 44, 12253–12264. [Google Scholar] [CrossRef]
- Hardjito, D.; Wallah, S.E.; Sumajouw, D.M.; Rangan, B.V. On the Development of Fly Ash-Based Geopolymer Concrete. Mater. J. 2004, 101, 467–472. [Google Scholar]
- Nguyen, K.T.; Nguyen, Q.D.; Le, T.A.; Shin, J.; Lee, K. Analyzing the Compressive Strength of Green Fly Ash Based Geopolymer Concrete Using Experiment and Machine Learning Approaches. Constr. Build. Mater. 2020, 247, 118581. [Google Scholar] [CrossRef]
- Noushini, A.; Castel, A.; Aldred, J.; Rawal, A. Chloride Diffusion Resistance and Chloride Binding Capacity of Fly Ash-Based Geopolymer Concrete. Cem. Concr. Compos. 2020, 105, 103290. [Google Scholar] [CrossRef]
- Rangan, B.V. Fly Ash-Based Geopolymer Concrete; Curtin University of Technology: Bentley, Australia, 2008. [Google Scholar]
- Gomes, K.C.; Carvalho, M.; Diniz, D. de P.; Abrantes, R. de C.C.; Branco, M.A.; Carvalho, P.R.O.d. Carbon Emissions Associated with Two Types of Foundations: CP-II Portland Cement-Based Composite vs. Geopolymer Concrete. Matéria (Rio De Jan.) 2019, 24. [Google Scholar] [CrossRef]
- Colangelo, F.; Roviello, G.; Ricciotti, L.; Ferrandiz-Mas, V.; Messina, F.; Ferone, C.; Tarallo, O.; Cioffi, R.; Cheeseman, C. Mechanical and Thermal Properties of Lightweight Geopolymer Composites. Cem. Concr. Compos. 2018, 86, 266–272. [Google Scholar] [CrossRef]
- Jaya, N.A.; Yun-Ming, L.; Cheng-Yong, H.; Abdullah, M.M.A.B.; Hussin, K. Correlation between Pore Structure, Compressive Strength and Thermal Conductivity of Porous Metakaolin Geopolymer. Constr. Build. Mater. 2020, 247, 118641. [Google Scholar] [CrossRef]
- Basu, M.; Pande, M.; Bhadoria, P.; Mahapatra, S. Potential Fly-Ash Utilization in Agriculture: A Global Review. Prog. Nat. Sci. 2009, 19, 1173–1186. [Google Scholar]
- Ramanathan, S.; Gopinath, S.C.; Arshad, M.M.; Poopalan, P. Nanostructured Aluminosilicate from Fly Ash: Potential Approach in Waste Utilization for Industrial and Medical Applications. J. Clean. Prod. 2020, 253, 119923. [Google Scholar]
- Sanalkumar, K.U.A.; Lahoti, M.; Yang, E.-H. Investigating the Potential Reactivity of Fly Ash for Geopolymerization. Constr. Build. Mater. 2019, 225, 283–291. [Google Scholar] [CrossRef]
- Giergiczny, Z. Fly Ash and Slag. Cem. Concr. Res. 2019, 124, 105826. [Google Scholar] [CrossRef]
- Elmrabet, R.; El Harfi, A.; El Youbi, M. Study of Properties of Fly Ash Cements. Mater. Today: Proc. 2019, 13, 850–856. [Google Scholar] [CrossRef]
- Biondi, L.; Perry, M.; Vlachakis, C.; Wu, Z.; Hamilton, A.; McAlorum, J. Ambient Cured Fly Ash Geopolymer Coatings for Concrete. Materials 2019, 12, 923. [Google Scholar] [CrossRef]
- Davidovits, J.; Davidovits, R.; Davidovits, M. Geopolymeric Cement Based on Fly Ash and Harmless to Use. U.S. Patent No. US8202362B2, 19 June 2012. [Google Scholar]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly Ash-Based Geopolymer: Clean Production, Properties and Applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Azad, N.M.; Samarakoon, S.S.M. Utilization of Industrial By-Products/Waste to Manufacture Geopolymer Cement/Concrete. Sustainability 2021, 13, 873. [Google Scholar] [CrossRef]
- Ali, N.; Jaffar, A.; Anwer, M.; Khan, S.; Anjum, M.; Hussain, A.; Raja, M.; Ming, X. The Greenhouse Gas Emissions Produced by Cement Production and Its Impact on Environment: A Review of Global Cement Processing. Int. J. Res. (IJR) 2015, 2, 488–500. [Google Scholar]
- Kajaste, R.; Hurme, M. Cement Industry Greenhouse Gas Emissions–Management Options and Abatement Cost. J. Clean. Prod. 2016, 112, 4041–4052. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Cement to Minimize Carbon-Dioxde Greenhouse-Warming. Ceram. Trans. 1993, 37, 165–182. [Google Scholar]
- Davidovits, J. Environmentally Driven Geopolymer Cement Applications. In Proceedings of the 2002 Geopolymer Conference, Melbourne, Australia, 28–29 October 2002. [Google Scholar]
- Zain, H.; Abdullah, M.M.A.B.; Hussin, K.; Ariffin, N.; Bayuaji, R. Review on Various Types of Geopolymer Materials with the Environmental Impact Assessment. EDP Sci. 2017, 97, 01021. [Google Scholar] [CrossRef]
- Meesala, C.R.; Verma, N.K.; Kumar, S. Critical Review on Fly-ash Based Geopolymer Concrete. Struct. Concr. 2020, 21, 1013–1028. [Google Scholar] [CrossRef]
- McLellan, B.C.; Williams, R.P.; Lay, J.; van Riessen, A.; Corder, G.D. Costs and Carbon Emissions for Geopolymer Pastes in Comparison to Ordinary Portland Cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]
- Das, S.; Saha, P.; Jena, S.P.; Panda, P. Geopolymer Concrete: Sustainable Green Concrete for Reduced Greenhouse Gas Emission–A Review. Mater. Today Proc. 2022, 60, 62–71. [Google Scholar] [CrossRef]
- Davidovits, J. False Values on CO2 Emission for Geopolymer Cement/Concrete Published in Scientific Papers. Tech. Pap. 2015, 24, 1–9. [Google Scholar]
- Choi, J.-H.; Ha, S.-J.; Bak, Y.-C.; Park, Y.-O. Particle Size Effect on the Filtration Drag of Fly Ash from a Coal Power Plant. Korean J. Chem. Eng. 2002, 19, 1085–1090. [Google Scholar] [CrossRef]
- Jaworek, A.; Sobczyk, A.; Krupa, A.; Marchewicz, A.; Czech, T.; Śliwiński, L. Hybrid Electrostatic Filtration Systems for Fly Ash Particles Emission Control. A Review. Sep. Purif. Technol. 2019, 213, 283–302. [Google Scholar]
- Lee, K.M.; Jo, Y.M.; Lee, J.H.; Raper, J.A. Assessment of Surface and Depth Filters by Filter Quality. Powder Technol. 2008, 185, 187–194. [Google Scholar] [CrossRef]
- Suryawanshi, S.; Shewale, V.; Thakare, R.; Yarasu, R. Parametric Study of Different Biomass Feedstocks Used for Gasification Process of Gasifier—A Literature Review. Biomass Convers. Biorefinery 2021, 13, 7689–7700. [Google Scholar] [CrossRef]
- Matveeva, V.G.; Bronstein, L.M. From Renewable Biomass to Nanomaterials: Does Biomass Origin Matter? Prog. Mater. Sci. 2022, 130, 100999. [Google Scholar]
- Gardner, N. Effect of Temperature on the Early-Age Properties of Type I, Type II, and Type III/Fly Ash Concretes with Temperature. Mater. J. 1990, 87, 68–78. [Google Scholar]
- Ghazali, N.; Muthusamy, K.; Ahmad, S.W. Utilization of Fly Ash in Construction; IOP Publishing: Bristol, UK, 2019; Volume 601, p. 012023. [Google Scholar]
- Joshi, R.; Nagaraj, T. Fly Ash Utilization for Soil Improvement. In Environmental Geotechnics and Problematic Soils and Rocks; CRC Press: Boca Raton, FL, USA, 2021; pp. 15–24. ISBN 1-00-321105-4. [Google Scholar]
- Ohenoja, K.; Pesonen, J.; Yliniemi, J.; Illikainen, M. Utilization of Fly Ashes from Fluidized Bed Combustion: A Review. Sustainability 2020, 12, 2988. [Google Scholar] [CrossRef]
- Onifade, M.; Genc, B. A Review of Research on Spontaneous Combustion of Coal. Int. J. Min. Sci. Technol. 2020, 30, 303–311. [Google Scholar] [CrossRef]
- Hansen, L.D.; Fisher, G.L. Elemental Distribution in Coal Fly Ash Particles. Environ. Sci. Technol. 1980, 14, 1111–1117. [Google Scholar] [CrossRef]
- Davidovits, J. Joseph Geopolymer Chemistry and Applications. Geopolymer Inst 2008, 2, 145–148. [Google Scholar]
- Qian, J.; Shi, C.; Wang, Z. Activation of Blended Cements Containing Fly Ash. Cem. Concr. Res. 2001, 31, 1121–1127. [Google Scholar] [CrossRef]
- Malhotra, V.M.; Ramezanianpour, A.A. Fly Ash in Concrete, 2nd ed.; CANMET: Ottawa, ON, Canada, 1994. [Google Scholar]
- Provis, J.L.; Van Deventer, J.S.J. Geopolymers: Structures, Processing, Properties and Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 1-84569-638-7. [Google Scholar]
- Bhatt, A.; Priyadarshini, S.; Mohanakrishnan, A.A.; Abri, A.; Sattler, M.; Techapaphawit, S. Physical, Chemical, and Geotechnical Properties of Coal Fly Ash: A Global Review. Case Stud. Constr. Mater. 2019, 11, e00263. [Google Scholar] [CrossRef]
- Wilén, C.; Moilanen, A.; Kurkela, E. Biomass Feedstock Analyses; Technical Research Centre: Espoo, Finland, 1996. [Google Scholar]
- Van Zandvoort, I.; Wang, Y.; Rasrendra, C.B.; van Eck, E.R.; Bruijnincx, P.C.; Heeres, H.J.; Weckhuysen, B.M. Formation, Molecular Structure, and Morphology of Humins in Biomass Conversion: Influence of Feedstock and Processing Conditions. ChemSusChem 2013, 6, 1745–1758. [Google Scholar] [CrossRef]
- Dimitriadis, A.; Bezergianni, S. Hydrothermal Liquefaction of Various Biomass and Waste Feedstocks for Biocrude Production: A State of the Art Review. Renew. Sustain. Energy Rev. 2017, 68, 113–125. [Google Scholar]
- Teixeira, E.R.; Camões, A.; Branco, F.G. Synergetic Effect of Biomass Fly Ash on Improvement of High-Volume Coal Fly Ash Concrete Properties. Constr. Build. Mater. 2022, 314, 125680. [Google Scholar] [CrossRef]
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical Characteristics of Biomass Ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- Hasler, P.; Nussbaumer, T. Particle Size Distribution of the Fly Ash from Biomass Combustion. In Proceedings of the Biomass for Energy and Industry, 10th European Conference and Technology Exhibition, Rimpar Carmen, Germany, 8–11 June 1998; pp. 8–11. [Google Scholar]
- Chen, H.; Liu, Z.; Chen, X.; Chen, Y.; Dong, Z.; Wang, X.; Yang, H. Comparative Pyrolysis Behaviors of Stalk, Wood and Shell Biomass: Correlation of Cellulose Crystallinity and Reaction Kinetics. Bioresour. Technol. 2020, 310, 123498. [Google Scholar] [CrossRef] [PubMed]
- Lozano, Y.M.; Lehnert, T.; Linck, L.T.; Lehmann, A.; Rillig, M.C. Microplastic Shape, Polymer Type, and Concentration Affect Soil Properties and Plant Biomass. Front. Plant Sci. 2021, 12, 616645. [Google Scholar] [CrossRef] [PubMed]
- Spirek, T. Využití odpadního produktu z kombinované výroby elektrické energie a tepla (Found EU, OP PIK) 2021, Green Energy Consulting, s.r.o. CZ.01.1.02/0.0/0.0/20_360/0023834. Available online: https://www.e-zakazky.cz/profil-zadavatele/495fd7c9-c323-4859-8d32-d170889b971b/zakazka/P21V00000001 (accessed on 2 July 2023).
- Zafar, S. An Introduction to Biomass Energy | BioEnergy Consult 2022. Available online: https://www.bioenergyconsult.com/biomass-energy-introduction/ (accessed on 7 April 2023).
- Iakovou, E.; Karagiannidis, A.; Vlachos, D.; Toka, A.; Malamakis, A. Waste Biomass-to-Energy Supply Chain Management: A Critical Synthesis. Waste Manag. 2010, 30, 1860–1870. [Google Scholar]
- Nunes, A.A.; Franca, A.S.; Oliveira, L.S. Activated Carbons from Waste Biomass: An Alternative Use for Biodiesel Production Solid Residues. Bioresour. Technol. 2009, 100, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Fořt, J.; Šál, J.; Ševčík, R.; Doleželová, M.; Keppert, M.; Jerman, M.; Záleská, M.; Stehel, V.; Černý, R. Biomass Fly Ash as an Alternative to Coal Fly Ash in Blended Cements: Functional Aspects. Constr. Build. Mater. 2021, 271, 121544. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Y.; Hu, Z.; Zhang, L.; Yang, S.; Ruan, R.; Bai, S.; Tan, H. Characteristics of Ash and Slag from Four Biomass-Fired Power Plants: Ash/Slag Ratio, Unburned Carbon, Leaching of Major and Trace Elements. Energy Convers. Manag. 2020, 214, 112897. [Google Scholar] [CrossRef]
- Demirbaş, A. Heavy Metal Contents of Fly Ashes from Selected Biomass Samples. Energy Sources 2005, 27, 1269–1276. [Google Scholar] [CrossRef]
- Pastircakova, K. Determination of Trace Metal Concentrations in Ashes from Various Biomass Materials. Energy Educ. Sci. Technol. 2004, 13, 97–104. [Google Scholar]
- Liao, C.; Wu, C.; Yan, Y. The Characteristics of Inorganic Elements in Ashes from a 1 MW CFB Biomass Gasification Power Generation Plant. Fuel Process. Technol. 2007, 88, 149–156. [Google Scholar] [CrossRef]
- Dwivedi, A.; Jain, M.K. Fly Ash–Waste Management and Overview: A Review. Recent Res. Sci. Technol. 2014, 6, 30–35. [Google Scholar]
- Chew, K.W.; Chia, S.R.; Yen, H.-W.; Nomanbhay, S.; Ho, Y.-C.; Show, P.L. Transformation of Biomass Waste into Sustainable Organic Fertilizers. Sustainability 2019, 11, 2266. [Google Scholar] [CrossRef]
- Chojnacka, K.; Gorazda, K.; Witek-Krowiak, A.; Moustakas, K. Recovery of Fertilizer Nutrients from Materials-Contradictions, Mistakes and Future Trends. Renew. Sustain. Energy Rev. 2019, 110, 485–498. [Google Scholar]
- Sun, X.; Li, J.; Zhao, X.; Zhu, B.; Zhang, G. A Review on the Management of Municipal Solid Waste Fly Ash in American. Procedia Environ. Sci. 2016, 31, 535–540. [Google Scholar] [CrossRef]
- Obernberger, I.; Supancic, K. Possibilities of Ash Utilisation from Biomass Combustion Plants. In Proceedings of the 17th European Biomass Conference & Exhibition, Hamburg, Germany, 29 June–3 July 2009; Volume 29. [Google Scholar]
- Zhang, Y.; Wang, L.; Chen, L.; Ma, B.; Zhang, Y.; Ni, W.; Tsang, D.C. Treatment of Municipal Solid Waste Incineration Fly Ash: State-of-the-Art Technologies and Future Perspectives. J. Hazard. Mater. 2021, 411, 125132. [Google Scholar] [PubMed]
- Saeli, M.; Senff, L.; Tobaldi, D.M.; Seabra, M.P.; Labrincha, J.A. Novel Biomass Fly Ash-Based Geopolymeric Mortars Using Lime Slaker Grits as Aggregate for Applications in Construction: Influence of Granulometry and Binder/Aggregate Ratio. Constr. Build. Mater. 2019, 227, 116643. [Google Scholar]
- Uliasz-Bocheńczyk, A.; Mazurkiewicz, M.; Mokrzycki, E. Fly Ash from Energy Production—A Waste, Byproduct and Raw Material. Gospod. Surowcami Miner. 2015, 31, 139–149. [Google Scholar] [CrossRef]
- Wang, S.; Miller, A.; Llamazos, E.; Fonseca, F.; Baxter, L. Biomass Fly Ash in Concrete: Mixture Proportioning and Mechanical Properties. Fuel 2008, 87, 365–371. [Google Scholar] [CrossRef]
- Wang, S.; Baxter, L.; Fonseca, F. Biomass Fly Ash in Concrete: SEM, EDX and ESEM Analysis. Fuel 2008, 87, 372–379. [Google Scholar] [CrossRef]
- Wang, S.; Baxter, L. Comprehensive Study of Biomass Fly Ash in Concrete: Strength, Microscopy, Kinetics and Durability. Fuel Process. Technol. 2007, 88, 1165–1170. [Google Scholar] [CrossRef]
- Jaturapitakkul, C.; Kiattikomol, K.; Tangchirapat, W.; Saeting, T. Evaluation of the Sulfate Resistance of Concrete Containing Palm Oil Fuel Ash. Constr. Build. Mater. 2007, 21, 1399–1405. [Google Scholar] [CrossRef]
- Johari, M.M.; Zeyad, A.; Bunnori, N.M.; Ariffin, K. Engineering and Transport Properties of High-Strength Green Concrete Containing High Volume of Ultrafine Palm Oil Fuel Ash. Constr. Build. Mater. 2012, 30, 281–288. [Google Scholar] [CrossRef]
- Memon, S.A.; Shaikh, M.A.; Akbar, H. Utilization of Rice Husk Ash as Viscosity Modifying Agent in Self Compacting Concrete. Constr. Build. Mater. 2011, 25, 1044–1048. [Google Scholar] [CrossRef]
- Tuan, N.V.; Ye, G.; van Breugel, K.; Fraaij, A.L.A.; Bui, D.D. The Study of Using Rice Husk Ash to Produce Ultra High Performance Concrete. Constr. Build. Mater. 2011, 25, 2030–2035. [Google Scholar] [CrossRef]
- Nagrockienė, D.; Daugėla, A. Investigation into the Properties of Concrete Modified with Biomass Combustion Fly Ash. Constr. Build. Mater. 2018, 174, 369–375. [Google Scholar] [CrossRef]
- Costopoulos, N.G.; Newhouse, H.K. Building Material Manufacturing from Fly Ash. U.S. Patent US4659385A, 21 April 1987. [Google Scholar]
- Liskowitz, J.W.; Wecharatana, M.; Jaturapitakkul, C.; Cerkanowicz, A.E. Compressive Strength of Concrete and Mortar Containing Fly Ash. U.S. Patent US5624491A, 29 April 1997. [Google Scholar]
- Perez-Pena, M. Fly Ash Based Lightweight Cementitious Composition with High Compressive Strength and Fast Set. U.S. Patent US8551241B2, 8 October 2013. [Google Scholar]
- Roddy, C.W. Well Cement Compositions Comprising Biowaste Ash and Methods of Use. U.S. Patent US8641818B2, 4 February 2014. [Google Scholar]
- Belviso, C. State-of-the-Art Applications of Fly Ash from Coal and Biomass: A Focus on Zeolite Synthesis Processes and Issues. Prog. Energy Combust. Sci. 2018, 65, 109–135. [Google Scholar]
- Pels, J.R.; de Nie, D.S.; Kiel, J.H. Utilization of Ashes from Biomass Combustion and Gasification. In Proceedings of the 14th European Biomass Conference & Exhibition, Paris, France, 10–17 October 2005; Volume 17, pp. 17–21. [Google Scholar]
- Novais, R.M.; Carvalheiras, J.; Tobaldi, D.M.; Seabra, M.P.; Pullar, R.C.; Labrincha, J.A. Synthesis of Porous Biomass Fly Ash-Based Geopolymer Spheres for Efficient Removal of Methylene Blue from Wastewaters. J. Clean. Prod. 2019, 207, 350–362. [Google Scholar]
- Sun, Y.; Liu, Z.; Fatehi, P. Treating Thermomechanical Pulping Wastewater with Biomass-Based Fly Ash: Modeling and Experimental Studies. Sep. Purif. Technol. 2017, 183, 106–116. [Google Scholar]
- Kwong, C.; Chao, C.Y.H. Fly-Ash Products from Biomass Co-Combustion for VOC Control. Bioresour. Technol. 2010, 101, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Asquer, C.; Cappai, G.; Carucci, A.; De Gioannis, G.; Muntoni, A.; Piredda, M.; Spiga, D. Biomass Ash Characterisation for Reuse as Additive in Composting Process. Biomass Bioenergy 2019, 123, 186–194. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L. Fly Ash-Based Geopolymer as a Novel Photocatalyst for Degradation of Dye from Wastewater. Particuology 2013, 11, 353–358. [Google Scholar]
- Bahadur, N.; Das, P.; Bhargava, N. Improving Energy Efficiency and Economic Feasibility of Photocatalytic Treatment of Synthetic and Real Textile Wastewater Using Bagasse Fly Ash Modified TiO2. Chem. Eng. J. Adv. 2020, 2, 100012. [Google Scholar] [CrossRef]
- Mushtaq, F.; Zahid, M.; Bhatti, I.A.; Nasir, S.; Hussain, T. Possible Applications of Coal Fly Ash in Wastewater Treatment. J. Environ. Manag. 2019, 240, 27–46. [Google Scholar]
- Karnchanawong, S.; Mongkontep, T.; Praphunsri, K. Effect of Green Waste Pretreatment by Sodium Hydroxide and Biomass Fly Ash on Composting Process. J. Clean. Prod. 2017, 146, 14–19. [Google Scholar]
- Davidovits, J. Why Alkali-Activated Materials (AAM) Are Not Geopolymers. Publ. Tech. Pap. 2018, 25. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers and Geopolymeric Materials. J. Therm. Anal. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Davidovits, J.; Davidovits, R. Ferro-Sialate Geopolymers (-Fe-O-Si-O-Al-O-); Geopolymer Institute Library: Paris, France, 2020. [Google Scholar]
- Ranjbar, N.; Mehrali, M.; Behnia, A.; Javadi Pordsari, A.; Mehrali, M.; Alengaram, U.J.; Jumaat, M.Z. A Comprehensive Study of the Polypropylene Fiber Reinforced Fly Ash Based Geopolymer. PLoS ONE 2016, 11, e0147546. [Google Scholar] [CrossRef]
- Villca, A.R.; Soriano, L.; Font, A.; Tashima, M.M.; Monzó, J.; Borrachero, M.V.; Payá, J. Lime/Pozzolan/Geopolymer Systems: Performance in Pastes and Mortars. Constr. Build. Mater. 2021, 276, 122208. [Google Scholar] [CrossRef]
- Alter, S.; Wright, M. Geopolymer Compositions 2010. Patent WO2010079414A2, 15 July 2010. [Google Scholar]
- Bakri, A.; Kamarudin, H.; Binhussain, M.; Nizar, I.K.; Rafiza, A.; Zarina, Y. Comparison of Geopolymer Fly Ash and Ordinary Portland Cement to the Strength of Concrete. Adv. Sci. Lett. 2013, 19, 3592–3595. [Google Scholar] [CrossRef]
- Schmücker, M.; MacKenzie, K.J. Microstructure of Sodium Polysialate Siloxo Geopolymer. Ceram. Int. 2005, 31, 433–437. [Google Scholar] [CrossRef]
- Temuujin, J.; Minjigmaa, A.; Rickard, W.; Lee, M.; Williams, I.; Van Riessen, A. Fly Ash Based Geopolymer Thin Coatings on Metal Substrates and Its Thermal Evaluation. J. Hazard. Mater. 2010, 180, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Bakharev, T. Resistance of Geopolymer Materials to Acid Attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Le Chi, H.; Louda, P.; Le Van, S.; Volesky, L.; Kovacic, V.; Bakalova, T. Composite Performance Evaluation of Basalt Textile-Reinforced Geopolymer Mortar. Fibers 2019, 7, 63. [Google Scholar] [CrossRef]
- Le Chi, H.; Louda, P.; Periyasamy, A.P.; Bakalova, T.; Kovacic, V. Flexural Behavior of Carbon Textile-Reinforced Geopolymer Composite Thin Plate. Fibers 2018, 6, 87. [Google Scholar] [CrossRef]
- Le Chi, H.; Louda, P. Experimental Investigation of Four-Point Flexural Behavior of Textile Reinforcement in Geopolymer Mortar. Int. J. Eng. Technol 2019, 11, 10–15. [Google Scholar] [CrossRef][Green Version]
- Payakaniti, P.; Pinitsoonthorn, S.; Thongbai, P.; Amornkitbamrung, V.; Chindaprasirt, P. Effects of Carbon Fiber on Mechanical and Electrical Properties of Fly Ash Geopolymer Composite. Mater. Today Proc. 2018, 5, 14017–14025. [Google Scholar] [CrossRef]
- Barsukov, V.; Senyk, I.; Kryukova, O.; Butenko, O. Composite Carbon-Polymer Materials for Electromagnetic Radiation Shielding. Mater. Today Proc. 2018, 5, 15909–15914. [Google Scholar] [CrossRef]
- Chung, D.; Eddib, A.A. Effect of Fiber Lay-up Configuration on the Electromagnetic Interference Shielding Effectiveness of Continuous Carbon Fiber Polymer-Matrix Composite. Carbon 2019, 141, 685–691. [Google Scholar] [CrossRef]
- He, P.; Jia, L.; Ma, G.; Wang, R.; Yuan, J.; Duan, X.; Yang, Z.; Jia, D. Effects of Fiber Contents on the Mechanical and Microwave Absorbent Properties of Carbon Fiber Felt Reinforced Geopolymer Composites. Ceram. Int. 2018, 44, 10726–10734. [Google Scholar] [CrossRef]
- Munalli, D.; Dimitrakis, G.; Chronopoulos, D.; Greedy, S.; Long, A. Electromagnetic Shielding Effectiveness of Carbon Fibre Reinforced Composites. Compos. Part B Eng. 2019, 173, 106906. [Google Scholar] [CrossRef]
- Singh, A.K.; Shishkin, A.; Koppel, T.; Gupta, N. A Review of Porous Lightweight Composite Materials for Electromagnetic Interference Shielding. Compos. Part B Eng. 2018, 149, 188–197. [Google Scholar] [CrossRef]
- Dhasindrakrishna, K.; Pasupathy, K.; Ramakrishnan, S.; Sanjayan, J. Progress, Current Thinking and Challenges in Geopolymer Foam Concrete Technology. Cem. Concr. Compos. 2021, 116, 103886. [Google Scholar] [CrossRef]
- Jindal, B.B. Investigations on the Properties of Geopolymer Mortar and Concrete with Mineral Admixtures: A Review. Constr. Build. Mater. 2019, 227, 116644. [Google Scholar] [CrossRef]
- Kumar, A.; Saravanan, T.J.; Bisht, K.; Kabeer, K.S.A. A Review on the Utilization of Red Mud for the Production of Geopolymer and Alkali Activated Concrete. Constr. Build. Mater. 2021, 302, 124170. [Google Scholar] [CrossRef]
- Novais, R.M.; Buruberri, L.H.; Ascensão, G.; Seabra, M.P.; Labrincha, J.A. Porous Biomass Fly Ash-Based Geopolymers with Tailored Thermal Conductivity. J. Clean. Prod. 2016, 119, 99–107. [Google Scholar] [CrossRef]
- Rossi, A.D.; Simão, L.; Ribeiro, M.J.; Novais, R.M.; Labrincha, J.A.; Hotza, D.; Moreira, R.F.P.M. In-Situ Synthesis of Zeolites by Geopolymerization of Biomass Fly Ash and Metakaolin. Mater. Lett. 2019, 236, 644–648. [Google Scholar] [CrossRef]
- Rossi, A.D.; Simão, L.; Ribeiro, M.J.; Hotza, D.; Moreira, R.F.P.M. Study of Cure Conditions Effect on the Properties of Wood Biomass Fly Ash Geopolymers. J. Mater. Res. Technol. 2020, 9, 7518–7528. [Google Scholar] [CrossRef]
- Bouzar, B.; Mamindy-Pajany, Y. Immobilization Study of As, Cr, Mo, Pb, Sb, Se and Zn in Geopolymer Matrix: Application to Shooting Range Soil and Biomass Fly Ash. Int. J. Environ. Sci. Technol. 2023. [Google Scholar] [CrossRef]
- Abdulkareem, O.A.; Ramli, M.; Matthews, J.C. Production of Geopolymer Mortar System Containing High Calcium Biomass Wood Ash as a Partial Substitution to Fly Ash: An Early Age Evaluation. Compos. Part B Eng. 2019, 174, 106941. [Google Scholar] [CrossRef]
- Jurado-Contreras, S.; Bonet-Martínez, E.; Sánchez-Soto, P.; Gencel, O.; Eliche-Quesada, D. Synthesis and Characterization of Alkali-Activated Materials Containing Biomass Fly Ash and Metakaolin: Effect of the Soluble Salt Content of the Residue. Arch. Civ. Mech. Eng. 2022, 22, 121. [Google Scholar] [CrossRef]
- Pérez-Villarejo, L.; Bonet-Martínez, E.; Eliche-Quesada, D.; Sánchez-Soto, P.J.; Rincón-López, J.M.; Castro-Galiano, E. Biomass Fly Ash and Aluminium Industry Slags-Based Geopolymers. Mater. Lett. 2018, 229, 6–12. [Google Scholar] [CrossRef]
- Rossi, A.D.; Ribeiro, M.J.; Labrincha, J.A.; Novais, R.M.; Hotza, D.; Moreira, R.F.P.M. Effect of the Particle Size Range of Construction and Demolition Waste on the Fresh and Hardened-State Properties of Fly Ash-Based Geopolymer Mortars with Total Replacement of Sand. Process Saf. Environ. Prot. 2019, 129, 130–137. [Google Scholar] [CrossRef]
- Novais, R.M.; Buruberri, L.H.; Seabra, M.P.; Bajare, D.; Labrincha, J.A. Novel Porous Fly Ash-Containing Geopolymers for PH Buffering Applications. J. Clean. Prod. 2016, 124, 395–404. [Google Scholar] [CrossRef]
- Gong, W.; Lutze, W.; Pegg, J. Tailored Geopolymer Composite Binders for Cement and Concrete Applications. U.S. Patent No. 13/138,233, 2 February 2012. [Google Scholar]
- Król, M.; Rożek, P.; Chlebda, D.; Mozgawa, W. Influence of Alkali Metal Cations/Type of Activator on the Structure of Alkali-Activated Fly Ash—ATR-FTIR Studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 198, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, H.; Provis, J.L. Quantitative Study of the Reactivity of Fly Ash in Geopolymerization by FTIR. J. Sustain. Cem. -Based Mater. 2012, 1, 154–166. [Google Scholar] [CrossRef]
- Svoboda, P.; Skvara, F.; Dolezal, J.; Dvoracek, K.; Lucuk, K. Fly-Ash Concrete Compositon, Method of Preparation by Geo-Polymeric Reaction of Activated Fly-Ash and Its Use. Patent EP1801084A1, 27 June 2007. [Google Scholar]
- Saeli, M.; Tobaldi, D.M.; Seabra, M.P.; Labrincha, J.A. Mix Design and Mechanical Performance of Geopolymeric Binders and Mortars Using Biomass Fly Ash and Alkaline Effluent from Paper-Pulp Industry. J. Clean. Prod. 2019, 208, 1188–1197. [Google Scholar] [CrossRef]
- Songpiriyakij, S.; Pulngern, T.; Pungpremtrakul, P.; Jaturapitakkul, C. Anchorage of Steel Bars in Concrete by Geopolymer Paste. Mater. Des. 2011, 32, 3021–3028. [Google Scholar] [CrossRef]
- Terrones-Saeta, J.M.; Suárez-Macías, J.; Iglesias-Godino, F.J.; Corpas-Iglesias, F.A. Development of Geopolymers as Substitutes for Traditional Ceramics for Bricks with Chamotte and Biomass Bottom Ash. Materials 2021, 14, 199. [Google Scholar] [CrossRef]
- Das, S.K.; Mishra, J.; Mustakim, S.M.; Adesina, A.; Kaze, C.R.; Das, D. Sustainable Utilization of Ultrafine Rice Husk Ash in Alkali Activated Concrete: Characterization and Performance Evaluation. J. Sustain. Cem.-Based Mater. 2022, 11, 100–112. [Google Scholar] [CrossRef]
- Diamond, S. Particle Morphologies in Fly Ash. Cem. Concr. Res. 1986, 16, 569–579. [Google Scholar] [CrossRef]
- Memon, S.A.; Khan, S.; Wahid, I.; Shestakova, Y.; Ashraf, M. Evaluating the Effect of Calcination and Grinding of Corn Stalk Ash on Pozzolanic Potential for Sustainable Cement-Based Materials. Adv. Mater. Sci. Eng. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Characterisation of Fly Ashes. Potential Reactivity as Alkaline Cements☆. Fuel 2003, 82, 2259–2265. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.; Van Deventer, J.; Lukey, G. The Characterisation of Source Materials in Fly Ash-Based Geopolymers. Mater. Lett. 2003, 57, 1272–1280. [Google Scholar] [CrossRef]
- Sharko, A.; Louda, P.; Nguyen, V.V.; Buczkowska, K.E.; Stepanchikov, D.; Ercoli, R.; Kascak, P.; Le, V.S. Multicriteria Assessment for Calculating the Optimal Content of Calcium-Rich Fly Ash in Metakaolin-Based Geopolymers. Ceramics 2023, 6, 525–537. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Geopolymer Foam Concrete: An Emerging Material for Sustainable Construction. Constr. Build. Mater. 2014, 56, 113–127. [Google Scholar] [CrossRef]
- Billong, N.; Kinuthia, J.; Oti, J.; Melo, U.C. Performance of Sodium Silicate Free Geopolymers from Metakaolin (MK) and Rice Husk Ash (RHA): Effect on Tensile Strength and Microstructure. Constr. Build. Mater. 2018, 189, 307–313. [Google Scholar] [CrossRef]
- Liang, G.; Zhu, H.; Zhang, Z.; Wu, Q.; Du, J. Investigation of the Waterproof Property of Alkali-Activated Metakaolin Geopolymer Added with Rice Husk Ash. J. Clean. Prod. 2019, 230, 603–612. [Google Scholar] [CrossRef]
- Liu, H.; Jing, W.; Qin, L.; Duan, P.; Zhang, Z.; Guo, R.; Li, W. Thermal Stability of Geopolymer Modified by Different Silicon Source Materials Prepared from Solid Wastes. Constr. Build. Mater. 2022, 315, 125709. [Google Scholar] [CrossRef]
- Lahoti, M.; Tan, K.H.; Yang, E.-H. A Critical Review of Geopolymer Properties for Structural Fire-Resistance Applications. Constr. Build. Mater. 2019, 221, 514–526. [Google Scholar] [CrossRef]
- Das, S.K.; Mishra, J.; Singh, S.K.; Mustakim, S.M.; Patel, A.; Das, S.K.; Behera, U. Characterization and Utilization of Rice Husk Ash (RHA) in Fly Ash–Blast Furnace Slag Based Geopolymer Concrete for Sustainable Future. Mater. Today Proc. 2020, 33, 5162–5167. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, J.; Zhang, H.; Li, M.; Wu, Y.; Guo, L.; Wang, W.; Duan, P.; Zhang, W.; Zhang, Z. Thermal Stability and Microstructure of Metakaolin-Based Geopolymer Blended with Rice Husk Ash. Appl. Clay Sci. 2020, 196, 105769. [Google Scholar] [CrossRef]
- Shearer, C.R.; Provis, J.L.; Bernal, S.A.; Kurtis, K.E. Alkali-Activation Potential of Biomass-Coal Co-Fired Fly Ash. Cem. Concr. Compos. 2016, 73, 62–74. [Google Scholar] [CrossRef]
- Lee, N.; Kim, E.; Lee, H.-K. Mechanical Properties and Setting Characteristics of Geopolymer Mortar Using Styrene-Butadiene (SB) Latex. Constr. Build. Mater. 2016, 113, 264–272. [Google Scholar] [CrossRef]
- Sumesh, M.; Alengaram, U.J.; Jumaat, M.Z.; Mo, K.H.; Alnahhal, M.F. Incorporation of Nano-Materials in Cement Composite and Geopolymer Based Paste and Mortar–A Review. Constr. Build. Mater. 2017, 148, 62–84. [Google Scholar] [CrossRef]
- Asim, N.; Alghoul, M.; Mohammad, M.; Amin, M.H.; Akhtaruzzaman, M.; Amin, N.; Sopian, K. Emerging Sustainable Solutions for Depollution: Geopolymers. Constr. Build. Mater. 2019, 199, 540–548. [Google Scholar] [CrossRef]
- Sandanayake, M.; Law, D.; Sargent, P. A New Framework for Assessing the Environmental Impacts of Circular Economy Friendly Soil Waste-Based Geopolymer Cements. Build. Environ. 2022, 210, 108702. [Google Scholar] [CrossRef]
- Amran, Y.M.; Alyousef, R.; Alabduljabbar, H.; El-Zeadani, M. Clean Production and Properties of Geopolymer Concrete; A Review. J. Clean. Prod. 2020, 251, 119679. [Google Scholar] [CrossRef]
- Tayeh, B.A.; Zeyad, A.M.; Agwa, I.S.; Amin, M. Effect of Elevated Temperatures on Mechanical Properties of Lightweight Geopolymer Concrete. Case Stud. Constr. Mater. 2021, 15, e00673. [Google Scholar] [CrossRef]
- Diaz-Loya, E.I.; Allouche, E.N.; Vaidya, S. Mechanical Properties of Fly-Ash-Based Geopolymer Concrete. ACI Mater. J. 2011, 108, 300. [Google Scholar]
- Pan, Z.; Sanjayan, J.G.; Rangan, B.V. Fracture Properties of Geopolymer Paste and Concrete. Mag. Concr. Res. 2011, 63, 763–771. [Google Scholar] [CrossRef]
- Lyon, R.E. Fire Response of Geopolymer Structural Composites; Federal Aviation Administration Washington DC Office of Aviation: Washington, DC, USA, 1996.
- Shahari, S.; Fathullah, M.; Abdullah, M.M.A.B.; Shayfull, Z.; Mia, M.; Darmawan, V.E.B. Recent Developments in Fire Retardant Glass Fibre Reinforced Epoxy Composite and Geopolymer as a Potential Fire-Retardant Material: A Review. Constr. Build. Mater. 2021, 277, 122246. [Google Scholar] [CrossRef]
- Novais, R.M.; Gameiro, T.; Carvalheiras, J.; Seabra, M.P.; Tarelho, L.A.; Labrincha, J.A.; Capela, I. High PH Buffer Capacity Biomass Fly Ash-Based Geopolymer Spheres to Boost Methane Yield in Anaerobic Digestion. J. Clean. Prod. 2018, 178, 258–267. [Google Scholar] [CrossRef]
- Novais, R.M.; Ascensao, G.; Tobaldi, D.M.; Seabra, M.P.; Labrincha, J.A. Biomass Fly Ash Geopolymer Monoliths for Effective Methylene Blue Removal from Wastewaters. J. Clean. Prod. 2018, 171, 783–794. [Google Scholar] [CrossRef]
- Novais, R.; Ribeiro, A.; Seabra, P.; Tarelho, L.; Labrincha, J. Novel Biomass Fly Ash-Based Geopolymers for Environmental Applications. Int. J. Renew. Energy Sources 2016, 1, 20–25. [Google Scholar]
- Li, C.J.; Zhang, Y.J.; Chen, H.; He, P.Y.; Meng, Q. Development of Porous and Reusable Geopolymer Adsorbents for Dye Wastewater Treatment. J. Clean. Prod. 2022, 348, 131278. [Google Scholar] [CrossRef]
- Habert, G.; De Lacaillerie, J.D.; Roussel, N. An Environmental Evaluation of Geopolymer Based Concrete Production: Reviewing Current Research Trends. J. Clean. Prod. 2011, 19, 1229–1238. [Google Scholar] [CrossRef]
- Nawaz, M.; Heitor, A.; Sivakumar, M. Geopolymers in Construction-Recent Developments. Constr. Build. Mater. 2020, 260, 120472. [Google Scholar] [CrossRef]
- Frydrych, M.; Hýsek, Š.; Fridrichová, L.; Le Van, S.; Herclík, M.; Pechočiaková, M.; Le Chi, H.; Louda, P. Impact of Flax and Basalt Fibre Reinforcement on Selected Properties of Geopolymer Composites. Sustainability 2019, 12, 118. [Google Scholar] [CrossRef]
- Ouellet-Plamondon, C.; Habert, G. 25—Life Cycle Assessment (LCA) of Alkali-Activated Cements and Concretes. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Pacheco-Torgal, F., Labrincha, J.A., Leonelli, C., Palomo, A., Chindaprasirt, P., Eds.; Woodhead Publishing: Oxford, UK, 2015; pp. 663–686. ISBN 978-1-78242-276-1. [Google Scholar]
- Turner, L.K.; Collins, F.G. Carbon Dioxide Equivalent (CO2-e) Emissions: A Comparison between Geopolymer and OPC Cement Concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Teh, S.H.; Wiedmann, T.; Castel, A.; Burgh, J. de Hybrid Life Cycle Assessment of Greenhouse Gas Emissions from Cement, Concrete and Geopolymer Concrete in Australia. J. Clean. Prod. 2017, 152, 312–320. [Google Scholar] [CrossRef]
- Heidrich, C.; Hinczak, I.; Ryan, B. SCM’s Potential to Lower Australia’s Greenhouse Gas Emissions Profile. In Proceedings of the Australasian Slag Association Conference 2005, Sydney, Australia, 19–21 September 2015. [Google Scholar]
- Wilson, J.L.; Tagaza, E. Green Buildings in Australia: Drivers and Barriers. Aust. J. Struct. Eng. 2006, 7, 57–63. [Google Scholar] [CrossRef]
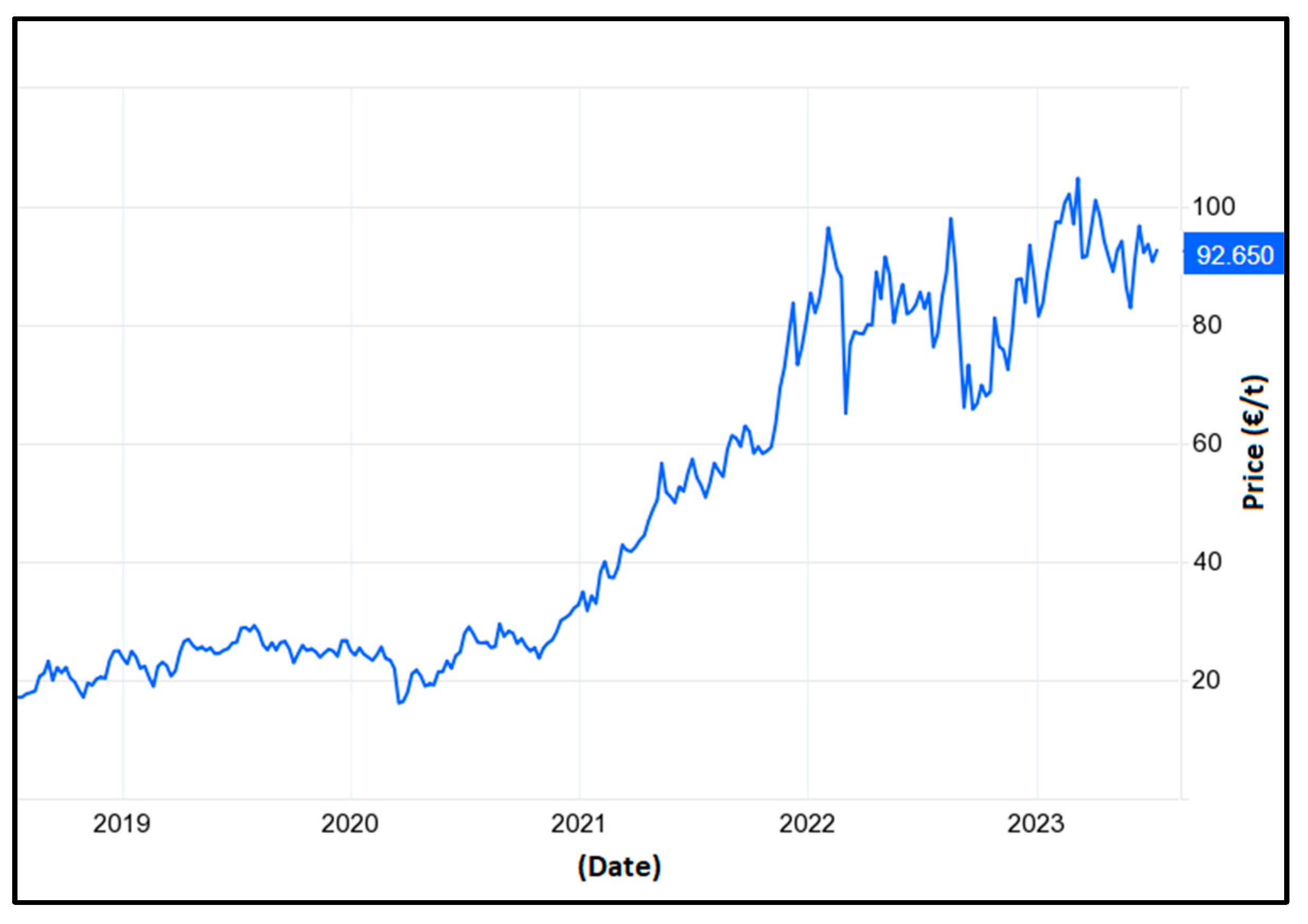
- EU Carbon Permits—2022 Data—2005–2021 Historical—2023 Forecast—Price—Quote. Available online: https://tradingeconomics.com/commodity/carbon (accessed on 23 December 2022).
- Thaarrini, J.; Dhivya, S. Comparative Study on the Production Cost of Geopolymer and Conventional Concretes. Int. J. Civ. Eng. Res. 2016, 7, 117–124. [Google Scholar]
- Verma, M.; Upreti, K.; Vats, P.; Singh, S.; Singh, P.; Dev, N.; Kumar Mishra, D.; Tiwari, B. Experimental Analysis of Geopolymer Concrete: A Sustainable and Economic Concrete Using the Cost Estimation Model. Adv. Mater. Sci. Eng. 2022, 2022, 7488254. [Google Scholar] [CrossRef]
- Vilamová, Š.; Piecha, M. Economic Evaluation of Using of Geopolymer from Coal Fly Ash in the Industry. Acta Montan. Slovaca 2016, 21, 139–145. [Google Scholar]
- Rintala, A.; Havukainen, J.; Abdulkareem, M. Estimating the Cost-Competitiveness of Recycling-Based Geopolymer Concretes. Recycling 2021, 6, 46. [Google Scholar] [CrossRef]
- Munir, Q.; Kärki, T. Cost Analysis of Various Factors for Geopolymer 3D Printing of Construction Products in Factories and on Construction Sites. Recycling 2021, 6, 60. [Google Scholar] [CrossRef]

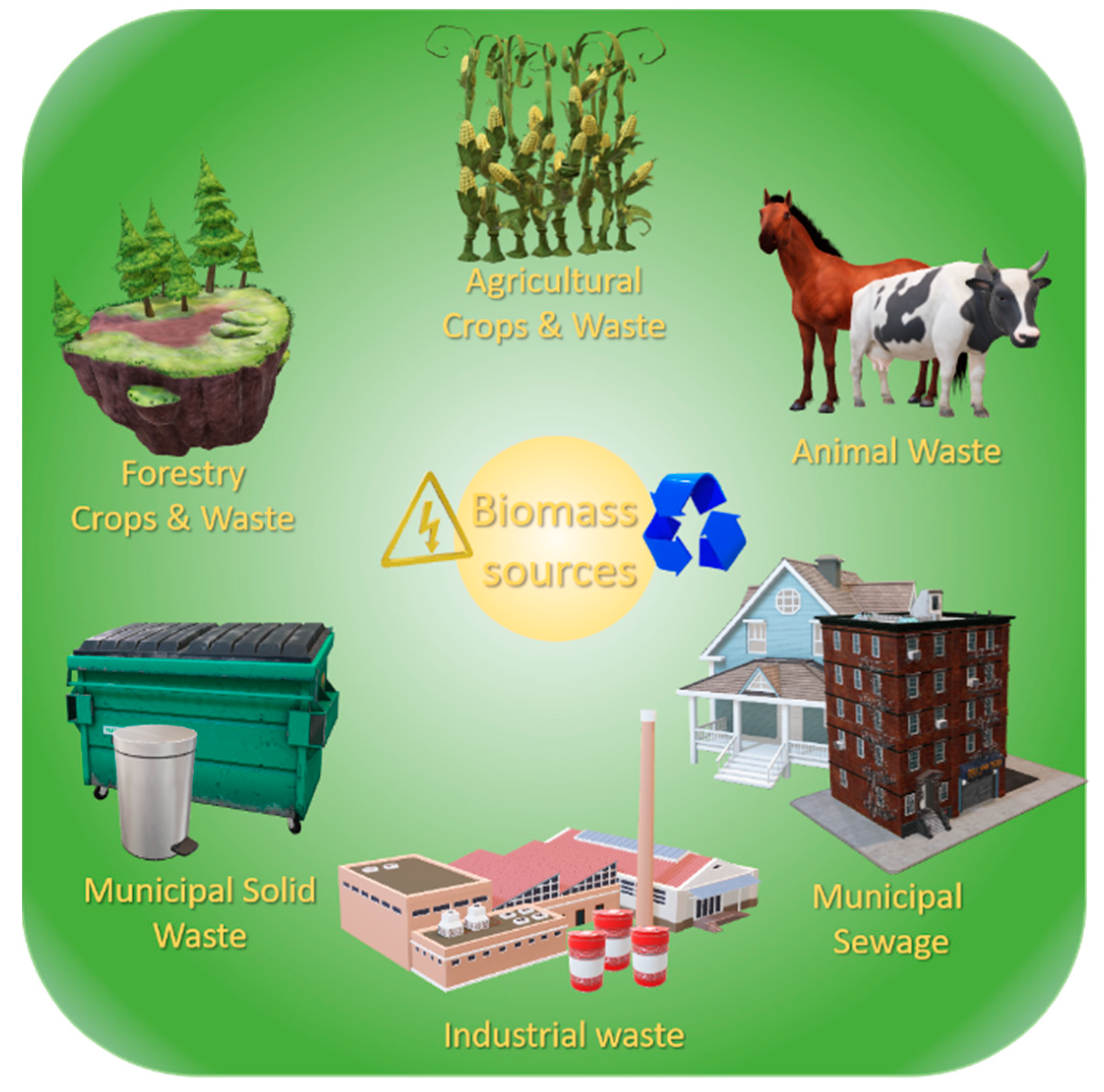
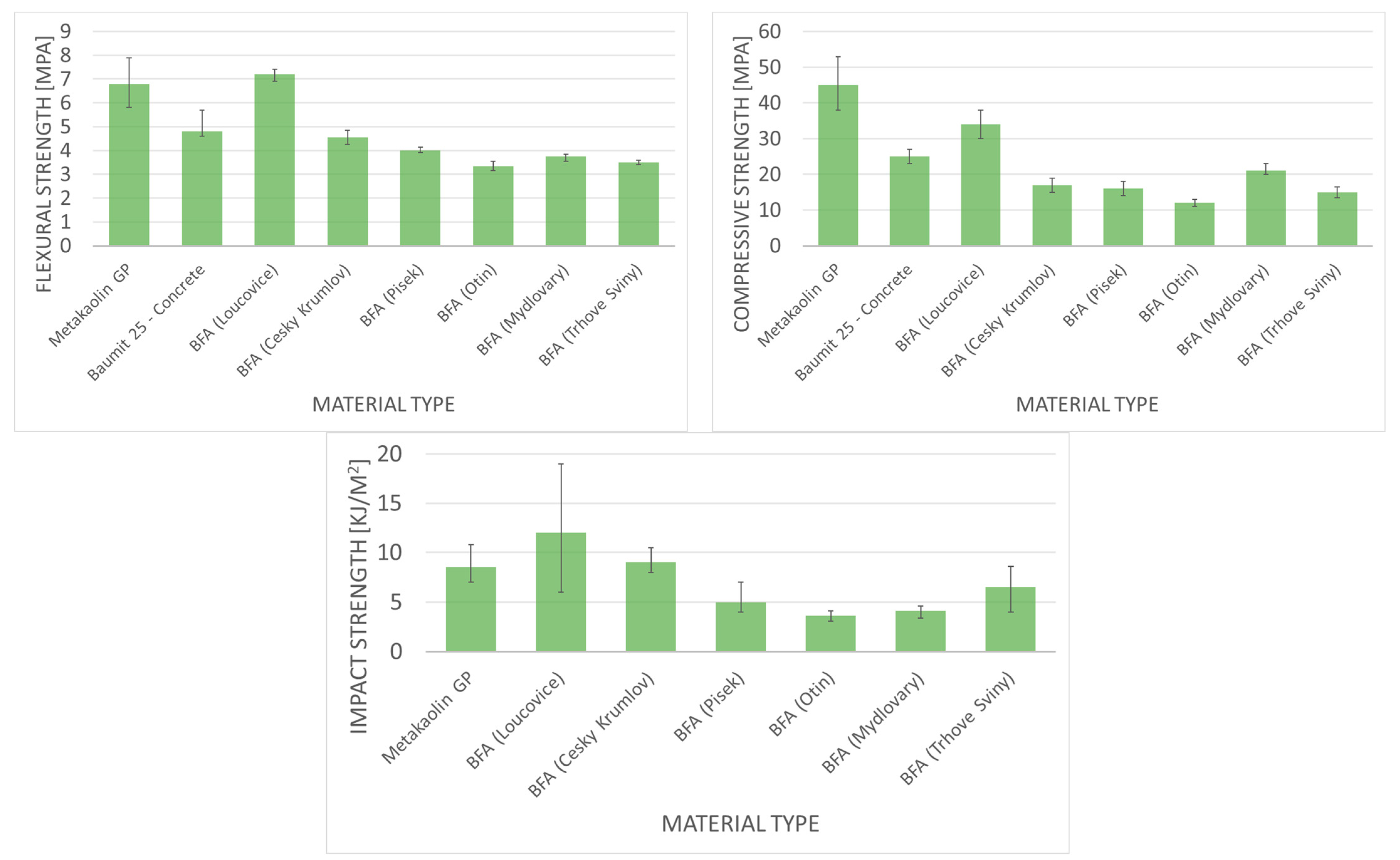
| Fast-Growing Woody Plants | Herbaceous Plants | Aquatic | Animal and Human | Contaminated Biomass |
|---|---|---|---|---|
| poplar | hemp | macroalgae | meat | fiberboard |
| willow | amaranth | seaweed | bone | chipboard |
| alder | sorrel | kelp | meal | waste paper |
| acacia | sedges | lake weed | poultry litter | plywood |
| hazel trees | fescues | |||
| stems | alfalfa | |||
| branches | arundo | |||
| foliage | bamboo | |||
| bark | beans | |||
| sawdust | flax | |||
| pellets | corn | |||
| lumps | rice |
| Element | Fly Ash from Brown Coal | Fly Ash from Biomass | Unit |
|---|---|---|---|
| Sb | 3.56 | 4.52 | mg/kg |
| As | 10.7 | 0.14 | mg/kg |
| Pb | 11.7 | 3.66 | mg/kg |
| Cd | 0.101 | 0.14 | mg/kg |
| Cr | 157 | 26.9 | mg/kg |
| Co | 59 | 2.5 | mg/kg |
| Cu | 131 | 26.1 | mg/kg |
| Mn | 650 | 48.5 | mg/kg |
| Ni | 145 | 7.82 | mg/kg |
| Hg | 0.006 | 0.0003 | mg/kg |
| Tl | 2 | 0.85 | mg/kg |
| V | 165 | 47.6 | mg/kg |
| F | 0.0493 | 0.0295 | % |
| Cl | 0.0044 | 0.0148 | % |
| Advantages | Disadvantages |
|---|---|
| The use of spherical ash particles improves workability. | The use of fly ash is not advisable in low-temperature concrete pours. |
| Fly ash improves the density of cementitious binder, and the tightness of hardened concrete surface layers inhibits carbonation of the hardened concrete surface. | An excessive amount of fly ash has a significant impact on the water content, rheological properties, and durability of concrete. It often leads to bleeding and a potential decrease in the hardened concrete’s durability, as well as increased permeability when exposed to pressurized water. |
| Concrete costs less because fly ash is less expensive than cement. | The high chloride content of fly ash can have a negative impact on building structures, such as the danger of corrosion of embedded reinforcing steel. |
| CO2 emissions have been reduced. | The strength and durability of cement concrete can be affected by the quality of fly ash. |
| Fly ash concrete shrinks far less than conventional concrete. | |
| Fly ash concrete is resistant to acid and sulfate attacks. |
| Sources of Fly Ash | Geopolymer Preparation Method | Precursor | Application/Goal of Geopolymer | References |
|---|---|---|---|---|
| Paper waste | A mixture consisting of 15 g of aluminosilicate precursors, comprising 50 wt.% metakaolin and 50 wt.% FA, was subjected to mechanical mixing with 24.38 g of alkaline solution, 4.15 g of water, and 0.75 g of pore-forming agent in order to generate the geopolymer slurry. | Metakaolin | Wastewater treatment | [89] |
| Paper waste | The SiO2/Al2O3 ratio was 3.1, the Na2O/Al2O3 ratio was 2.0, and the Na2O/SiO2 ratio was 0.6. To investigate the influence of the pore-former on porous geopolymer materials, different quantities of H2O2 were utilized. Sodium silicate was replaced in these compositions by 0.03, 0.15, 0.30, 0.90, and 1.2 wt.% H2O2. | Metakaolin | Board and wall panels | [119] |
| Co-generation plant (BA) | Here, 75 wt.% BA and 25 wt.% MK were employed in the formulation. The solids were combined for 1 min at 60 rpm in a Kenwood planetary mixer before adding the alkaline activators for 10 min at the same agitation. Stirring was maintained for another 5 min at 95 rpm with the addition of H2O2 as needed. | Metakaolin (MK) | Filtration and separation | [120] |
| Kraft pulp mill (BFA) | The manufacturing process of GP mortars involves several steps. First, MK and BFA were hand mixed for a duration of 1 min to achieve a consistent blend. Second, sodium hydroxide and silicate were homogenized at a speed of 60 rpm for 5 min. Next, the alkaline solution was mixed with the solid precursors (BFA + MK) in a Hobart-type mixer at a speed of 60 rpm for 9 min. Finally, lime slaker grits were added to the mixture and mixed for an additional 1 min at the same speed to ensure uniformity. | Metakaolin(MK) | Construction and masonry | [73] |
| Wood biomass (BA) | The alkaline activators were added while still being stirred for 10 min after the solids (BA and MK) had been combined for 1 min at 60 rpm in a Kenwood planetary mixer. The mixture was stirred for 5 more min at 95 rpm. | Metakaolin(MK) | Reducing cost of geopolymer | [121] |
| Mixed waste from Hauts-de-France (BFA) | NaOH (20 wt.% of the activation solution) and Na2SiO3 (80 wt.%) are the chemicals used to initiate the geopolymerization process. Na2SiO3 was added with the goal of raising the concentration of soluble silicates and the pace of the reaction. A magnetic agitator was used to combine the 2 reagents in a glass container for 6 h before resting the solution in a plastic bottle for 24 h. The alkaline solution was then combined for about 3 min in a mixer with metakaolin and SRS or BFA at a rotating speed of 300 rpm. | Metakaolin (MK) and shooting range soil (SRS) | Immobilization of heavy metal | [122] |
| Wood biomass (BWA) | Three replacement ratios of FA by BWA were used in the blended biomass wood fly ash–fly ash geopolymer mortars: 10%, 20%, and 30% of the total binder. The activator (Na2SiO3 NaOH)/binder and fine aggregate/binder mass ratios for the geopolymer mortars were fixed at 0.5 and 2.0, respectively. | Fly ash | Economic and environmental benefits | [123] |
| Mix of pine pruning, forest residues | The solid precursors were combined with the activating solution. The concentration of the sodium hydroxide solution was 8 M, and the ratio of sodium silicate to sodium hydroxide was 1.15, which represents the modulus of the activator. The activator was introduced into the precursors that had been previously combined for a duration of 2 min. Subsequently, the mixture was subjected to agitation for an approximate duration of 5 min using a Proeti planetary mixer. | Metakaolin | Building materials, bricks | [124] |
| Olive and forest pruning (FBA) | The geopolymers were prepared using five different compositions. These compositions included pure MK, as well as four other compositions referred to as GP1, GP2, GP3, and GP4. GP1 consisted of 50% MK, 25% AIS, and 25% FBA. GP2 consisted of 50% MK, 33% AIS, and 17% FBA. GP3 consisted of 40% MK, 35% AIS, and 25% FBA. GP4 consisted of 40% MK, 25% AIS, and 35% FBA. | Metakaolinaluminum industry slags (AIS) | Partial substitutes for metakaolin and Portland cement | [125] |
| Burned eucalyptus biomass | The geopolymer mortars were prepared according to a mix design that followed a binder-to-aggregate weight ratio of 1:3. The mixer was supplemented with alkaline activators according to the following procedure: (i) the sodium silicate and NaOH solution were initially homogenized at a rotational speed of 60 rpm for a duration of 5 min; (ii) the alkaline solution was then mixed with the solid materials at the same rotational speed for a period of 10 min; and (iii) the mixture underwent further homogenization and mixing at a rotational speed of 95 rpm for an additional 5 min. | Metakaolin andconstruction and demolition waste | Applications in building, replacing conventional mortars | [126] |
| Wood biomass | Geopolymers were synthesized by combining a mixture consisting of 2/3 wt.% metakaolin (MK) and 1/3 wt.% biomass FA, which served as an aluminosilicate source. In the present study, various compositions were examined by replacing sodium silicate with different weight percentages (0.03, 0.15, 0.30, 0.60, 0.90, and 1.2 wt.%) of hydrogen peroxide (H2O2). The blending of the mixtures was conducted using a mechanical procedure consisting of the following steps: (i) the sodium silicate and NaOH solution were homogenized at a rotational speed of 60 revolutions per min (rpm) for a duration of 5 min; (ii) the alkaline solution was then mixed with biomass FA and MK at the same rotational speed for a period of 10 min; and (iii) H2O2 was added to the blend in an amount determined by the formulation, followed by an additional mixing period of 2 min at a rotational speed of 95 rpm. | Metakaolin | pH regulators for biogas reactors or wastewater treatment | [127] |
| Thermal Power Plants | Elements (wt.%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | C | Ca | Si | K | Al | S | Mg | Cl | Na | Fe | P | Mn | Zn | Ti | |
| BFA (Loucovice) | 40.4 | 32.9 | 9.4 | 6.6 | 3.6 | 1.9 | 1.4 | 1.0 | 0.8 | - | 0.6 | - | - | - | - |
| BFA (Cesky Krumlov) | 32.3 | 50.0 | 9.8 | 2.4 | 1.9 | 0.9 | 0.9 | 0.5 | 0.5 | 0.4 | 0.4 | 0.2 | - | - | - |
| BFA (Pisek) | 32.7 | 50.7 | 3.3 | 3.7 | 3.6 | 1.1 | 1.2 | 0.9 | 1.0 | 0.7 | 0.4 | 0.3 | 0.3 | 0.1 | 0.1 |
| BFA (Otin) | 39.5 | 32.5 | 10.7 | 5.7 | 2.9 | 1.5 | 1.0 | 2.1 | 0.5 | 0.4 | 0.9 | 1.1 | 1.0 | 0.2 | - |
| BFA (Mydlovary) | 60.3 | - | 9.4 | 9.9 | 8.9 | 1.2 | 2.8 | 1.5 | 2.3 | 1.2 | 0.9 | 0.5 | 0.5 | 0.6 | - |
| BFA (Trhove Sviny) | 33.1 | 43.2 | 5.4 | 2.1 | 7.2 | 0.9 | 3.2 | 1.3 | 1.4 | 0.8 | 0.6 | 0.4 | 0.4 | - | - |
| Material Name Reinforced Concrete | Weight (kg) | Weight Ratio | Density kg/m3 | Emission Factor kg CO2 eq/kg |
|---|---|---|---|---|
| Mixture of sands | 303.47 | 30.3% | 1650 | 0.147 |
| Cement (75 MPa) | 298.47 | 29.8% | 3050 | 1.250 |
| Water | 276.51 | 27.7% | 1000 | 0.00059 |
| Binder mixture | 8.74 | 0.9% | 2685 | 3.210 |
| Steel rods | 112.80 | 11.3% | 7850 | 2.890 |
| Density of concrete | 2596.6 | |||
| Total mixture (kg) | 1000.0 | |||
| Total CO2 (for 1 ton) | 771.932 | |||
| kg CO2 eq/kg | 0.772 |
| Material Name Geopolymer | Weight (kg) | Weight Ratio | Density (kg/m3) | Emission Factor (kg CO2 eq/kg) |
|---|---|---|---|---|
| Metakaolin | 292.903 | 29.3% | 1850 | 0.245 |
| Reagent for alkalinization | 287.279 | 28.7% | 1050 | 0.424 |
| SiO2 | 31.406 | 3.1% | 319 | 2.890 |
| Carbon fiber | 8.010 | 0.8% | 350 | 0.051 |
| Sands | 94.340 | 9.4% | 1650 | 0.147 |
| Biomass fly ash | 283.019 | 28.3% | 425 | 0.0 |
| Aluminum | 3.043 | 0.3% | 2700 | 12.790 |
| Density of geopolymer | 1140.5 | |||
| Total mixture (kg) | 1000.0 | |||
| Total CO2 (for 1 ton) | 337.530 | |||
| kg CO2 eq/kg | 0.338 |
| Material Name Reinforced Concrete | Weight (kg) | Weight Ratio | Price €/kg |
|---|---|---|---|
| Mixture of sands | 303.47 | 30.3% | 0.01 |
| Cement (75 Pa) | 298.47 | 29.8% | 0.88 |
| Water | 276.51 | 27.7% | 1.97 |
| Binder mixture | 8.74 | 0.9% | 1.36 |
| Steel rods | 112.80 | 11.3% | 2.25 |
| Total mixture (kg) | 1000.0 | ||
| €/t | 1076.1 |
| Material Name Geopolymer | Weight (kg) | Weight Ratio | Price €/kg |
|---|---|---|---|
| Metakaolin | 292.903 | 29.3% | 0.40 |
| Reagent for alkalinization | 287.279 | 28.7% | 1.95 |
| SiO2 | 31.406 | 3.1% | 2.25 |
| Carbon fiber | 8.010 | 0.8% | 8.1 |
| Sands | 94.340 | 9.4% | 0.01 |
| Ash | 283.019 | 28.3% | (−)0.02 |
| Aluminum | 3.043 | 0.3% | 1.40 |
| Total mixture (kg) | 1000.0 | ||
| €/t | 812.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalcinkaya, B.; Spirek, T.; Bousa, M.; Louda, P.; Růžek, V.; Rapiejko, C.; Buczkowska, K.E. Unlocking the Potential of Biomass Fly Ash: Exploring Its Application in Geopolymeric Materials and a Comparative Case Study of BFA-Based Geopolymeric Concrete against Conventional Concrete. Ceramics 2023, 6, 1682-1704. https://doi.org/10.3390/ceramics6030104
Yalcinkaya B, Spirek T, Bousa M, Louda P, Růžek V, Rapiejko C, Buczkowska KE. Unlocking the Potential of Biomass Fly Ash: Exploring Its Application in Geopolymeric Materials and a Comparative Case Study of BFA-Based Geopolymeric Concrete against Conventional Concrete. Ceramics. 2023; 6(3):1682-1704. https://doi.org/10.3390/ceramics6030104
Chicago/Turabian StyleYalcinkaya, Baturalp, Tomas Spirek, Milan Bousa, Petr Louda, Vojtěch Růžek, Cezary Rapiejko, and Katarzyna Ewa Buczkowska. 2023. "Unlocking the Potential of Biomass Fly Ash: Exploring Its Application in Geopolymeric Materials and a Comparative Case Study of BFA-Based Geopolymeric Concrete against Conventional Concrete" Ceramics 6, no. 3: 1682-1704. https://doi.org/10.3390/ceramics6030104
APA StyleYalcinkaya, B., Spirek, T., Bousa, M., Louda, P., Růžek, V., Rapiejko, C., & Buczkowska, K. E. (2023). Unlocking the Potential of Biomass Fly Ash: Exploring Its Application in Geopolymeric Materials and a Comparative Case Study of BFA-Based Geopolymeric Concrete against Conventional Concrete. Ceramics, 6(3), 1682-1704. https://doi.org/10.3390/ceramics6030104