Semiconducting Metal Oxides Nanocomposites for Enhanced Detection of Explosive Vapors
Abstract
:1. Introduction
- High response to the target agent,
- High selectivity to target gas in the presence of a mixture of gases,
- Fast and reversible interaction with analyte,
- Low sensitivity of the signal to a change in air humidity,
- Absence of long-term drift,
- Short time to operational status,
- Effective low-cost technology,
- High reproducibility,
- Uniform and strong binding to the surface of the substrate.
2. Most Promising Semiconducting Metal Oxides for Explosive Vapor Detection
2.1. Zinc Oxide Composites
2.2. Zeolites and Metal Organic Frameworks (MOFs)
2.3. WO3 Composites
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, R.; Wei, Y.; Yu, Y.; Gao, C.; Wang, L.; Liu, J.-H.; Huang, X.-J. Make it different: The plasma treated multi-walled carbon nanotubes improve electrochemical performances toward nitroaromatic compounds. Electrochim. Acta 2012, 76, 354–362. [Google Scholar] [CrossRef]
- Kaplan, D.L.; Kaplan, A.M. 2,4,6-Trinitrotoluene-Surfactant Complexes: Decomposition, Mutagenicity, and Soil Leaching Studies. Environ. Sci. Technol. 1982, 16, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.C.; Commons, B.J.; Bausum, H.T.; Abemathy, C.O.; Murphy, J.J.; Khanna, K.; Ohanian, E.V. Overview of the Health Effects of Selected Munitions Chemicals. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/20003MXC.TXT?ZyActionD=ZyDocument&Client=EPA&Index=1991+Thru+1994&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C91thru94%5CTxt%5C00000007%5C20003MXC.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL (accessed on 10 June 2018).
- Strle, D.; Štefane, B.; Nahtigal, U.; Zupanič, E.; Pozgan, F.; Kvasič, I.; Maček, M.; Trontelj, J.; Muševič, I. Surface-Functionalized COMB Capacitive Sensors and CMOS Electronics for Vapor Trace Detection of Explosives. IEEE Sens. J 2012, 12, 1048–1057. [Google Scholar] [CrossRef]
- Bielecki, Z.; Janucki, J.; Kawalec, A.; Mikołajczyk, J.; Pałka, N.; Pasternak, M.; Pustelny, T.; Stacewicz, T.; Wojtas, J. Sensors and systems for the detection of explosive devices – An overview. Metrol. Meas. Syst. 2012, 19, 3–28. [Google Scholar] [CrossRef]
- Ewing, R.G.; Waltman, M.J.; Atkinson, D.A.; Grate, J.W.; Hotchkiss, P.J. The vapor pressures of explosives. Trends Anal. Chem. 2013, 42, 35–48. [Google Scholar] [CrossRef]
- Östmark, H.; Wallin, S.; Ang, H.G. Vapor Pressure of Explosives: A Critical Review. Propellants Explos. Pyrotech. 2012, 37, 12–23. [Google Scholar] [CrossRef]
- Sekhar, P.K.; Wignes, F. Trace detection of research department explosive (RDX) using electrochemical gas sensor. Sens. Actuators B Chem. 2016, 227, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.; Ge, Y.; Zu, B.; Li, Y.; Dou, X. Transition-Metal-Doped p-Type ZnO Nanoparticle-Based Sensory Array for Instant Discrimination of Explosive Vapors. Small 2016, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.; Hackner, A.; Beer, S.; Göbel, J. Solid-State Gas Sensors: Sensor System Challenges in the Civil Security Domain. Materials 2016, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Atkins, P.; Jones, L. Chimica Generale, Seconda Edizione Italiana; Zanichelli Editore: Bologna, Italy, 1998; p. 253. [Google Scholar]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Receptor function of small semiconductor crystals with clean and electron-traps dispersed surfaces. Thin Solid Films 2009, 517, 6148–6155. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Pronin, I.A.; Ham, M.H.; Cho, B.K. Metal Oxides for Application in Conductometric Gas Sensors: How to Choose? Solid State Phenom. 2017, 266, 187–195. [Google Scholar] [CrossRef]
- Bârsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Gőpel, W.; Schierbaum, K.D. SnO2 sensors: Current status and future prospects. Sens. Actuators B Chem. 1995, 26–27, 1–12. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Liu, S.-B.; Meng, F.-B.; Liu, J.-Y.; Jin, Z.; Kong, L.-T.; Liu, J.-H. Metal Oxide Nanostructures and Their Gas Sensing Properties: A Review. Sensors 2012, 12, 2610–2631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamazoe, N.; Shimanoe, K. Receptor Function and Response of Semiconductor Gas Sensor. J. Sens. 2009, 875704. [Google Scholar] [CrossRef]
- Xu, C.; Tamaki, J.; Miura, N.; Yamazoe, N. Grain size effects on gas sensitivity of porous SnO2-based elements. Sens. Actuators B Chem. 1991, 3, 147–155. [Google Scholar] [CrossRef]
- Bârsan, N.; Schweizer-Berberich, M.; Göpel, W. Fundamental and practical aspects in the design of nanoscaled SnO2 gas sensors: A status report. Fresenius J. Anal. Chem. 1999, 365, 287–304. [Google Scholar] [CrossRef]
- Oprea, I.A.; Bârsan, N.; Weimar, U. Work function changes in gas sensitive materials: Fundamentals and applications. Sens. Actuators B Chem. 2009, 142, 470–493. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Hübner, M.; Simion, C.E.; Tomescu-Stănoiu, A.; Pokhrel, S.; Bârsan, N.; Weimar, U. Influence of humidity on CO sensing with p-type CuO thick film gas sensors. Sens. Actuators B Chem. 2011, 153, 347–353. [Google Scholar] [CrossRef]
- Brattain, W.H.; Bardeen, J. Surface Properties of Germanium. Bell Syst. Tech. J. 1953, 32, 1–41. [Google Scholar] [CrossRef]
- Seiyama, T.; Kato, A.; Fujushi, K.; Nagatani, M. A new detector for gaseous components using semiconductive thin films. Anal. Chem. 1962, 34, 1502–1503. [Google Scholar] [CrossRef]
- Gas Sensors Market by Gas Type (Oxygen, Carbon Monoxide, Carbon Dioxide, Ammonia, Chlorine, Hydrogen Sulfide, Nitrogen Oxide, Volatile organic Compounds, Hydrocarbons), Technology, End-Use Application, Geography—Global Forecast 2023. Available online: https://www.marketsandmarkets.com/Market-Reports/gas-sensor-market-245141093.html?gclid=EAIaIQobChMI5Lb-jtLI2wIVlIRwCh3teAkOEAAYASAAEgKT7_D_BwE (accessed on 10 June 2018).
- Drobek, M.; Kim, J.-H.; Bechelany, M.; Vallicari, C.; Julbe, A.; Kim, S.S. MOF-Based Membrane Encapsulated ZnO Nanowires for Enhanced Gas Sensor Selectivity. ACS Appl. Mater. Interfaces 2016, 8, 8323–8328. [Google Scholar] [CrossRef] [PubMed]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Doll, T.; Eisele, I. Gas detection with work function sensors. Proc. SPIE 1998, 3539. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Boris, Y.; Ivanov, M.; Schwank, J.; Morante, J. Influence of surface Pd doping on gas sensing characteristics of SnO2 thin films deposited by spray pirolysis. Thin Solid Films 2003, 436, 119–126. [Google Scholar] [CrossRef]
- Horsfall, L.A.; Pugh, D.C.; Blackman, C.S.; Parkin, I.P. An array of WO3 and CTO heterojunction semiconducting metal oxide gas sensors used as a tool for explosive detection. J. Mater. Chem. A 2017, 5, 2172–2179. [Google Scholar] [CrossRef]
- Yamazoe, N.; Kurokawa, Y.; Seiyama, T. Effects of additives on semiconductor gas sensors. Sens. Actuators 1983, 4, 283–289. [Google Scholar] [CrossRef]
- Korotcenkov, G. Gas response control through structural and chemical modification of metal oxide films: State of the art and approaches. Sens. Actuators B Chem. 2005, 107, 209–232. [Google Scholar] [CrossRef]
- Makimoto, O.; Arakawa, T. Sensing properties of Ln2CuO4-SnO2, (Ln =rare earth) having a heterojunction. Sens. Actuators B 1993, 13–14, 585–586. [Google Scholar] [CrossRef]
- Marichy, C.; Pinna, N. Atomic Layer Deposition to Materials for Gas Sensing Applications. Adv. Mater. Interfaces 2016, 3, 1–20. [Google Scholar] [CrossRef]
- Cabot, A.; Arbiol, J.; Morante, J.R.; Weimar, U.; Bârsan, N.; Göpel, W. Analysis of the noble metal catalytic additives introduced by impregnation of as obtained SnO2 sol–gel nanocrystals for gas sensors. Sens. Actuators B Chem. 2000, 70, 87–100. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Cho, B.K. Conductometric gas sensors based on metal oxides modified with gold nanoparticles: A review. Microchim. Acta 2016, 183, 1033–1054. [Google Scholar] [CrossRef]
- Marikutsa, A.V.; Rumyantseva, M.N.; Gaskov, A.M.; Samoylov, A.M. Nanocrystalline Tin Dioxide: Basics in Relation with Gas Sensing Phenomena Part II. Active Centers and Sensor Behavior. Inorg. Mater. 2016, 52, 1311–1338. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Han, S.-D.; Cho, B.K.; Brinzari, V. Grain Size Effects in Sensor Response of Nanostructured SnO2- and In2O3-Based Conductometric. Thin Film Gas Sensor. Crit. Rev. Solid State Mater. Sci. 2009, 34, 1–17. [Google Scholar] [CrossRef]
- Rumyantseva, M.N.; Gas´kov, A.M. Chemical modification of nanocrystalline metal oxides: Effect of the real structure and surface chemistry on the sensor properties. Russ. Chem. Bull. Int. Ed. 2008, 57, 1106–1125. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: A review. Sens. Actuators A-Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
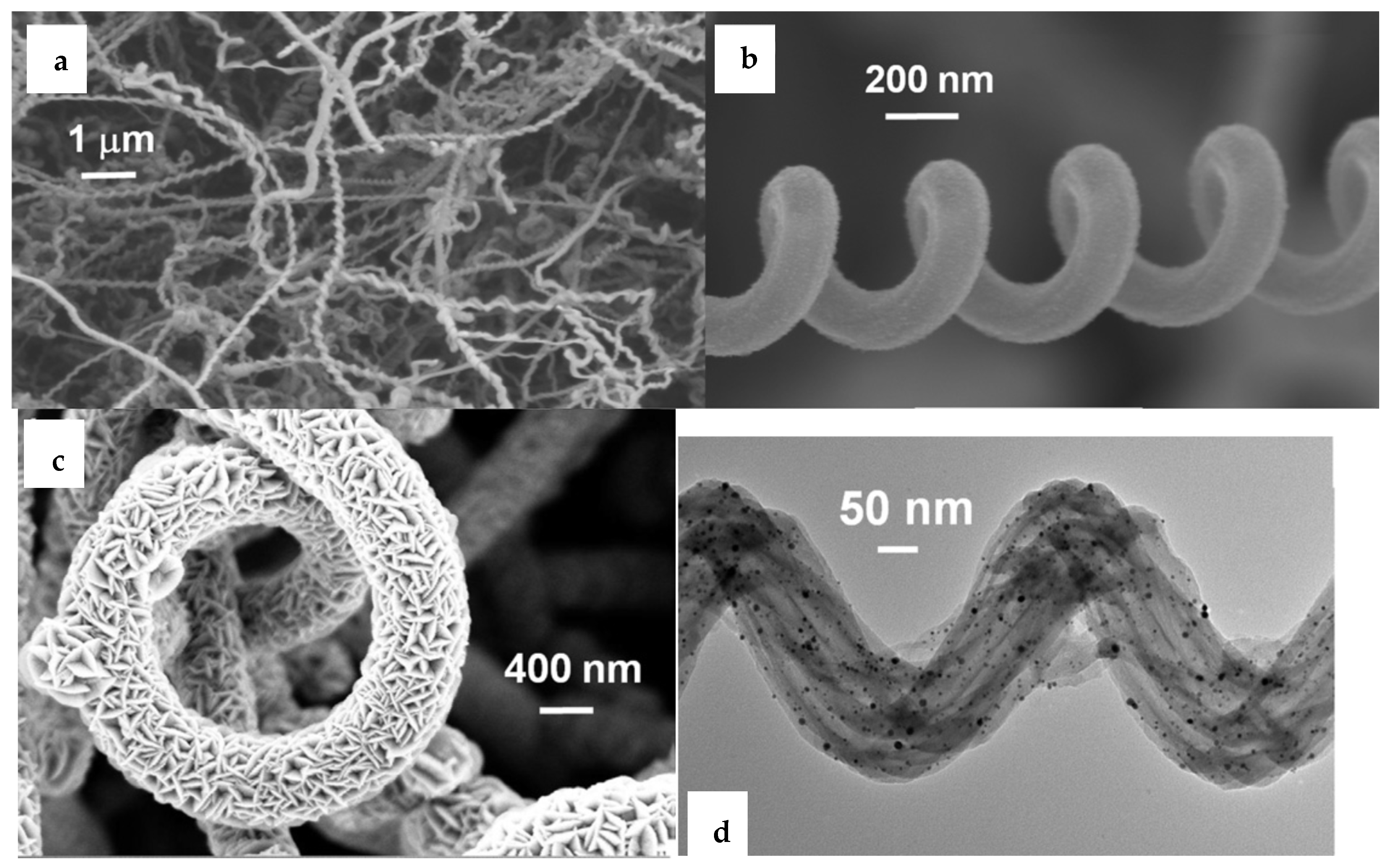
- Dobrokhotov, V.; Oakes, L.; Sowell, D.; Larin, A.; Hall, J.; Kengne, A.; Bakharev, P.L.; Corti, G.; Cantrell, T.; Prakash, T.; et al. Toward the nanospring-based artificial olfactory system for trace-detection of flammable and explosive vapors. Sens. Actuators B Chem. 2012, 168, 138–148. [Google Scholar] [CrossRef]
- Guo, L.; Yang, Z.; Li, Y.; Zu, B.; Dou, X. Sensitive, real-time and anti-interfering detection of nitro-explosive vapors realized by ZnO/rGO core/shell micro-Schottky junction. Sens. Actuators B Chem. 2017, 239, 286–294. [Google Scholar] [CrossRef]
- Gui, Y.; Xie, C.; Xu, J.; Wang, G. Detection and discrimination of low concentration explosives using MOS nanoparticle sensors. J. Hazard. Mater. 2009, 164, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Peveler, W.J.; Binions, R.; Hailes, S.M.V.; Parkin, I.P. Detection of explosive markers using zeolite modified gas sensors. J. Mater. Chem. A 2013, 1, 2613–2620. [Google Scholar] [CrossRef] [Green Version]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. MetalOrganic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Deep, A.; Kim, K.-H. Metal organic frameworks for sensing applications. Trends Anal. Chem. 2015, 73, 39–53. [Google Scholar] [CrossRef]
- Moseley, P.T. Progress in the development of semiconducting metal oxide gas sensors: A review. Meas. Sci. Technol. 2018, 28, 082001. [Google Scholar] [CrossRef]
- Wang, D.; Chen, A.; Jen, A.K.-Y. Reducing cross-sensitivity of TiO2-(B) nanowires to humidity using ultraviolet illumination for trace explosive detection. Phys. Chem. Chem. Phys. 2013, 15, 5017–5021. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, M.; Kornely, S.; Weh, T.; Frank, J.; Meixner, H. Selective gas detection with high-temperature operated metal oxides using catalytic filters. Sens. Actuators B Chem. 2000, 69, 205–210. [Google Scholar] [CrossRef]
- Tulliani, J.M.; Moggi, P. Development of a porous layer catalytically activated for improving gas sensors performances. Ceram. Int. 2007, 33, 1199–1203. [Google Scholar] [CrossRef]
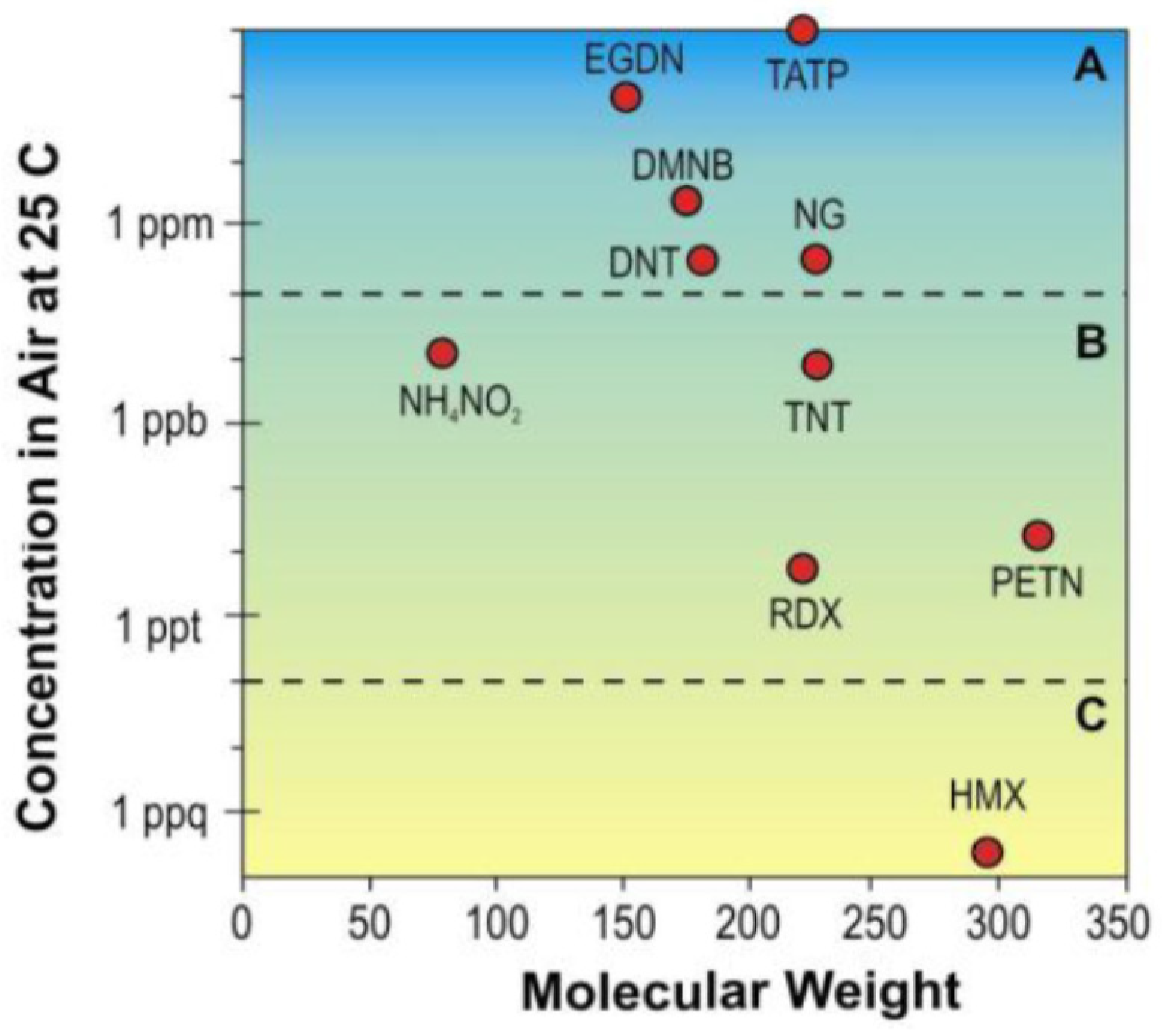
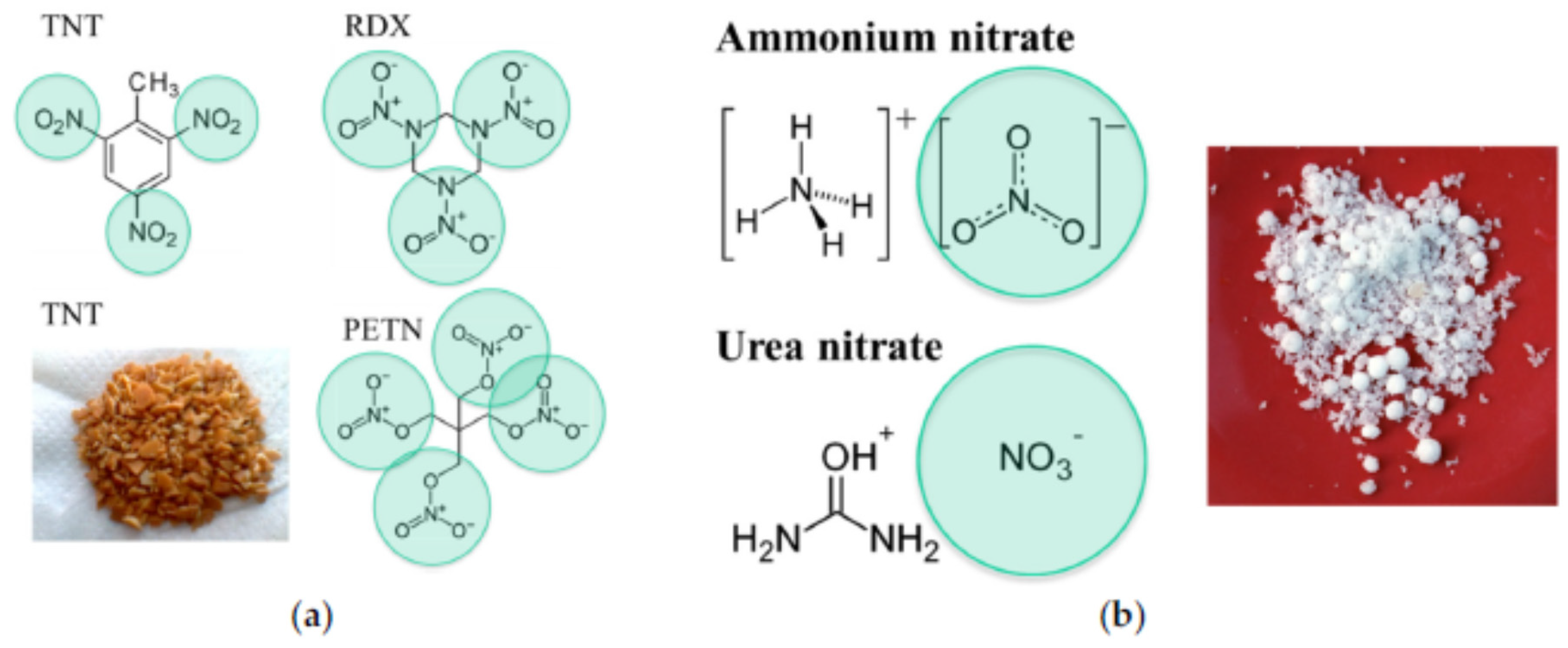



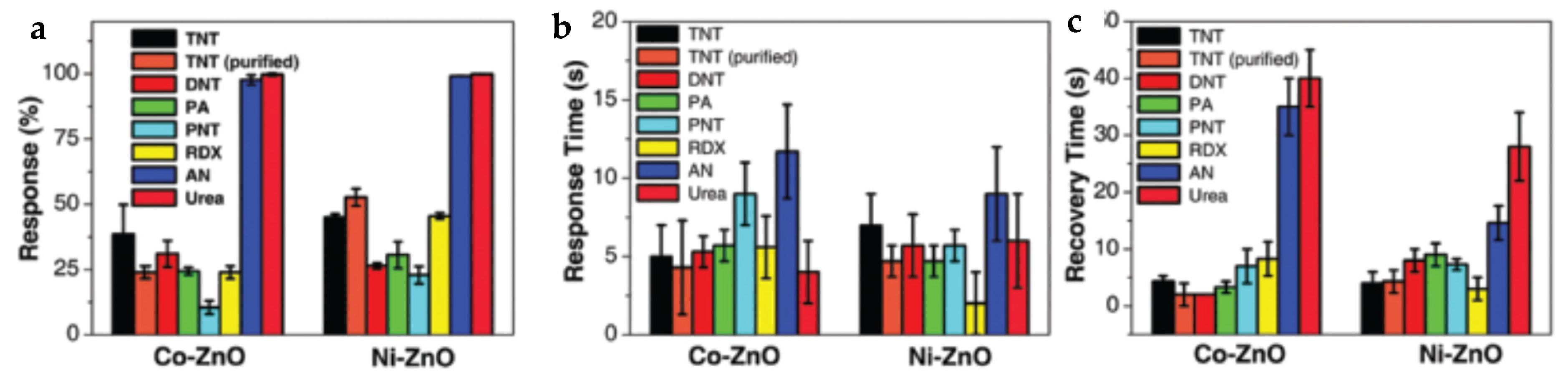
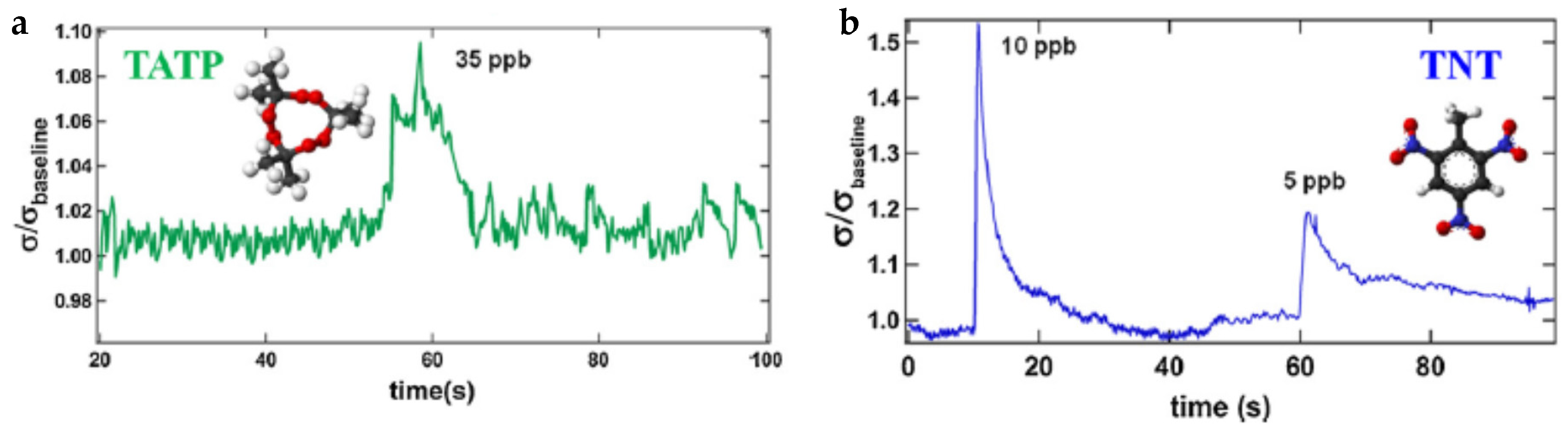
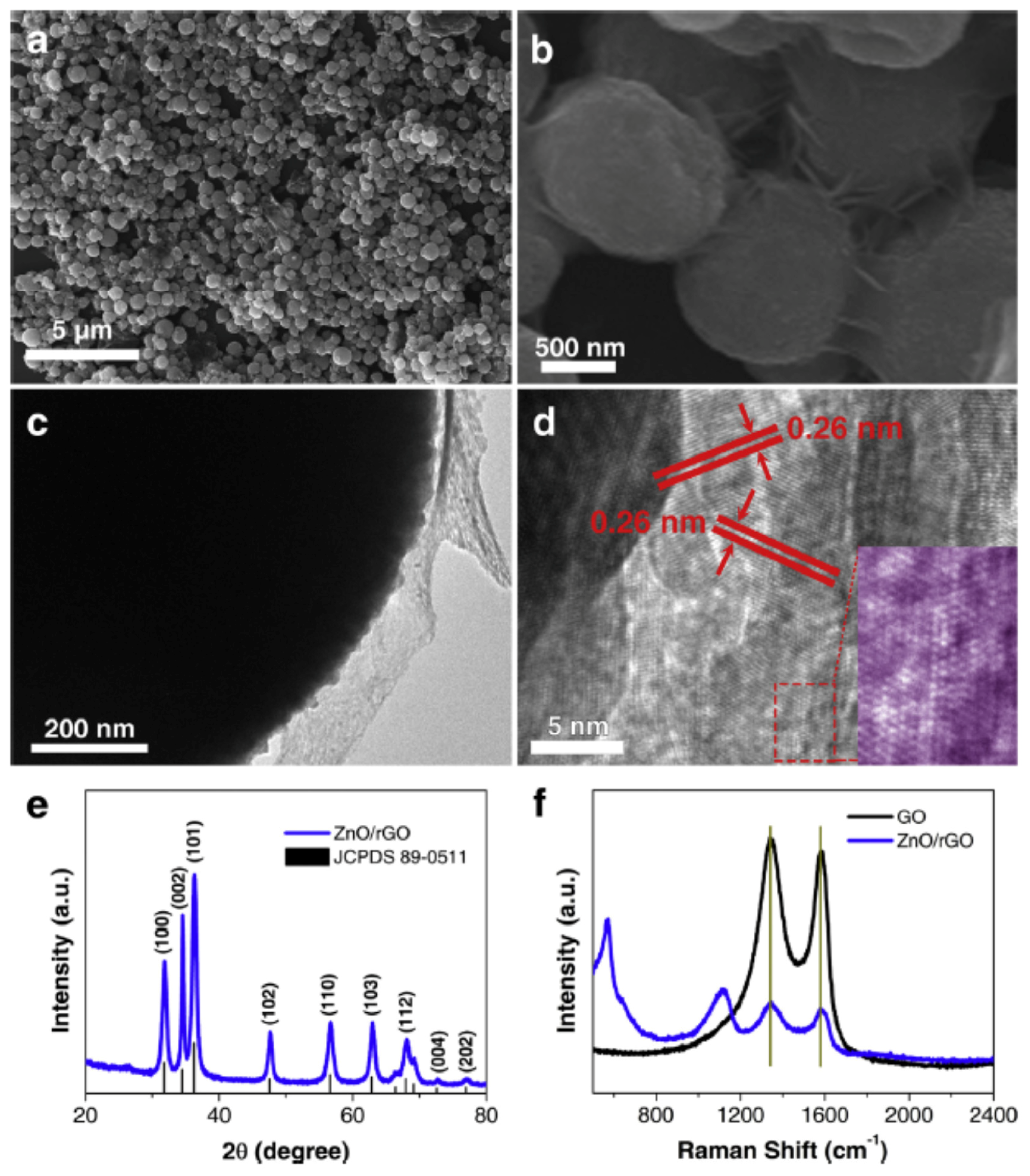
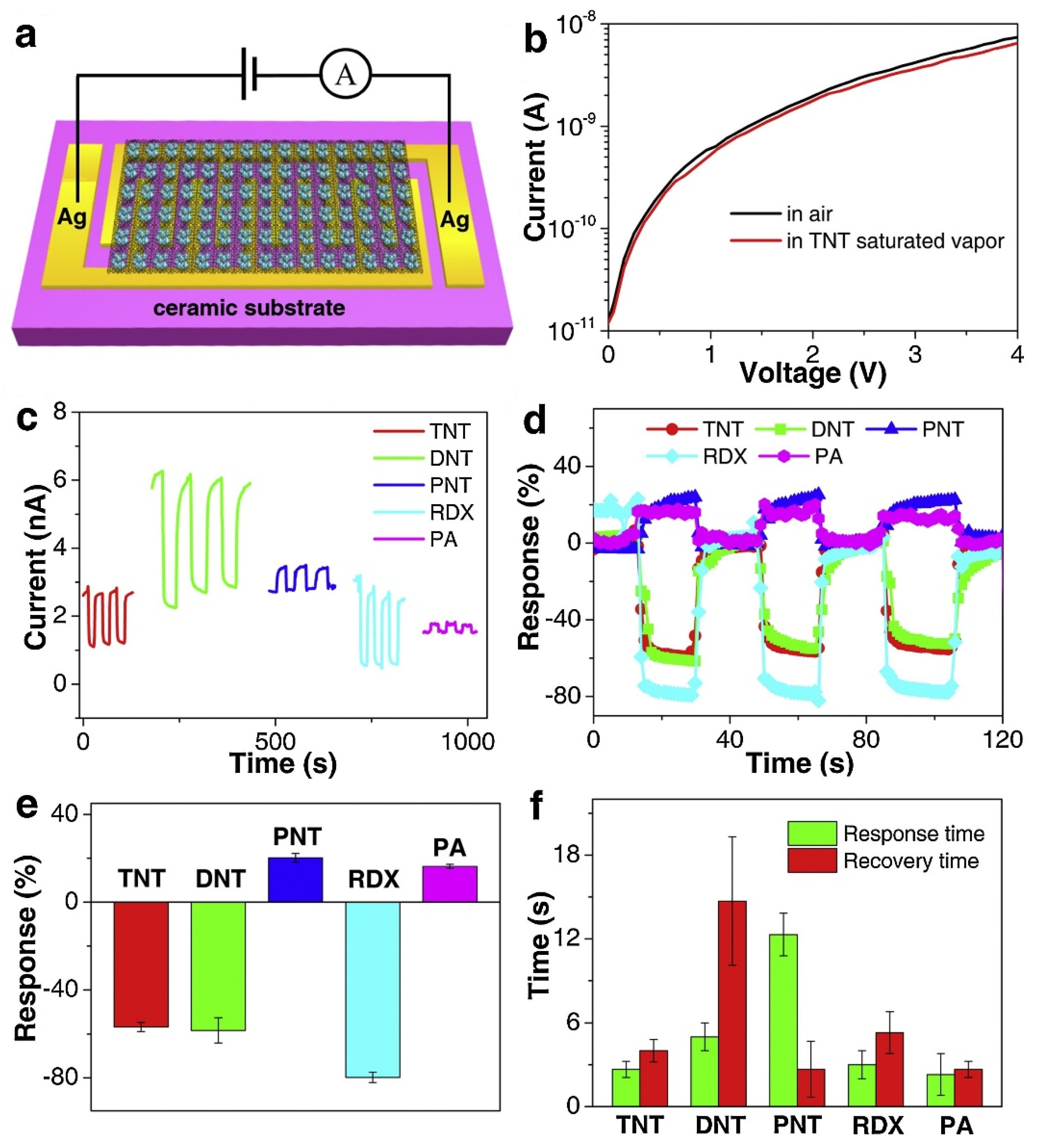
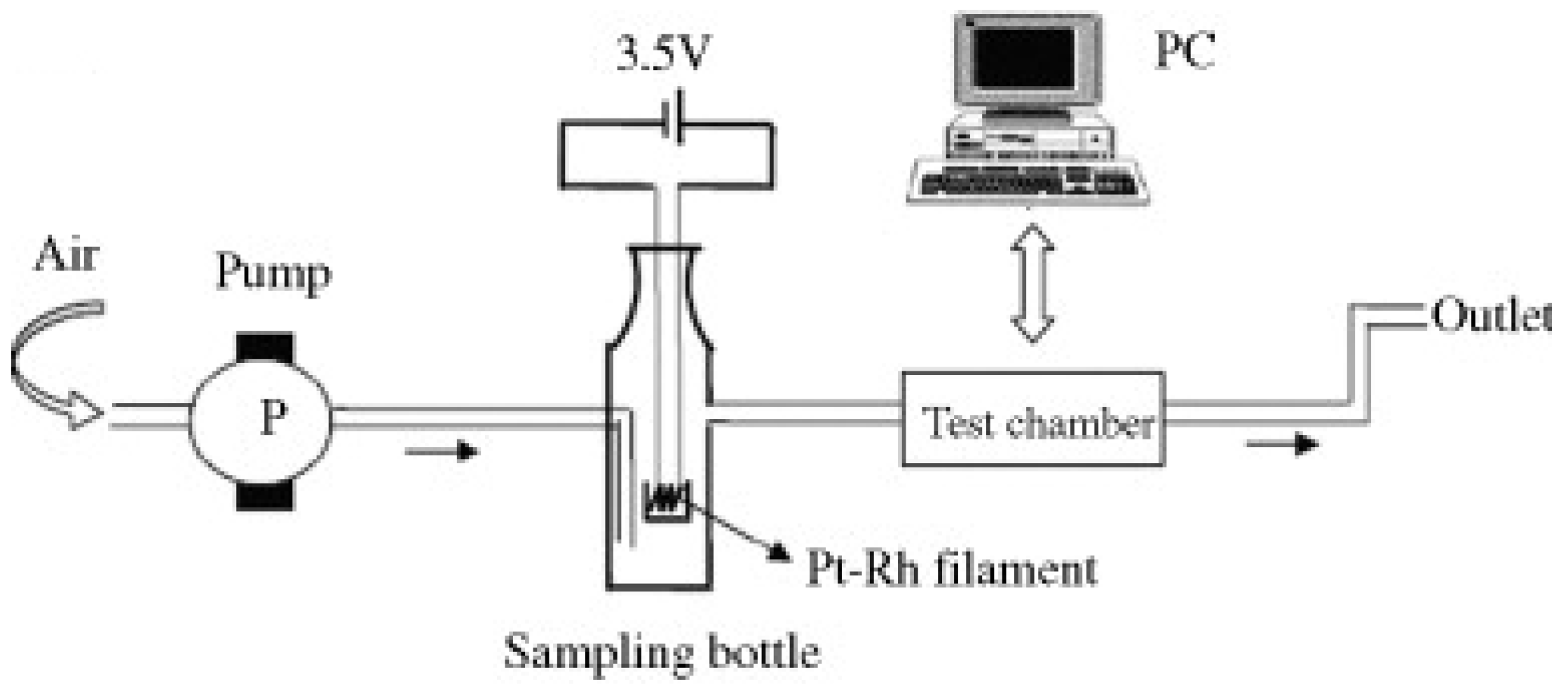


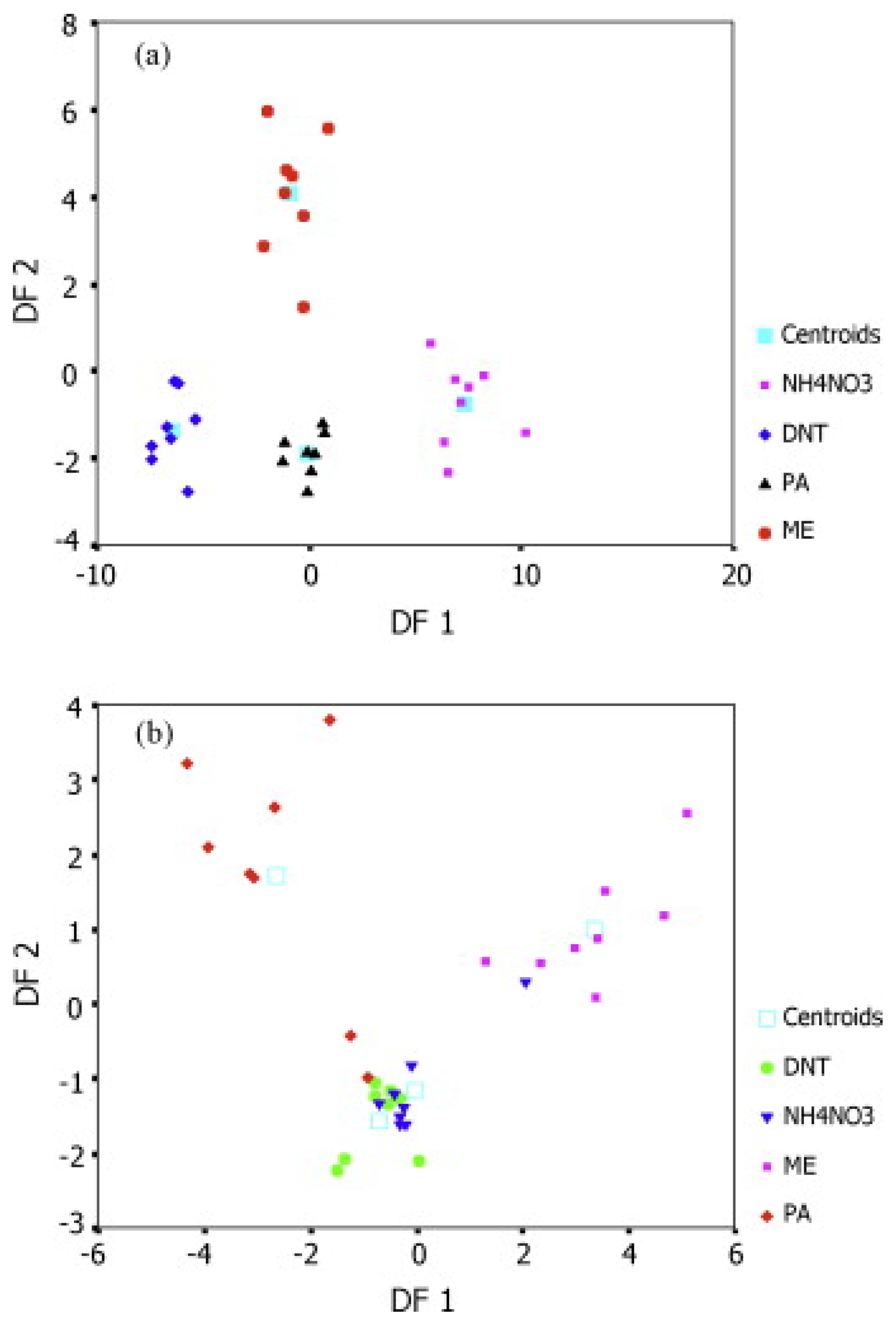
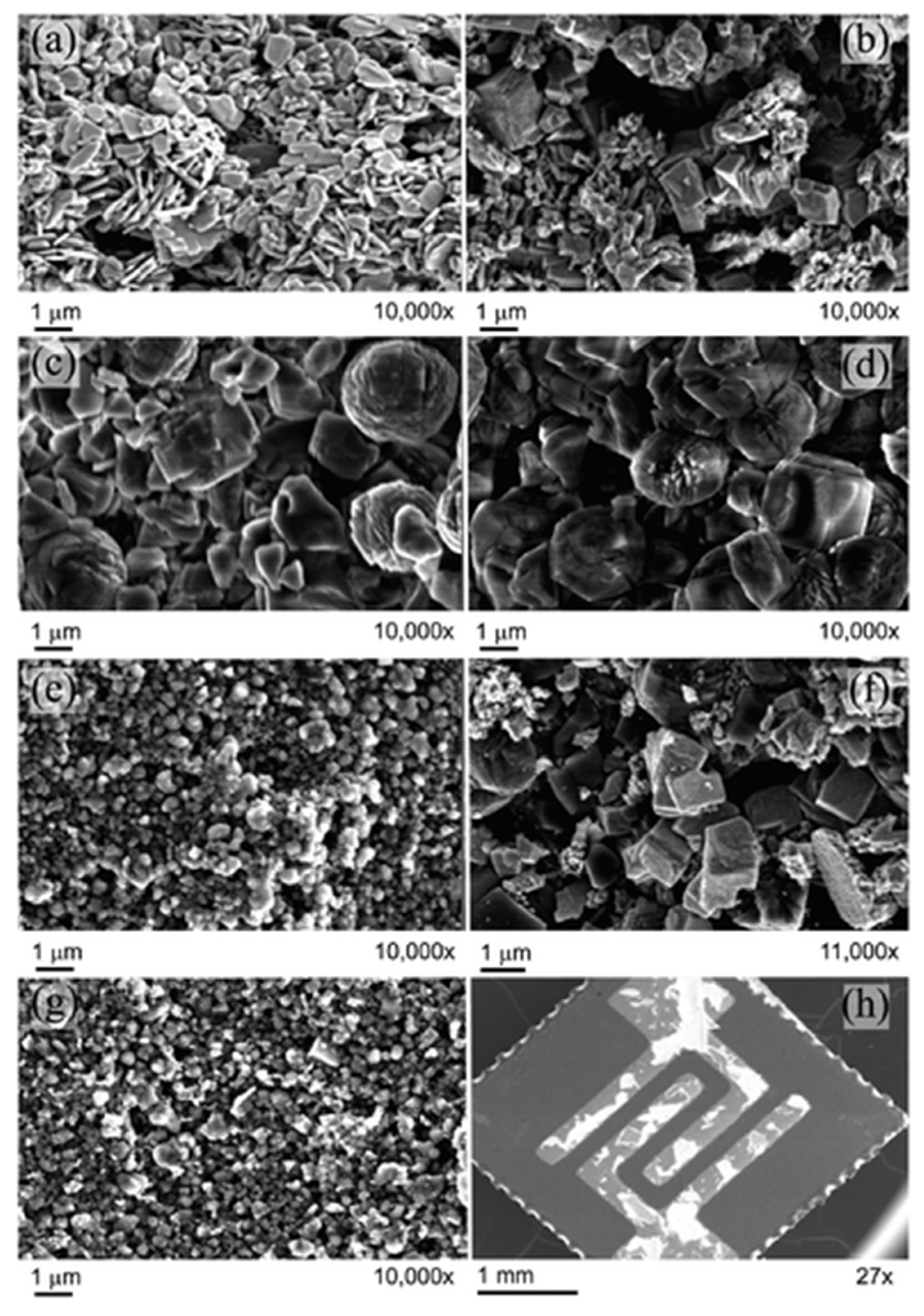

| Name | Aggregate Form | Ea (eV) | Vapor Pressure at 25 °C (ppb) | Melting Point (°C) | Boiling Point (°C) | Product Gas |
|---|---|---|---|---|---|---|
| TNT | Solid | ~2.3 | 9.55 | 80 | 295 (igniting) | NO2 |
| RDX | Solid | ~1.2 | 0.006 | 205 | 235 | NO2 |
| PETN | Solid | ~1.6 | 0.18 | 141 | 150 (decomposes) | NO2 |
| AN | Solid | 3.58 | 12.3 | 170 | 210 | NO2 |
| UNi | Solid | ~3.7 | 0.009 | 163 | unknown | NO2 |
| Gas | Name | Ea (eV) |
|---|---|---|
| N2 | Nitrogen | −0.72 |
| O2 | Oxygen | 0.448 |
| H2O | Water vapor | negative |
| CO2 | Carbon dioxide | −0.6 |
| H2, HC | Hydrogen, most hydrocarbons | negative |
| NO2 | Nitrogen dioxide | 2.273 |
| O3 | Ozone | 2.103 |
| Type of Metal Oxide | SMO |
|---|---|
| Binary oxides | ZnO, SnO2, In2O3, WO3, TiO2, Ga2O3, Fe2O3, CuO, NiO, ZrO2, Co3O4, Cr2O3, Mn3O4 |
| Ternary oxides and solid solutions | CdIn2O4, Cr2−xTixO3, NiTa2O6, CoTa2O6, CuTa2O6, BaSnO3, LaFeO3, CdFe2O4, Bi2Sn2O7, BixMoyO2, Sn1−xFexOy, NiFe2O4, CaFe2O4, ZnFe2O4; SnO2-Fe2O3, In2O3-Fe2O3, SnO2-CuO, SnO2-AgOx, In2O3-Ga2O3; TiO2-NiO; TiO2-V2O5; ZnO-CuO; SnO2-SiO2 |
| More complex oxides | Li-SmFe2O4, YBa2Cu3O7−δ, Ni0.99Co0.01M0.01Fe1.99O4, BaSn0.95Zr0.05O3, Na0.1Nb0.1W0.8O3, CS4SiW12O40, CoMn0.65Fe1.35O4, BaTiO3-CuO-La2O3; In2O3-SnO2-TiO2; In2O3-ZnO-SnO2 |
| Sensor’s Number | Components of Raw Material | Ea (eV) |
|---|---|---|
| 1 | Zn (1% PdCl2) | Prepared from Zn nanoparticle flurry and then quick immersion of dry film into 1% PdCl2 solution and out. |
| 2 | Zn | Prepared from Zn nanoparticle flurry. |
| 3 | 1 at% TiO2 + Zn | Prepared from mixture flurry of TiO2 and Zn nanoparticles with atomic ratio Ti:Zn of 1:99. |
| 4 | 1 at% WO3 + Zn | Prepared from mixture flurry of WO3 and Zn nanoparticles with atomic ratio W:Zn of 1:99. |
| 5 | 5 at% V2O5 + Zn (1% PdCl2) | Prepared from mixture flurry of V2O5 and Zn nanoparticles with atomic ratio V:Zn of 5:95 and then quickly immersed into 1% PdCl2 solution and out. |
| 6 | 5 at% Sb2O3 + Zn | Prepared from mixture flurry of Sb2O3 and Zn nanoparticles with atomic ratio Sb:Zn of 5:95. |
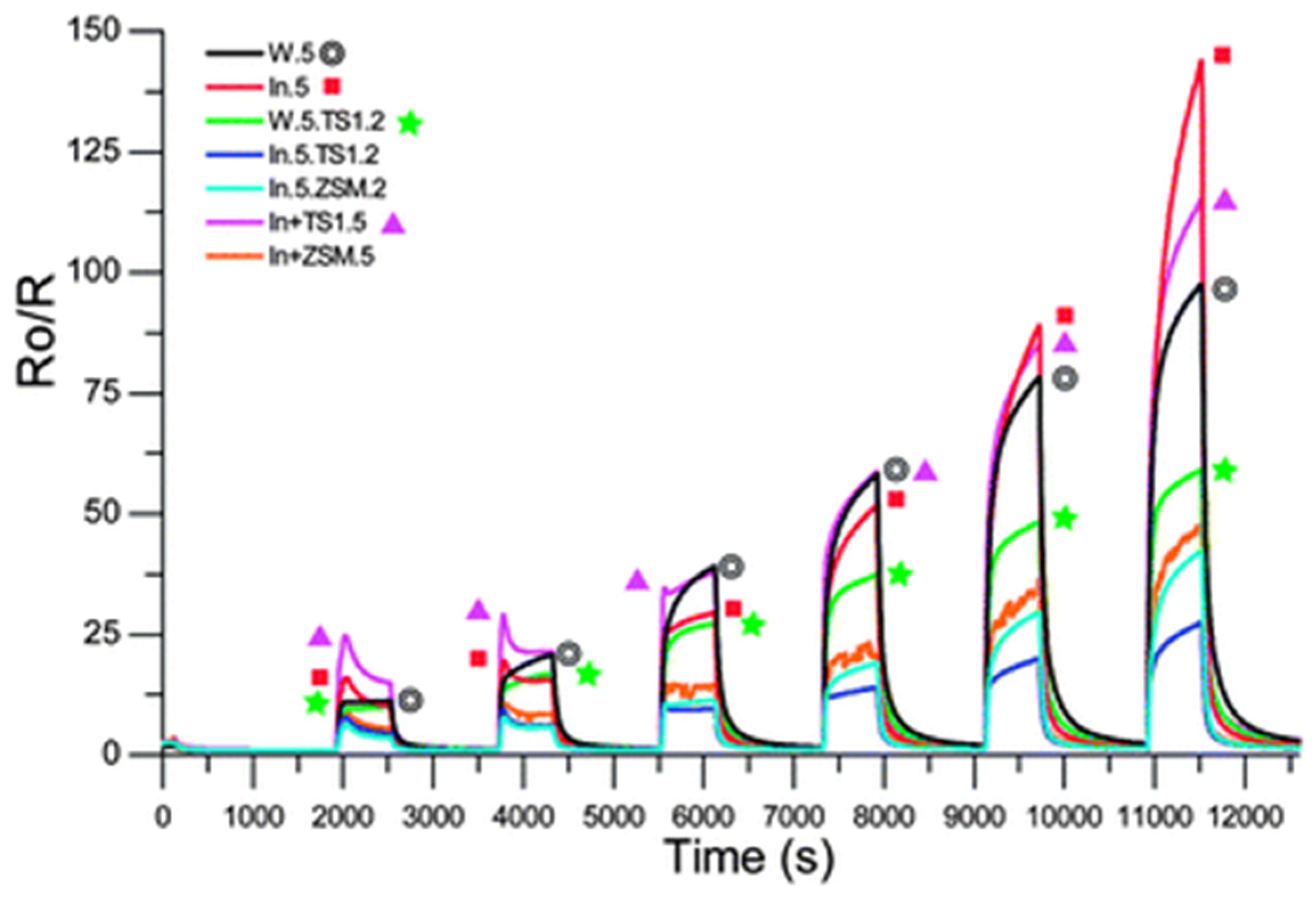
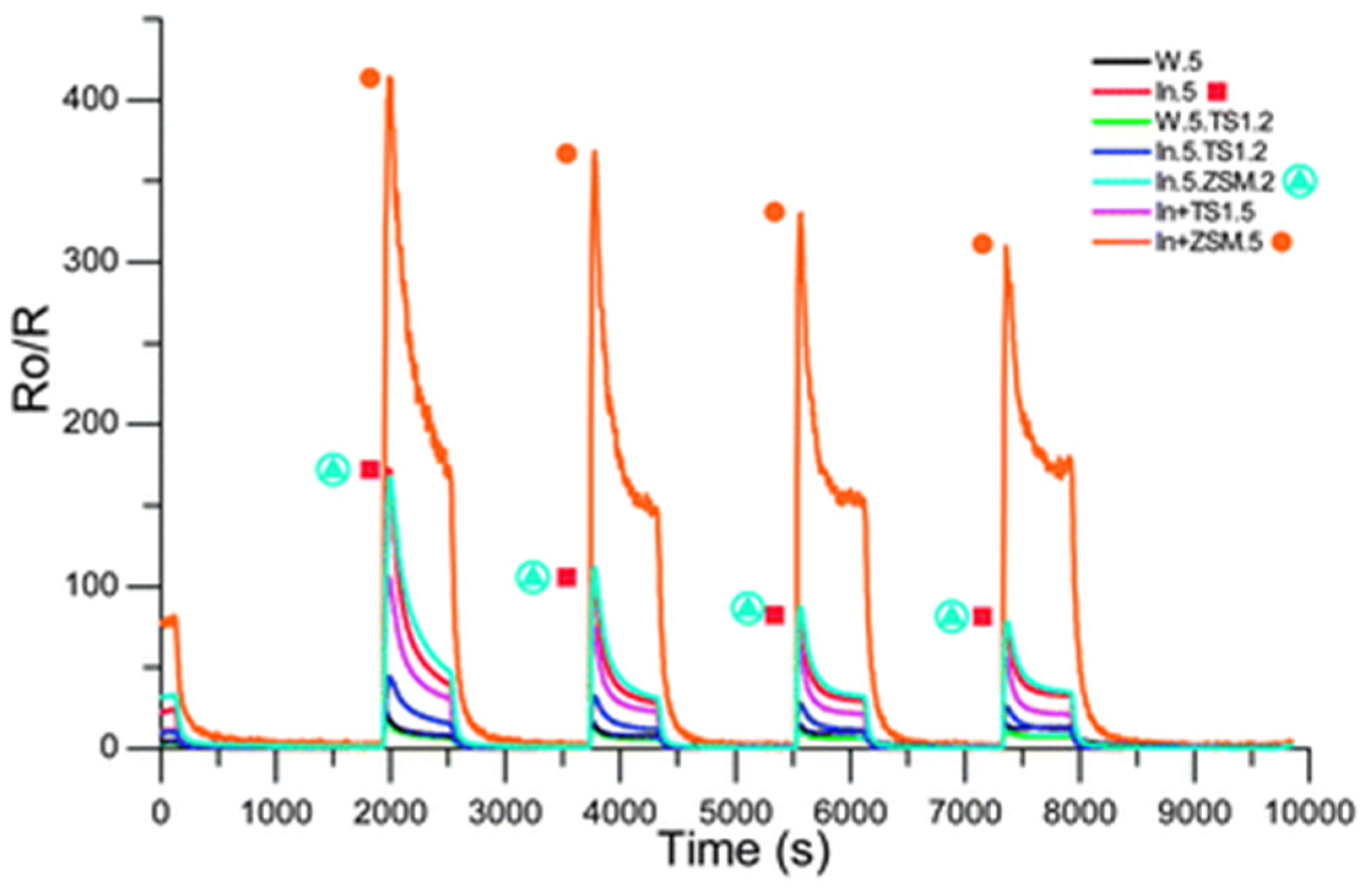
| Sensor | Metal Oxide | Overlay |
|---|---|---|
| W.5 | WO3 (5) | Nil |
| In.5 | In2O3 (5) | Nil |
| W.5.TS1.2 | WO3 (5) | TS-1 (2) |
| In.5.TS1.2 | In2O3 (5) | TS-1 (2) |
| In.5.ZSM.2 | In2O3 (5) | H-ZSM-5 (2) |
| In+TS1.5 | In2O3, 30% TS-1 (5) | Nil |
| In+ZSM.5 | In2O3, 30% H-ZSM-5 (5) | Nil |
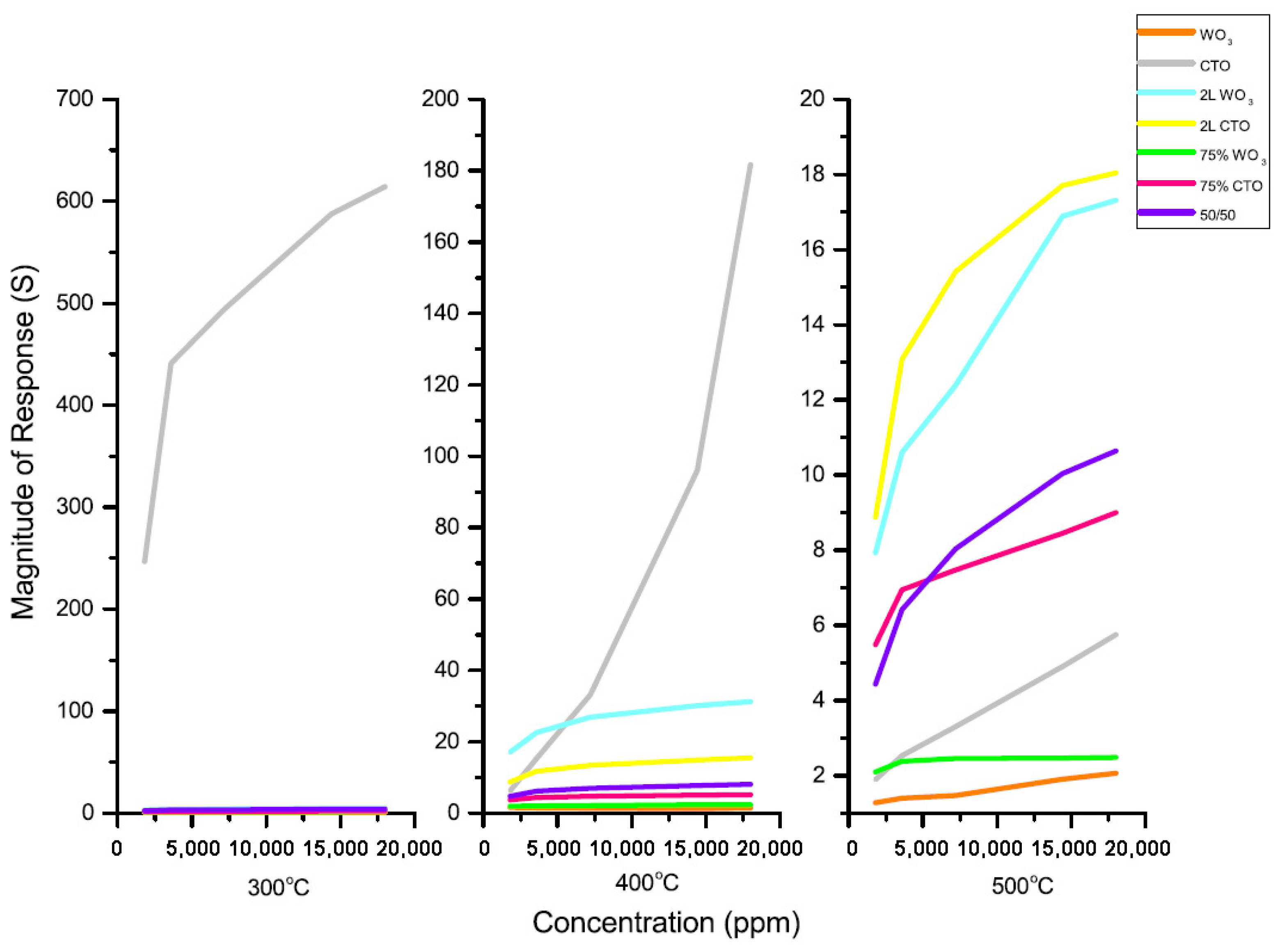
| Sensor Abbreviation | Metal Oxide (4 Layers) |
|---|---|
| WO3 | WO3 100% |
| CTO | Chromium titanium oxide (100%) |
| 2L WO3 | 2 layers of WO3 over 2 layers of CTO |
| 2L CTO | 2 layers of CTO over 2 layers of WO3 |
| 75% WO3 | 75% WO3, 25% CTO |
| 75% CTO | 75% CTO, 25% WO3 |
| 50/50 | 50% CTO, 50% WO3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchisio, A.; Tulliani, J.-M. Semiconducting Metal Oxides Nanocomposites for Enhanced Detection of Explosive Vapors. Ceramics 2018, 1, 98-119. https://doi.org/10.3390/ceramics1010009
Marchisio A, Tulliani J-M. Semiconducting Metal Oxides Nanocomposites for Enhanced Detection of Explosive Vapors. Ceramics. 2018; 1(1):98-119. https://doi.org/10.3390/ceramics1010009
Chicago/Turabian StyleMarchisio, Andrea, and Jean-Marc Tulliani. 2018. "Semiconducting Metal Oxides Nanocomposites for Enhanced Detection of Explosive Vapors" Ceramics 1, no. 1: 98-119. https://doi.org/10.3390/ceramics1010009
APA StyleMarchisio, A., & Tulliani, J.-M. (2018). Semiconducting Metal Oxides Nanocomposites for Enhanced Detection of Explosive Vapors. Ceramics, 1(1), 98-119. https://doi.org/10.3390/ceramics1010009





