Abstract
Glass is a familiar material that requires abundant mineral sources, with devastating consequences for the environment. Rice husk ash (RHA) presents a very high silica content (>95%) and it can be a very promising alternative source for silica in silica-based glass. However, impurities like manganese and iron, which depend on the rice harvest, might limit RHA use, particularly in the production of optical transparent glasses. In this work, we discussed how Mn and Fe can affect the coloring of the produced glass, and how the effect of these impurities can be removed. First, the RHA was treated with acid solutions, leading to the production of a soda-lime glass with similar transparency to commercial glass (>70%). Secondly, another simpler approach was studied: a small amount of antimony oxide was added in the composition of the glass, obtaining a transparent glass (>80%, same thickness) with RHA.
1. Introduction
In the last few decades, researchers have created a growing consciousness about science having to seek alternatives and sustainable materials for those products that are largely used in our society. Among others, agricultural waste and food byproducts have gained increasing attention [1,2,3,4,5,6,7]. Avocado cores, sugar cane bagasse and corn cobs have been involved in biofuel production, while rice husk ash (RHA) is currently being used in high performance concrete or as a rubber filler for tires, among other applications [1,2,3,4,5,6,7].
Another extremely common and demanded material in our daily lives is glass. Its major component, silica, is mainly obtained by the mineral quartz (sand). In Brazil only, the glass industry requires 1.7 million tons of silica every year [8]. The mining of this material can lead to several environmental problems, like erosion, river deviation and native vegetation removal [9]. Therefore, it would be of extreme interest to have an alternative source for silica; this is found in rice husk ashes. As already reported in the literature, RHA may contain more than 80% of silica, depending on the purification steps adopted [6,9,10]. Considering that Brazil harvests 13 million tons/year of rice [9,11], 4% of which can be RHA, it is possible to obtain almost 500 million tons of RHA every year. This means that a third of the country’s glass production could be sustained by this biomass reutilization, which would otherwise be discarded in nature, causing soil desertification and air pollution [12].
Most of the commercial glasses are transparent, which means that silica and the other components used for their production have to be extremely pure. Being a natural product, RHA retains some oxides and minerals that were useful to the original plant physiology and that also depend on soil composition, agronomic handling and climate [13]. Generally, this implies the presence of aluminum, sodium, calcium, phosphate and iron oxides in traces [6,7,9]. The concentrations vary from region to region, and other elements may also appear to differentiate a particular harvest. The Rio Grande do Sul state, the author’s location, is the most rice-productive region in Brazil [11], and it has been reported that the RHA from this region typically also contains small percentages of manganese oxide (II) [14]. The presence of transition metal oxides and how they react and oxidize in the melt (ie., Mn2+/Mn3+ and Fe2+/Fe3+ ratio) can affect the transparency of the glass, giving colored hues to the final material [15,16]. Thus, the crucial step for the effective industrial application of RHA as a source of silica for transparent glass production is its purification.
As reported by Hossain et al., silica purification can be obtained either by controlling the combustion conditions in a fluidized bed (fluidizing velocity, gas flux, temperature and time) or by adding a chemical pretreatment step to the rice husk (RH) before calcination to RHA [9]. This second option is more accessible to a wider range of industries, and various treatments have already been applied with success, resulting in >95% of pure silica [9]. In the literature, we can find simple lixiviation methods, involving only one purification step with either a strong base or acid solutions [10,17,18], together with more complicated methods composed of several steps and optimized by the Taguchi method [19]. Due to the possible toxicity of acid treatments and the subsequent waste management related to it, alternative methods may be more efficient and more ecofriendly in achieving the same purpose. Particularly, the glass melt is an extremely complex system, where many oxidation-reduction equilibria take place [20,21]. It can, to an extent, be possible to control and tailor these reactions by simply adding other reagents. Antimony oxides have been used for many years in the glass industry as a fining agent, and, as already demonstrated in the literature, antimony (Sb) can react with iron and particularly manganese to change their oxidation state (and thus their coloration) [22,23,24]. For this reason, the addition of antimony oxide (III) to the melt was also explored as a method to eliminate the effect of manganese on the final glass production.
Therefore, in this work, we explored and compared the efficiency of a simple acidic lixiviation pretreatment with hydrochloric or sulfuric acid with the addition of antimony oxide (III) in the production of discolored glasses from RHA with a considerable content of manganese. A thorough discussion on the influence of this transition metal on the final glass color is also presented.
2. Materials and Methods
The rice husk used in this work was donated by CERGRAL LTDA, from Itaqui, a city in the western part of the Rio Grande do Sul state, Brazil. Hydrochloric acid (HCl) 37% PA/ACS grade was purchased from Neon (Brazil), and sulfuric acid (H2SO4) 1N from Synth (Brazil). Sodium carbonate (Na2CO3), calcium carbonate (CaCO3) and sand (99,5% SiO2 and 600 ppm iron) were donated by the glass industry Verallia–Brasil, from Campo Bom, Rio Grande do Sul, Brazil.
2.1. Rice Husk Treatment
The rice husk, cleaned and dried, was treated with hydrochloric acid (HCl) or sulfuric acid (H2SO4), followed by a calcination step in order to produce high purity silica. Particularly, two different concentrations, 4 and 10 w/w%, were used for the different acid baths, in a proportion of 1:9 between the rice husk and the acid solutions. The mixtures were heated on a hot plate at 50 ± 5 °C for 1 h, under stirring. Afterwards, the rice husk was filtered and washed with distilled water until neutral pH. The treated husk was then dried at 100 °C for 24 h in order to remove moisture. High purity silica was obtained by calcination of the treated rice husk at 800 °C for 5 h in a muffle. The ashes were kept inside the muffle until being cooled at room temperature. For comparison, a sample of RH was calcined at the same temperature without any previous acid treatment, and it was considered as a control (A1). Moreover, pure commercial sand was also used to produce a glass sample for comparison, designed as a standard (STD). A summary of sample names and treatments can be found in Table 1.

Table 1.
Sample treatments and designations.
2.2. Glass Production
Simple soda-lime glass was produced by mixing 50 mol% of SiO2 exclusively from RHA, 35 mol% of sodium oxide, Na2O, generated by decomposition of sodium carbonate (Na2CO3) and 15 mol% of calcium oxide, CaO, derived from calcium carbonate (CaCO3). The relatively lower amount of silica with respect to the normally used amount in the industry for this type of glass (70–75 mol%) was chosen to decrease the melting point of the melt, based on the equipment available. The well-homogenized mixture was placed into a platinum-gold crucible and heated up to 1400 °C and kept at this temperature for 2 h. The heating rate was 10 °C/min, and the overall process lasted 5 h. The melt was poured into a preheated mold at 400 °C to reduce the superficial stress of the formed glass. Moreover, the glasses were annealed at 350 °C for 6 h to reduce tensions. The final dimensions of the produced glass were 15 × 15 × 10 mm. In order to be able to compare it with a standard soda-lime glass found on the market, a sample with commercial sand (STD) as the silica source was also produced, maintaining the same batch proportions.
2.3. Characterization
The composition of RHA was obtained by X-Ray Fluorescence, XRF (BRUKER, model Turbo SD, silver anode, SSD detector). The composition was measured three times for the same sample. For the thermogravimetry analyses (TGA), the equipment from TA INSTRUMENT, model SDT 600, was used. Approximately 8 mg of RHA were weighted, and the analyses were carried out under an N2 atmosphere, with a gas flow of 100 mL·min−1 and heating rate of 10 °C·min−1. The explored temperature interval was from 30 to 1000 °C. For the differential scanning calorimetry (DSC) analysis, 8 mg of grounded sample were tested in a Shimadzu DSC-60 equipment, in nitrogen atmosphere, from 180 °C to 600 °C with a heating rate of 10 °C/min.
To evaluate the optical transparency, the produced glass was analyzed by UV-Vis (BEL PHOTONICS, model UV-M51) in the visible range of 400–680 nm. Each sample was cut to roughly a size of 2 cm × 3 cm × 3 mm, sanded and polished before being analyzed optically. The transmittance measurements were also carried out three times for each glass sample, and the average spectra were plotted.
3. Results
As a first step, it was important to better understand the thermal stability of the used RH and to decide which calcination temperature range should be the optimal one in order to obtain a high percentage of amorphous silica for glass production. Based on thermogravimetric and derivative thermogravimetric analyses (TGA-DTG) of our pure and treated RH (described in Appendix A), it was confirmed that the best trade-off between removing all the organic matter and obtaining high purity amorphous silica was a temperature between 600 and 800 °C.
Table 2 collects the data obtained by X-ray Fluorescence, after calcination, for the RHA composition in terms of percentage by weight (wt%), with its standard deviation. First of all, it can be seen that the degree of purity of the obtained silica is extremely high, meaning that one can obtain a market-ready silica from RH with a simple procedure, as reported in other works [17,18,19]. Secondly, it is possible to see how the acid treatment effectively removes manganese and iron from the ashes, improving the silica purity. Particularly, manganese oxide (II)-(MnO) is almost completely removed, where only a 0.01 wt% persists when RH is treated with HCl and the 10 w/w% concentration of sulfuric acid. In the case of iron oxide (III)-(Fe2O3), the amount found in the sample without treatment is halved after acid lixiviation; however, a ~0.20 wt% is still detectable. Moreover, it is possible to perceive that the acidic treatment completely removes (if any) the alkali and alkali earth metal oxides, while it does not affect the amount of alumina present. Although this small proportion of Al2O3 is not sufficient to alter the composition of the melt (i.e., to aluminosilicate glass), it is worth mentioning that it might influence its overall basicity.

Table 2.
Composition of RHA samples used in glass fabrication by X-ray Fluorescence in wt%.
The effects of the acidic lixiviation on the RHA composition and consequently the final glass coloration can be seen in Figure 1; Figure 2. Figure 1 collects all the produced glass samples. Clearly, it is possible to find a strict correlation between the amount of iron and manganese in each sample and its coloration. Particularly, the sample A3 (treatment 10 w/w% HCl) displays the best transparency among all the fabricated samples with RHA; the slightly yellow hue can be assigned to the presence of residual manganese and iron, as reported in Table 2, which can also be confirmed by the typical absorption band of Fe3+ in the UV-edge range [15]. It is important to mention here that all the samples have a thickness of 2.5 mm, which is considerable and close to the thickness of commercial window glass (3–6 mm).

Figure 1.
Display of the fabricated soda-lime glass samples with RHA.
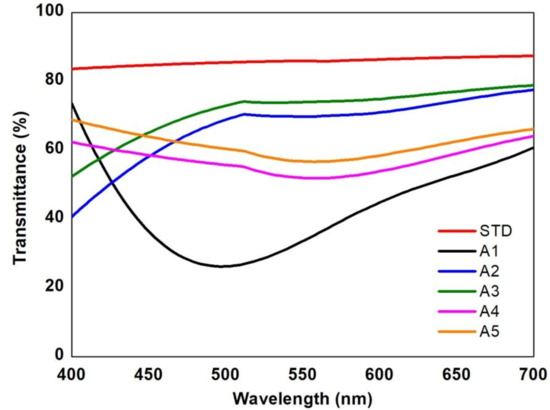
Figure 2.
Visible transmittance spectra of the produced glass samples.
Moreover, the UV-vis spectra shown in Figure 2 demonstrate in more details the high absorbance of the untreated sample (A1, black line) around 500 nm due to the Mn3+ ions, and the improved percentage of light transmittance in the treated samples. After the treatment with sulfuric acid, samples A4 and A5 (pink and orange lines) presented the most residual amount of Mn3+, which can be detected by the persistent broad peak at 500 nm [25]. Overall, these samples presented a light transmittance of almost 60%. Samples A2 (blue line) and A3 (green line), with less residual manganese content, display the highest transmittance over all the visible spectrum. Particularly, sample A3 demonstrates an overall transmission of 70% or more, which is very close to the STD sample, which is at 83% on average (red line).
Additionally, the thermal stability of the produced glasses was evaluated by DSC (for details, please see Appendix A), and the glass transition temperature was found to be around 478–510 °C for all samples.
As an alternative method for fabricating optically transparent glass with RHA, the work of Kaewkhao et al. came to our attention, where a transparent boron-soda-lime silica glass was produced with RHA presenting traces of MnO [25]. Particularly, the composition of the glass was constituted by 55SiO2(from RHA):13B2O3:1Al2O3:6.3CaO:4.5BaO:20Na2O:0.2Sb2O3 (antimony oxide) in mol%. The addition of this last element was not discussed in the paper, but we believe it plays a crucial role in the final glass transparency, as already related in the literature [22].
In order to prove this point, we produced a silicate glass with pure RHA (without any pretreatment) and Sb2O3 added in excess. As can be seen in Figure 3, the addition of 0.5 mol% of Sb2O3, ((SiO2–x) composition) was sufficient to reduce Mn3+ to Mn2+ and thus to obtain colorless glass. These melting conditions were repeated three times, and the same results were found.
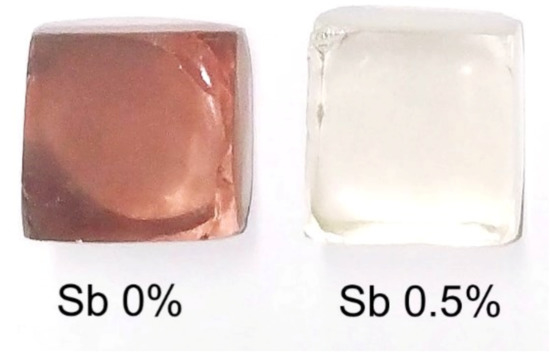
Figure 3.
Display of the fabricated soda-lime glass samples with RHA and an antimony addition.
In Figure 4, it is possible to see once more the high absorbance around 500 nm for the untreated RHA glass sample (black line). On the other hand, with the addition of 0.5 mol% Sb2O3, a transmission of more than 80% (orange line) was obtained.
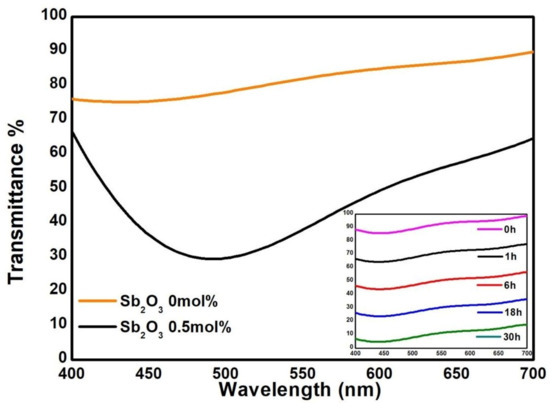
Figure 4.
Visible transmittance spectra of the glass samples produced with the addition of Sb2O3. Inset: transmittance spectra of the 0.5% Sb2O3 glass sample after the solarization test, measured at 0 h, 1 h, 6 h, 18 h and 30 h of UV-A lamp exposure. Spectral lines shifted for clarity.
Moreover, the glass produced with 0.5 mol% of Sb2O3 was tested against solarization (reversion of the equilibrium Mn-Sb) and, as a consequence, the reappearance of the Mn3+ absorbance band. The sample was exposed to UV-A radiation (50 W) for 30 h, and the UV-Vis spectra were taken after 1, 6, 18 and 30 h. As shown in the inset in Figure 4, there is no variation in the optical transparency of the glass after the exposure (the spectral lines were shifted for better clarity). We can state that, at least for the time measured, solarization did not occur.
As a last consideration, it was perceived that the 0.5% Sb2O3 glass sample presented a bright orange fluorescence when exposed to UV light during the solarization analysis, as represented in Figure A3 (Appendix A). The same effect was not visible for the glass samples prepared without antimony oxide or with sand.
4. Discussion
In the literature, it is possible to find other studies in which glasses were fabricated using RHA as a silica precursor, sometimes with a very similar pretreatment for the RHA [26,27]. Most of these glasses were fabricated with specific technological purposes, like photoluminescent glass [26], radiation shielding (presenting a 60% to 70% transmittance in the visible range) [27] and bioactive glasses [28]. However, these materials are not simple soda-lime glasses, and for this reason the eventual transparency cannot be compared with the glass reported in this work. Other works reported the fabrication of soda-lime glasses, like Berkin et al. and Maia et al.; however, due to the presence of chromium, iron and manganese, the results were green [7], dark grey [29] or brown [30] glasses, presenting less than 10% and ~20% light transmittance, respectively [29,30]. The only case about a transparent soda-lime glass with RHA (proven by photos) found in the literature, by Cordejo et al., did not mention the presence of manganese impurities in the used RHA [31,32].
Thus, in this work, it is in our interest to study more deeply the connection between the presence of Fe and mainly Mn impurities, typical of our raw material, with the overall transparency of the produced glass.
In the initial RHA composition, shown in Table 2, Fe3+ and Mn2+ ions were detected. While the most oxidized state of iron (Fe3+) has a pale yellow coloration, the manganese ions Mn2+ present a very weak yellow coloration due to a forbidden d-d electronic transition [15]. On the other hand, it is known from the literature that it is the cation Mn3+ that is responsible for a purple coloration, as it absorbs around 500 nm, due to the 5Eg → 5T2g transition [25]. As can be seen from Figure 1 and Figure 2, the glass sample fabricated with RHA without acid pretreatment presented a strong purple coloration due to the presence of Mn3+ ions.
Srisittipokakun et al. also studied soda-lime glasses made with pure quartz silica with different additions of MnO4 (Mn4+) and obtained purple-colored glasses [33]. They reported, in fact, that the most abundant species after the melting process was Mn3+. Thus, we might assume that in our soda-lime glass forming conditions, part, if not all of Mn2+, is also oxidized to Mn3+. In the case of iron, Donald et al. stated that the redox pair Fe3+/Fe2+ depends on the oxidation conditions [24]. Particularly, the more O2 is present, the more the equilibrium is shifted to higher concentrations of Fe3+. Yamashita et al. also stated that 90% of Fe in soda-lime glasses made with pure silica exists in the species Fe3+ [34], while, as reported by Chen et al., the addition of Sb2O3 tends to oxidize Fe2+ to Fe3+ [22]. For these reasons, we might assume that, in our glass, iron impurities are maintained predominantly as Fe3+ ions.
However, this might be a simplistic way to see the situation. In fact, studies on soda-lime silica glasses containing the Mn-Fe system actually state that the reaction between the redox pairs Mn3+/Mn2+ and Fe3+/Fe2+ (Equation (1)) favor the presence of Fe3+ and Mn2+, the opposite of what was found in this work regarding the manganese oxidation state [23]:
Mn3+ + Fe2+ ⇄ Mn2+ + Fe3+
Yamashita et al., i.e., reported that these species on the right side are more thermodynamically stable, particularly with high metal impurity concentrations and reductive conditions [34]. On the other hand, these authors also state that their soda-lime glass with Mn-Fe impurities solarizes under UV light (Equation (1) is shifted to the left), while Long et al. did not report this behavior in glasses with an almost identical composition [23]. Moreover, Long et al. did not see a purple coloration in their soda-lime glasses with only manganese as an impurity, contradicting what was found by Srisittipokakun et al. using very similar glass composition and glass melting conditions [33]. In general, oxidation-reduction reactions in a complex amorphous matrix such as glass are difficult to target and depend on many variables, like the metal concentration, matrix basicity, viscosity, oxygen and CO2 partial pressures, melting time, etc. [20,21]. Particularly in this work, the use of untreated RHA as a silica substitute might add amounts of alkali or alkali earth metal oxides, like CaO, that might affect the basicity of the glass matrix, affecting the redox equilibria. By calculating the optical basicity following Duffy’s method, using Equation (9) and Table 1 in [35], it was possible to find a value of 0.715 for untreated RHA glass. The value obtained is relatively high with respect to commercial soda-lime glasses, which is in the range 0.63–0.68, depending on the composition. The elevated optical basicity sustains the fact that the melt environment favors the most oxidized species of Fe and Mn [36]. Last but not least, the reaction between Mn and Fe will depend on the concentration of each species. In the case of our untreated RHA, if we consider that only 10% is Fe2+, as stated above [24,37], manganese ions will be 15× in excess (in mol%). For this reason, even if reaction 1 occurs, we will still have a purple glass due to the presence of unreacted Mn3+.
Considering all of the above, it appears that only by reducing the amount of metal impurities through a pretreatment step will it be possible to obtain decolorized glasses with RHA presenting significant amounts of manganese. However, as shown by Kaewkhao et al. [25] and also as proven by our results, it might be possible to convert Mn3+ to Mn2+ by altering the redox equilibrium of the melt. In fact, from the literature, it is known that antimony can react with manganese following the redox reaction (Equation (2)) [23]:
Sb3+ + 2Mn3+ ⇄ Sb5+ + 2Mn2+
Thus, it is possible to take advantage of this reaction, and with the addition of antimony oxide (III)-Sb2O3 the purple effect of Mn3+ may, at the right redox conditions, be eliminated by its reduction to Mn2+, which is colorless, avoiding further steps and pretreatments in the glass formation process. Moreover, the optical transparency is permanent, and the glass does not suffer solarization, even when exposed to UV light (50 W) for 30 h. At last, the fact that under UV-light exposure only the 0.5% Sb2O3 glass sample presented a visible orange fluorescence (Figure A3), typical of the Mn2+ species [38], further confirms that Equation (2) took place.
As a final note, comparing the two methods presented in this work to eliminate metal oxide impurities and their effect on the final color of the glass produced, it seems that the addition of elements that alter the redox equilibria of the melt, without an RH pretreatment, might be a more efficient, cheaper and more environmentally friendly way to produce transparent soda-lime silica glasses with RHA and Mn impurities. Different concentrations of Sb2O3 and other redox agents will be further explored in future works.
5. Conclusions
In this work, we reported the fabrication of a transparent soda-lime silica glass using rice husk ashes as the only silica source. Because of the metal impurities in the organic-derived silica, like manganese and iron oxides, the original glass presented a deep purple coloration, which limits its market application. Among the discussions behind the reasons for this coloration, two different methods were explored in order to remove the presence of Mn3+. First, it was proven that acid lixiviation with HCl 10 w/w% was successful in highly reducing metal oxide impurities, leading to a glass with more than 70% light transmission. The result was very close to the 80–85% transmission detected from the same glass composition and thickness but using pure quartz as a silica source. Secondly, the addition of a chromatic element that alters the redox equilibria of the melt, such as antimony oxide, was explored, and it was demonstrated that it could be an efficient method to remove the Mn3+ presence. In this sense, it was shown that with the addition of only 0.5 mol% of Sb2O3, a transparent (>80% transmission) soda-lime silica glass was obtained, opening the possibility of easily producing a transparent, cost-effective and sustainable glass using only RHA.
Author Contributions
Conceptualization, J.W.M. and C.V.; methodology, J.G. and G.d.S.; validation, J.M., D.M. and M.N.; formal analysis, J.G., L.L. and L.A.; investigation, L.E.G.A., L.L. and D.M.; data curation, J.G., G.d.S. L.L. and D.M.; writing—original draft preparation, J.G.,C.V.; writing—review and editing, C.V., M.N. and J.M.; supervision, J.W.M.; funding acquisition, C.V. and M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fapergs, grant number 19/2551-0001245-4 to the author C.V. and by FAPESP, grants number 2013/07793-6 to the author M.N.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A
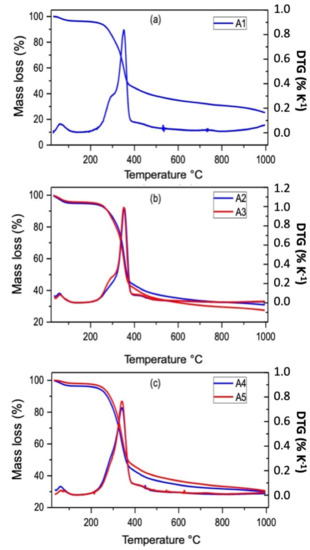
Figure A1.
TGA (Thermal Gravimetric Analysis) and DTG (Derivative Thermogravimetric) curves of the rice husk samples (a) A1, without acid treatment; (b) A2, A3, treated with HCl 4% and 10% solutions; (c) A4, A5, treated with H2SO4 4% and 10% solutions.
As can be seen from Figure A1, the untreated and all the treated RH samples presented the same thermogravimetric behavior, which can be divided into three main stages. The first stage occurs between room temperature and 100 °C, and it corresponds to moisture elimination, with a mass loss of about 3%. The second stage can be defined as occurring between 210 and 410 °C, and it is characterized by an abrupt mass loss, also identified by the peak at ~370 °C in the DTG analysis; the mass loss in this stage can amount to about 65%. In this range of temperature, we have the degradation of the major organic components of the rice husk, cellulose and hemicellulose. Particularly, the degradation of hemicellulose starts earlier than cellulose, as can be seen by the shoulder before the peak at around 300 °C. In the last stage, between 400 and 1000 °C, we can see a slower mass loss of about 15%, in which the lignin starts to decompose [17]. The calcination temperature for forming RHA was chosen to be between 600 and 800 °C, as the majority of the organic components have been eliminated, and the temperature is not sufficiently high to lead to silica crystallization (known to start at 900 °C [10]).
Figure A2 presents the DSC analysis for the untreated RHA glass sample (A0) and the HCl 10 w/w%-treated RHA glass sample (A3).
The dashed vertical lines between 480 and 500 °C highlight the sample Tg transitions. Particularly, for the glass sample produced with untreated RHA, Tg was found to be at 483 °C, while the Tg for the treated RHA with HCl 10 w/w% was found at 505 °C. The Tg typical value for commercial flat soda-lime glass is around 560 °C [38]. This large difference can be assigned to the fact that the concentration of SiO2 in our samples is much lower (about 43% lower) than the commercial standard (70%). Silica being the component with the higher melting temperature in the melt, this large effect on the Tg values is acceptable. Lastly, at around 570 °C (as highlighted with a star) it is possible to see an endothermic transition for the untreated RHA sample, not visible for the treated sample. This might be due to a stress release after Tg, also called apparent melting [39]. Unfortunately, due to the abrupt ending of the analysis (the available crucible did not support temperatures above 600 °C), this consideration should be taken with caution.
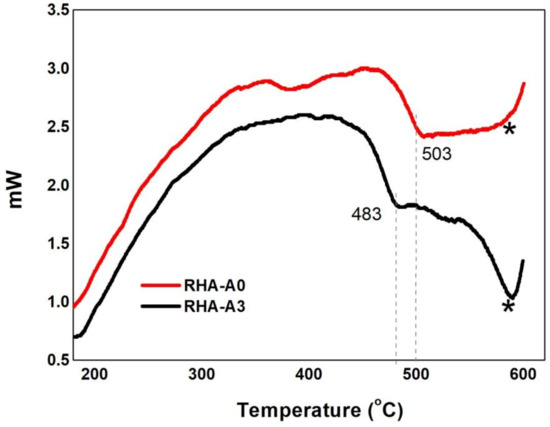
Figure A2.
DSC analysis of the untreated RHA glass sample (A0) and HCl 10 w/w%-treated RHA glass sample (A3).
Figure A3 shows that the 0.5% Sb2O3 glass sample presented a bright orange fluorescence when exposed to UV light during the solarization analysis. The same effect was not visible for the glass samples prepared without antimony oxide or with sand substituting RHA.
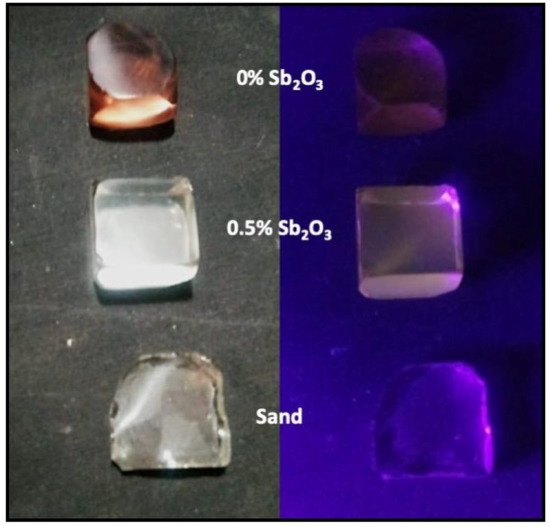
Figure A3.
Photograph of the 0% Sb2O3 (top), 0.5% Sb2O3 (middle) and sand (bottom) glass samples before and under UV-A exposure.
References
- Chandel, A.K.; da Silva, S.S.; Carvalho, W.; Singh, O.V. Sugarcane bagasse and leaves: Foreseeable biomass of biofuel and bio-products. J. Chem. Technol. Biotechnol. 2011, 87, 11–20. [Google Scholar] [CrossRef]
- Perea-Moreno, A.-J.; Aguilera-Ureña, M.-J.; Manzano-Agugliaro, F. Fuel properties of avocado stone. Fuel 2016, 186, 358–364. [Google Scholar] [CrossRef]
- Zhang, W.L.; Liu, Z.Y.; Liu, Z.; Li, F.L. Butanol production from corncob residue using Clostridium beijerinckii NCIMB 8052. Lett. Appl. Microbiol. 2012, 55, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Rajagopal, K.; Thangavel, K. Rice husk ash blended cement: Assessment of optimal level of replacement for strength and permeability properties of concrete. Constr. Build. Mater. 2008, 22, 1675–1683. [Google Scholar] [CrossRef]
- García, D.; López, J.; Balart, R.; Ruseckaite, R.A.; Stefani, P.M. Composites based on sintering rice husk–waste tire rubber mixtures. Mater. Des. 2007, 28, 2234–2238. [Google Scholar] [CrossRef]
- Danewalia, S.S.; Sharma, G.; Thakur, S.; Singh, K. Agricultural wastes as a resource of raw materials for developing low dielectric glass-ceramics. Sci. Rep. 2016, 6, 24617. [Google Scholar] [CrossRef]
- Deng, W.; Spathi, C.; Coulbeck, T.; Erhan, K.; Backhouse, D.; Marshall, M.; Ireson, R.; Bingham, P.A. Exploratory research in alternative raw material sources and reformulation for industrial soda-lime-silica glass batches. Int. J. Appl. Glass Sci. 2020, 11, 340–356. [Google Scholar] [CrossRef]
- Associação Brasileira de Distribuidores e Processadores de Vidros Planos—Abravidro (Brazilian Association of Distributors and Producer of Flat Glasses). Available online: https://abravidro.org.br/como-anda-o-setor-vidreiro-nacional/ (accessed on 15 March 2020).
- Hossain, S.K.S.; Mathur, L.; Roy, P.K. Rice husk/rice husk ash as an alternative source of silica in ceramics: A review. J. Asian Ceram. Soc. 2018, 6, 299–313. [Google Scholar] [CrossRef]
- Patel, K.G.; Shettigar, R.R.; Misra, N.M. Recent advance in silica production technologies from agricultural waste stream—Review. J. Adv. Agric. Technol. 2017, 4, 274–279. [Google Scholar] [CrossRef]
- Companhia Nacional de Abastecimento—CONAB (National Company of Supply—Brazilian Grain Harvest). Acompanhamento da Safra Brasileira de Grãos. Available online: https://www.conab.gov.br/info-agro/safras/graos/boletim-da-safra-de-graos (accessed on 13 January 2020).
- Pode, R. Potential applications of rice husk ash waste from rice husk biomass power plant. Renew. Sustain. Energy Rev. 2016, 53, 1468–1485. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Satyanarayana, K.G.; Pramada, P.N.; Raghavan, P. Review processing, properties and applications of reactive silica from rice husk—An overview. J. Mater. Sci. 2003, 38, 3159–3168. [Google Scholar] [CrossRef]
- Gonzalves, M.R.F.; Bergmann, C.P. Thermal insulators made with rice husk ashes: Production and correlation between properties and microstructure. Constr. Build. Mater. 2007, 21, 2059–2065. [Google Scholar] [CrossRef]
- Paul, A. Chemistry of Glasses, 1st ed.; Chapman and Hall: London, UK, 1982; pp. 204–276. [Google Scholar]
- Min’ko, N.I.; Morozova, I.I. Effect of the redox potential on the cooking and properties of glass. Glass Ceram. 2014, 71, 229–232. [Google Scholar] [CrossRef]
- Liou, T.-H. Preparation and characterization of nano-structured silica from rice husk. Mater. Sci. Eng. A 2004, 364, 313–323. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Pramada, P.N.; Praveen, L. Effect of organic acid treatment on the properties of rice husk silica. J. Mater. Sci. 2005, 40, 6535–6544. [Google Scholar] [CrossRef]
- Song, S.; Cho, H.B.; Kim, H.T. Surfactant-free synthesis of high surface area silica nanoparticles derived from rice husks by employing the Taguchi approach. J. Ind. Eng. Chem. 2018, 61, 281–287. [Google Scholar] [CrossRef]
- Thiemsorn, W.; Keowkamnerd, K.; Phanichphant, S.; Suwannathada, P.; Hessenkemper, H. Influence of glass basicity on redox interactions of iron-manganese-copper ion pairs in soda-lime-silica glass. Glass Phys. Chem. 2008, 34, 19–29. [Google Scholar] [CrossRef]
- Yamashita, M.; Akai, T.; Sawa, R.; Abe, J.; Matsumura, M. Effect of preparation procedure on redox states of iron in soda-lime silicate glass. J. Non-Cryst. Solids 2008, 354, 4534–4538. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Rautiyal, P.; Vaishnav, S.; Gupta, G.; Schlegl, H.; Dawson, R.J.; Evans, A.W.; Kamali, S.; Johnson, J.A.; Johnson, C.E.; et al. Composition-structure-property effects of antimony in soda-lime-silica glasses. J. Non-Cryst. Solids 2020, 544, 120184. [Google Scholar] [CrossRef]
- Long, B.T.; Peters, L.J.; Schreiber, H.D. Solarization of soda-lime-silicate glass containing manganese. J. Non-Cryst. Solids 1998, 239, 126–130. [Google Scholar] [CrossRef]
- Donald, S.B.; Swink, A.M.; Schreiber, H.D. High-iron ferric glass. J. Non-Cryst. Solids 2006, 352, 539–543. [Google Scholar] [CrossRef]
- Kaewkhao, J.; Limsuwan, P. Utilization of rice husk fly ash in the color glass production. Procedia Eng. 2012, 32, 670–675. [Google Scholar] [CrossRef]
- Lee, T.; Othman, R.; Yeoh, F.-Y. Development of photoluminescent glass derived from rice husk. Biomass Bioenergy 2013, 59, 380–392. [Google Scholar] [CrossRef]
- Ruengsri, S.; Insiripong, S.; Sangwaranatee, N.; Kaewkhao, J. Development of barium borosilicate glasses for radiation shielding materials using rice husk ash as a silica source. Prog. Nucl. Energy 2015, 83, 99–104. [Google Scholar] [CrossRef]
- Sierra, L.A.Q.; Sierra, D.M.E. Synthesis and bioactivity evaluation of a rice husk-derived bioactive glass. JOM 2018, 71, 302–307. [Google Scholar] [CrossRef]
- Berkin, G. Heat absorbing glass from rice husk ash for a sustainable environment. In WIT Transactions on Ecology and the Environment, Proceedings of the Fourth International Conference on Waste Management and the Environment, Granada, Spain, 2–4 June 2008; WIT Press: Southampton, UK, 2008. [Google Scholar] [CrossRef]
- Maia, B.G.O.; Souza, M.T.; Arcaro, S.; de Oliveira, T.M.N.; Wermuth, T.B.; Novaes de Oliveira, A.P.; Rodrigues Neto, J.B. Caracterização de vidros sódico-cálcicos produzidos a partir de resíduos sólidos. Cerâm. Ind. 2017, 22, 32–39. [Google Scholar] [CrossRef]
- Cornejo, I.A.; Ramalingam, S.; Fish, J.S.; Reimanis, I.E. Hidden treasures: Turning food waste into glass. Am. Ceram. Soc. Bull. 2014, 93, 24–27. [Google Scholar]
- Cornejo, I.A.; Reimanis, I.E.; Ramalingam, S. Methods of making glass from organic waste food streams. U.S. 2015/0065329A1, 05 March 2015. [Google Scholar]
- Srisittipokakun, N.; Kedkaew, C.; Kaewkhao, J.; Limsuwan, P. Coloration in soda-lime-silicate glass system containing manganese. Adv. Mater. Res. 2010, 93–94, 206–209. [Google Scholar] [CrossRef]
- Yamashita, M.; Yao, Z.; Matsumoto, Y.; Utagawa, Y.; Kadono, K.; Yazawa, T. X-ray irradiation-induced coloration of manganese in soda-lime silicate glass. J. Non-Cryst. Solids 2004, 333, 37–43. [Google Scholar] [CrossRef]
- Duffy, J.A. Optical Basicity: A practical acid-base theory for oxides and oxyanions. J. Chem. Educ. 1996, 73, 1138–1142. [Google Scholar] [CrossRef]
- Chimalawong, P.; Kirdsiri, K.; Kaewkhao, J.; Limsuwan, P. Investigation on the Physical and Optical Properties of Dy3+ Doped Soda-Lime-Silicate Glasses. Procedia Eng. 2012, 32, 690–698. [Google Scholar] [CrossRef]
- Wright, A.C.; Clarke, S.J.; Howard, C.K.; Bingham, P.A.; Forder, S.D.; Holland, D.; Martlew, D.; Fischer, H.E. The environment of Fe/Fe cations in a soda–lime–silica glass. Phys. Chem. Glasses: Eur. J. Glass Sci. Technol. B 2014, 55, 243–252. [Google Scholar]
- Möncke, D.; Kamitsos, E.I.; Herrmann, A.; Ehrt, D.; Friedrich, M. Bonding and ion–ion interactions of Mn2+ ions in fluoride-phosphate and boro-silicate glasses probed by EPR and fluorescence spectroscopy. J. Non-Cryst. Solids 2011, 357, 2542–2551. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, Y.; Montazerian, M.; Gulbiten, O.; Mauro, J.C.; Zanotto, E.D.; Yue, Y. Understanding Glass through Differential Scanning Calorimetry. Chem. Rev. 2019, 119, 7848–7939. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).