The Apparent Resilience of the Dry Tropical Forests of the Nicaraguan Region of the Central American Dry Corridor to Variations in Climate Over the Last C. 1200 Years
Abstract
1. Introduction
1.1. Climate
1.2. Vegetation
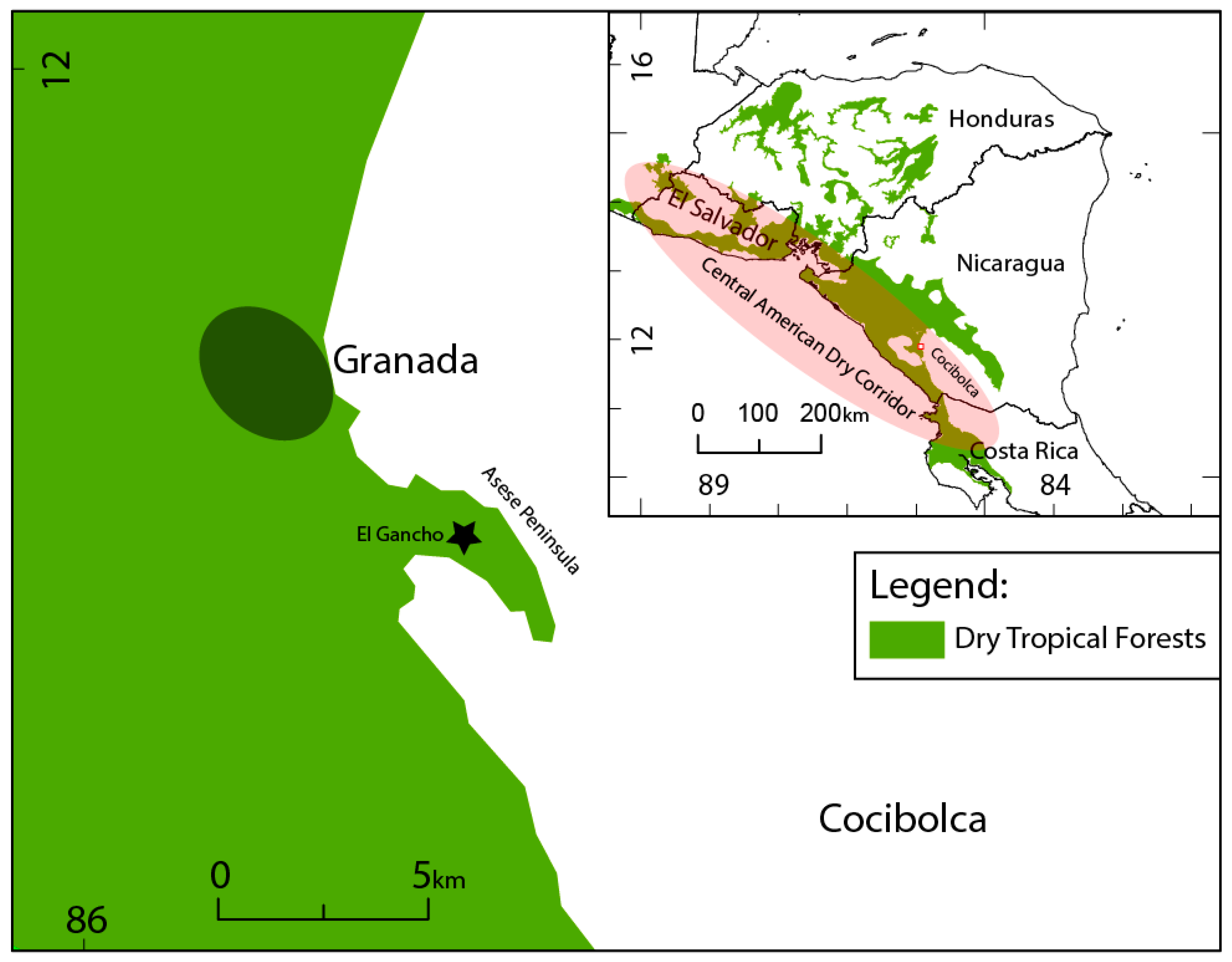
1.3. Study Area
1.4. El Gancho
2. Methods
2.1. Field and Sampling Techniques
2.2. Chronology
2.3. Diatom Analysis
2.4. Fossil Pollen and Dung-Spore Analysis
2.5. Macroscopic Charcoal Analysis
2.6. Data Handling
3. Results
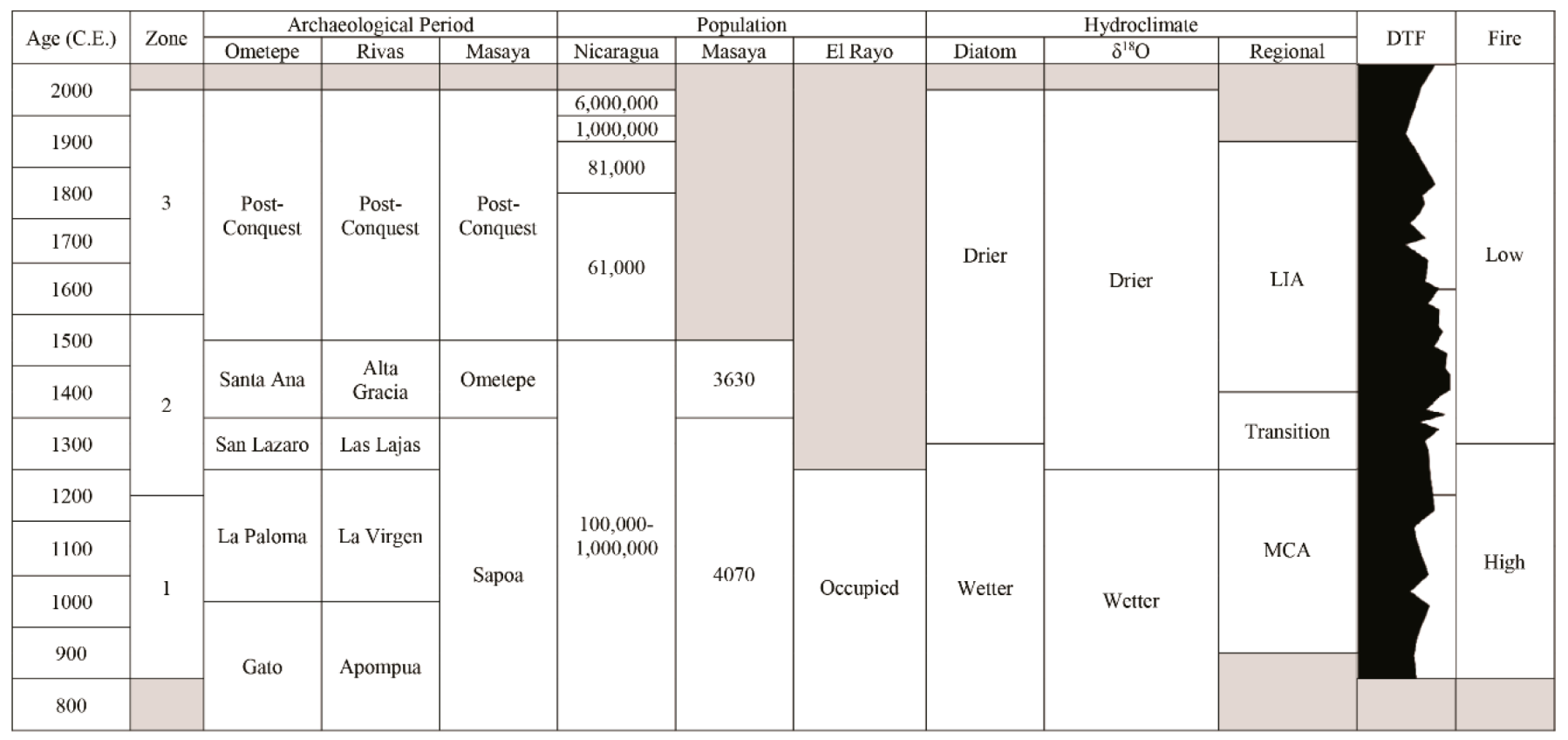
3.1. Chronology and Resolution
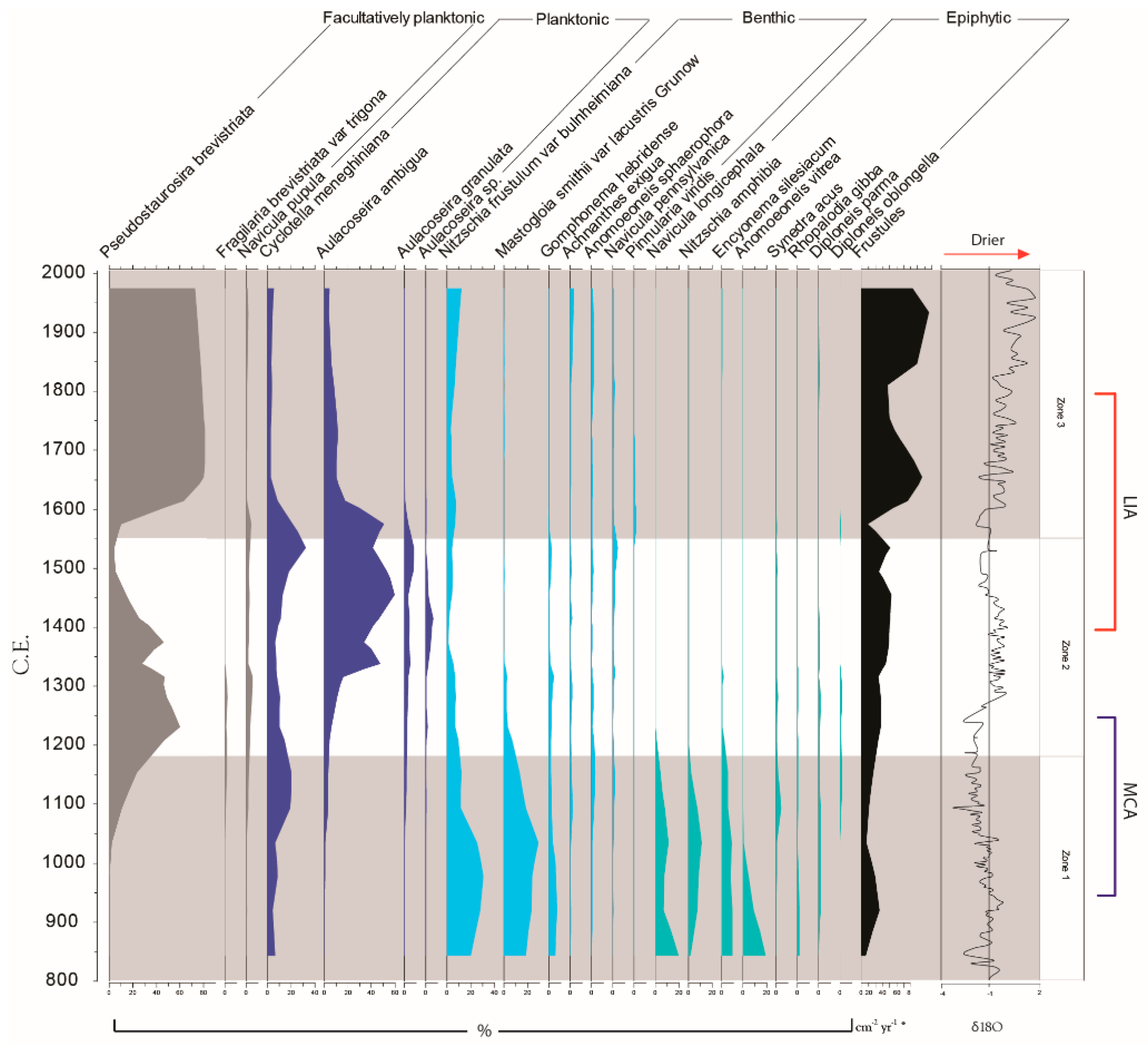
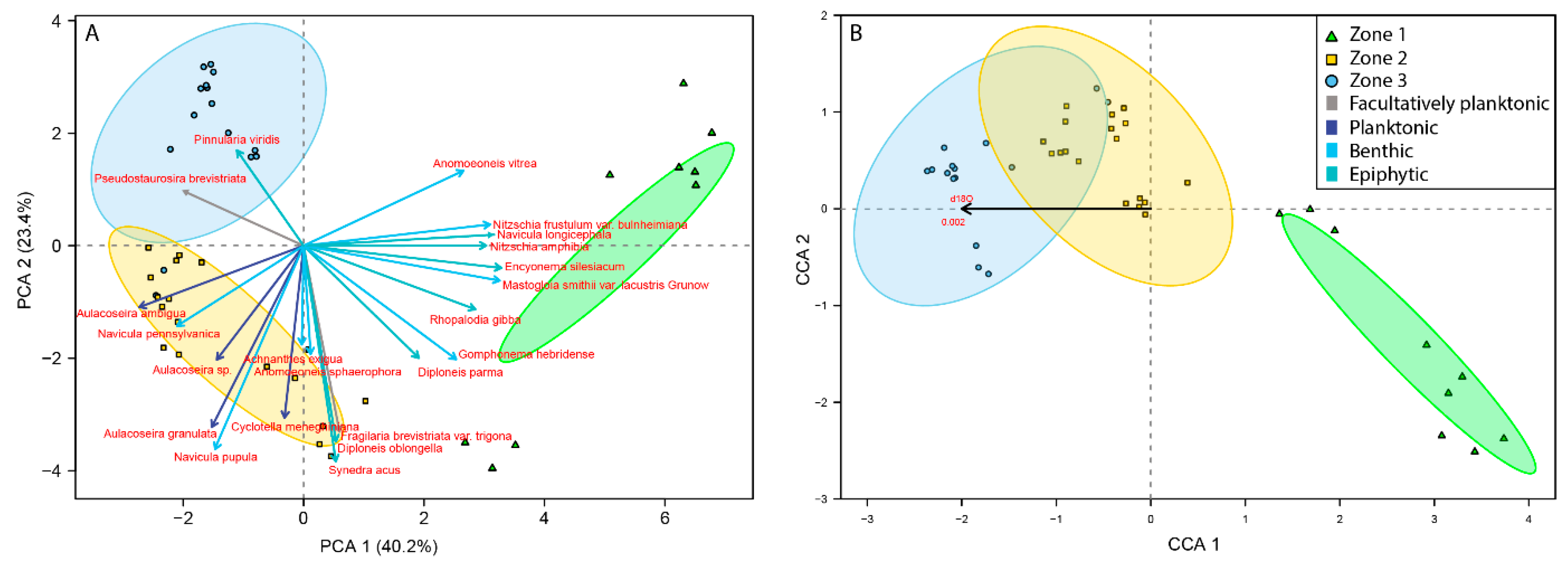
3.2. Palaeoecological Trends: Diatoms
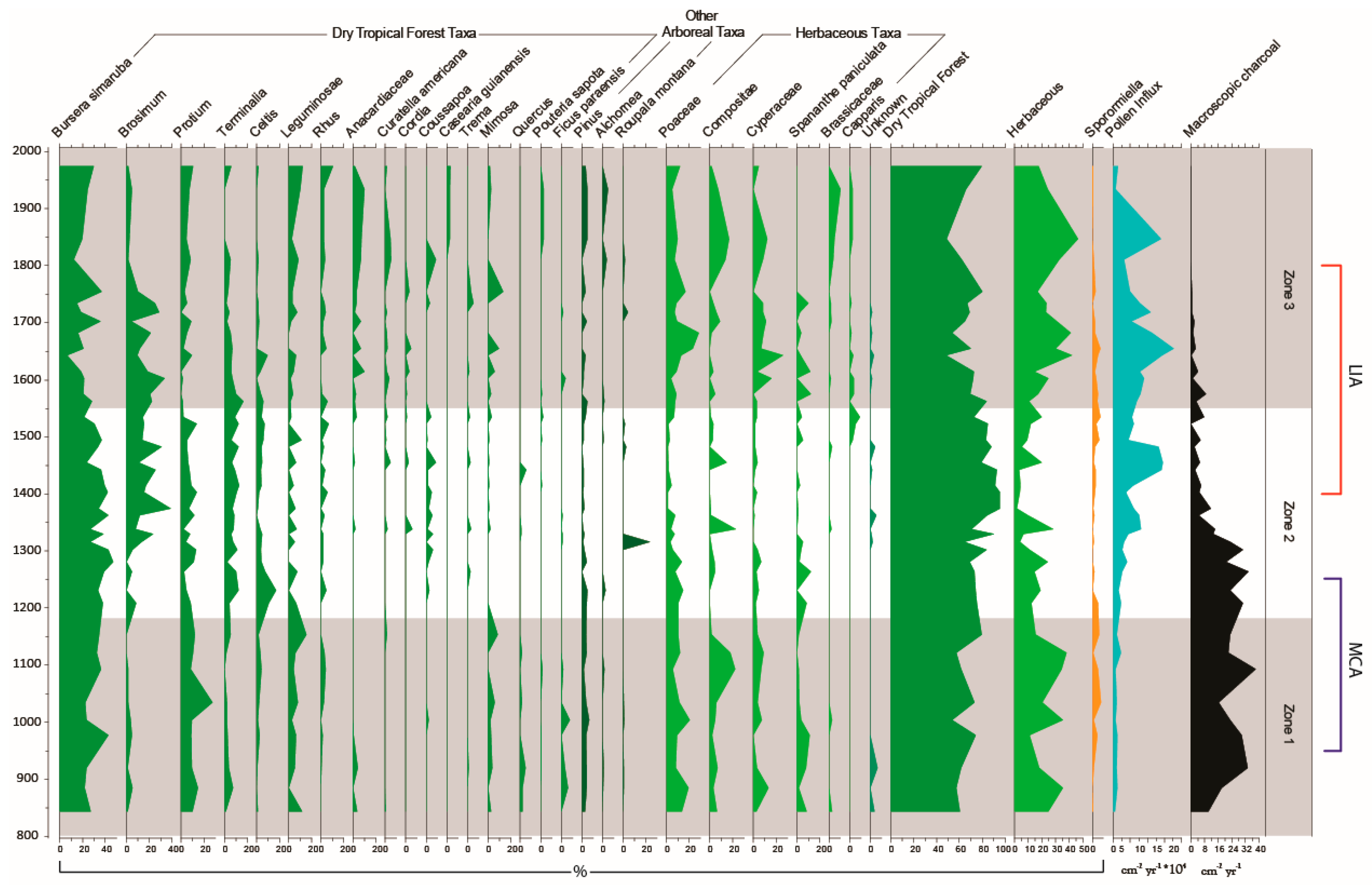
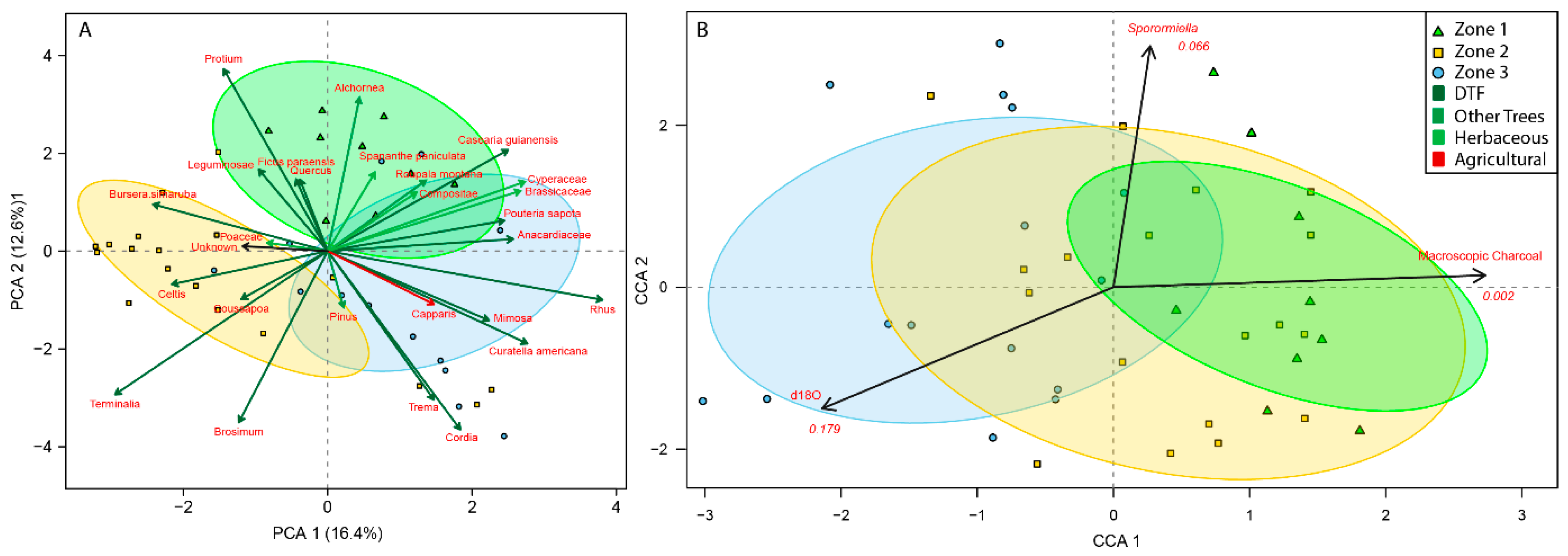
3.3. Palaeoecological Trends: Pollen, Sporormiella and Macroscopic Charcoal
4. Discussion
4.1. Hydroclimate Changes in the Nicaraguan Region of the Central American Dry Corridor during the Medieval Climate Anomaly and Little Ice Age
4.2. How Did the Dry Tropical Forests Respond to These Wet–Dry Climatic Shifts?
4.3. Anthropogenic Activity in the Dry Tropical Forests: Population and Land Management
4.4. Resilience of the Dry Tropical Forests Surrounding the Asese Peninsula
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wold Food Programme. Analysis of the Impact of the drought on food security in Guatemala, El Salvador, and Honduras. World Food Programme—Latin America and the Caribbean. Available online: https://documents.wforg/stellent/groups/public/documents/ena/wfp277948.pdf?_ga=2.194671341.1933972640.1559121603-515777643.1559121603 (accessed on 25 April 2018).
- Imbach, P.; Locatelli, B.; Zamora, J.C.; Fung, E.; Calderer, L.; Molina, L.; Ciais, P. Impacts of Climate Change on Ecosystem Hydrological Services of Central America: Water Availability. In Climate Change Impacts on Tropical Forests in Central America; Routledge: Abingdon, UK, 2015; pp. 81–106. [Google Scholar]
- Durán-Quesada, A.M.; Gimeno, L.; Amador, J. Role of moisture transport for Central American precipitation. Earth Syst. Dyn. 2017, 8, 147. [Google Scholar] [CrossRef]
- Hodell, D.A.; Curtis, J.H.; Brenner, M. Possible role of climate in the collapse of Classic Maya civilization. Nature 1995, 375, 391. [Google Scholar] [CrossRef]
- Hodell, D.A.; Brenner, M.; Curtis, J.H.; Medina-Gonzalez, R.; Can, E.I.C.; Albornaz-Pat, A.; Guilderson, T.P. Climate change on the Yucatan Peninsula during the little ice age. Quat. Res. 2005, 63, 109–121. [Google Scholar] [CrossRef]
- Hodell, D.A.; Brenner, M.; Curtis, J.H. Climate and cultural history of the northeastern Yucatan Peninsula, Quintana Roo, Mexico. Clim. Chang. 2007, 83, 215–240. [Google Scholar] [CrossRef]
- Curtis, J.H.; Hodell, D.A.; Brenner, M. Climate variability on the Yucatan Peninsula (Mexico) during the past 3500 years, and implications for Maya cultural evolution. Quat. Res. 1996, 46, 37–47. [Google Scholar] [CrossRef]
- Curtis, J.H.; Brenner, M.; Hodell, D.A.; Balser, R.A.; Islebe, G.A.; Hooghiemstra, H. A multi-proxy study of Holocene environmental change in the Maya lowlands of Peten, Guatemala. J. Paleolimnol. 1998, 19, 139–159. [Google Scholar] [CrossRef]
- Rosenmeier, M.F.; Hodell, D.A.; Brenner, M.; Curtis, J.H.; Martin, J.B.; Anselmetti, F.S.; Ariztegui, D.; Guilderson, T.P. Influence of vegetation change on watershed hydrology: Implications for paleoclimatic interpretation of lacustrine δ18O records. J. Paleolimnol. 2002, 27, 117–131. [Google Scholar] [CrossRef]
- Lachniet, M.S.; Burns, S.J.; Piperno, D.R.; Asmerom, Y.; Polyak, V.J.; Moy, C.M.; Christenson, K. A 1500-year El Niño/Southern Oscillation and rainfall history for the Isthmus of Panama from speleothem calcite. J. Geophys. Res. Atmos. 2004, 109. [Google Scholar] [CrossRef]
- Webster, J.W.; Brook, G.A.; Railsback, L.B.; Cheng, H.; Edwards, R.L.; Alexander, C.; Reeder, P.P. Stalagmite evidence from Belize indicating significant droughts at the time of Preclassic Abandonment, the Maya Hiatus, and the Classic Maya collapse. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 250, 1–17. [Google Scholar] [CrossRef]
- Metcalfe, S.; Breen, A.; Murray, M.; Furley, P.; Fallick, A.; McKenzie, A. Environmental change in northern Belize since the latest Pleistocene. J. Quat. Sci. 2009, 24, 627–641. [Google Scholar] [CrossRef]
- Carrillo-Bastos, A.; Islebe, G.A.; Torrescano-Valle, N.; González, N.E. Holocene vegetation and climate history of central Quintana Roo, Yucatán Península, Mexico. Rev. Palaeobot. Palynol. 2010, 160, 189–196. [Google Scholar] [CrossRef]
- Medina-Elizalde, M.; Burns, S.J.; Lea, D.W.; Asmerom, Y.; von Gunten, L.; Polyak, V.; Vuille, M.; Karmalkar, A. High resolution stalagmite climate record from the Yucatán Peninsula spanning the Maya terminal classic period. Earth Planet. Sci. Lett. 2010, 298, 255–262. [Google Scholar] [CrossRef]
- Velez, M.I.; Curtis, J.H.; Brenner, M.; Escobar, J.; Leyden, B.W.; Popenoe de Hatch, M. Environmental and cultural changes in highland Guatemala inferred from Lake Amatitlán sediments. Geoarchaeology 2011, 26, 346–364. [Google Scholar] [CrossRef]
- Stansell, N.D.; Steinman, B.A.; Abbott, M.B.; Rubinov, M.; Roman-Lacayo, M. Lacustrine stable isotope record of precipitation changes in Nicaragua during the Little Ice Age and Medieval Climate Anomaly. Geology 2013, 41, 151–154. [Google Scholar] [CrossRef]
- Kennett, D.J.; Breitenbach, S.F.; Aquino, V.V.; Asmerom, Y.; Awe, J.; Baldini, J.U.; Bartlein, P.; Culleton, B.J.; Ebert, C.; Jazwa, C.; et al. Development and disintegration of Maya political systems in response to climate change. Science 2012, 338, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Akers, D.; Brook, G.A.; Railsback, L.B.; Liang, F.; Iannone, G.; Webster, J.W.; Reeder, P.; Cheng, H.; Edwards, R.L. An extended and higher-resolution record of climate and land use from stalagmite MC01 from Macal Chasm, Belize, revealing connections between major dry events, overall climate variability, and Maya sociopolitical changes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 459, 268–288. [Google Scholar] [CrossRef]
- Correa-Metrio, A.; Vélez, M.I.; Escobar, J.; St-Jacques, J.M.; López-Pérez, M.; Curtis, J.; Cosford, J. Mid-elevation ecosystems of Panama: Future uncertainties in light of past global climatic variability. J. Quat. Sci. 2016, 31, 731–740. [Google Scholar] [CrossRef]
- Smyth, M.P.; Dunning, N.P.; Weaver, E.M.; van Beynen, P.; Zapata, D.O. The perfect storm: Climate change and ancient Maya response in the Puuc Hills region of Yucatán. Antiquity 2017, 91, 490–509. [Google Scholar] [CrossRef]
- Graham, N.E.; Hughes, M.K.; Ammann, C.M.; Cobb, K.M.; Hoerling, M.P.; Kennett, D.J.; Kennett, J.P.; Rein, B.; Stott, L.; Wigand, E.; et al. Tropical Pacific–mid-latitude teleconnections in medieval times. Clim. Chang. 2007, 83, 241–285. [Google Scholar] [CrossRef]
- Metcalfe, S.E.; Barron, J.A.; Davies, S.J. The Holocene history of the North American Monsoon: ‘Known knowns’ and ‘known unknowns’ in understanding its spatial and temporal complexity. Quat. Sci. Rev. 2015, 120, 1–27. [Google Scholar] [CrossRef]
- Richey, J.N.; Poore, R.Z.; Flower, B.P.; Quinn, T.M. 1400 yr multiproxy record of climate variability from the northern Gulf of Mexico. Geology 2007, 35, 423–426. [Google Scholar] [CrossRef]
- Haug, G.H.; Hughen, K.A.; Sigman, D.M.; Peterson, L.C.; Röhl, U. Southward migration of the intertropical convergence zone through the Holocene. Science 2001, 293, 1304. [Google Scholar] [CrossRef] [PubMed]
- Dinerstein, E.; Olson, D.; Joshi, A.; Vynne, C.; Burgess, N.D.; Wikramanayake, E.; Hahn, N.; Palminteri, S.; Hedao, P.; Noss, R.; et al. An ecoregion-based approach to protecting half the terrestrial realm. BioScience 2017, 67, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.; Nigh, R. Origins of the Maya forest garden: Maya resource management. J. Ethnobiol. 2009, 29, 213–236. [Google Scholar] [CrossRef]
- Dull, R.A. An 8000-year record of vegetation, climate, and human disturbance from the Sierra de Apaneca, El Salvador. Quat. Res. 2004, 61, 159–167. [Google Scholar] [CrossRef]
- Dull, R.A. A Holocene record of Neotropical savanna dynamics from El Salvador. J. Paleolimnol. 2004, 32, 219–231. [Google Scholar] [CrossRef]
- Dull, R.A. Evidence for Forest Clearance, Agriculture, and Human-Induced Erosion in Precolumbian El Salvador. Ann. Assoc. Am. Geogr. 2007, 97, 127–141. [Google Scholar] [CrossRef]
- Willis, K.J.; Jeffers, E.S.; Tovar, C. What makes a terrestrial ecosystem resilient? Science 2018, 359, 988–989. [Google Scholar] [CrossRef]
- Murphy, P.G.; Lugo, A.E. Ecology of tropical dry forest. Annual. Rev. Ecol. Syst. 1986, 17, 67–88. [Google Scholar] [CrossRef]
- Janzen, D. Management of habitat fragments in a tropical dry forest: Growth. Ann. Mo. Bot. Gard. 1988, 75, 105–116. [Google Scholar] [CrossRef]
- Gillespie, T.W.; Grijalva, A.; Farris, C.N. Diversity, composition, and structure of tropical dry forests in Central America. Plant Ecol. 2000, 147, 37–47. [Google Scholar] [CrossRef]
- Miles, L.; Newton, A.C.; DeFries, R.S.; Ravilious, C.; May, I.; Blyth, S.; Kapos, V.; Gordon, J.E. A global overview of the conservation status of tropical dry forests. J. Biogeogr. 2006, 33, 491–505. [Google Scholar] [CrossRef]
- Allen, K.; Dupuy, J.M.; Gei, M.G.; Hulshof, C.; Medvigy, D.; Pizano, C.; Salgado-Negret, B.; Smith, C.M.; Trierweiler, A.; Van Bloem, S.J.; et al. Will seasonally dry tropical forests be sensitive or resistant to future changes in rainfall regimes? Environ. Res. Lett. 2017, 12, 2. [Google Scholar] [CrossRef]
- Carpenter, S.; Walker, B.; Anderies, J.M.; Abel, N. From Metaphor to Measurement: Resilience of What to What? Ecosystems 2001, 4, 765–781. [Google Scholar] [CrossRef]
- Chiabai, A. (Ed.) Climate Change Impacts on Tropical Forests in Central America: An Ecosystem Service Perspective; Routledge: Abingdon, UK, 2015. [Google Scholar]
- Guevara-Murua, A.; Williams, C.A.; Hendy, E.J.; Imbach, P. 300 years of hydrological records and societal responses to droughts and floods on the Pacific coast of Central America. Clim. Past 2018, 14, 175–191. [Google Scholar] [CrossRef]
- Taylor, B.W. An outline of the vegetation of Nicaragua. J. Ecol. 1963, 51, 27–54. [Google Scholar] [CrossRef]
- González, R.B. Tree Species Diversity and Regeneration of Tropical Dry Forest in Nicaragua. Ph.D. Thesis, Swedish University of Agricultural Sciences, Umea, Sweden, 2005. [Google Scholar]
- Harcourt, C.S.; Sayer, J.A. The Conservation Atlas of Tropical Forest. The Americas; Simon & Schuster: New York, NY, USA, 1996; pp. 206–211. [Google Scholar]
- Denevan, W.M. The pristine myth: The landscape of the Americas in 1492. Annu. Assoc. Am. Geogr. 1992, 82, 369–385. [Google Scholar] [CrossRef]
- Cooke, R.; Ranere, A.J. Prehistoric human adaptations to the seasonally dry forests of Panama. World Archaeol. 1992, 24, 114–133. [Google Scholar] [CrossRef]
- Daniels, A.E.; Painter, K.; Southworth, J. Milpa imprint on the tropical dry forest landscape in Yucatan, Mexico: Remote sensing and field measurement of edge vegetation. Agric. Ecosyst. Environ. 2008, 123, 293–304. [Google Scholar] [CrossRef]
- Newson, L.A. Indian Survival in Colonial Nicaragua; University of Oklahoma Press: Norman, OK, USA, 1987. [Google Scholar]
- Denevan, W.M. The Native Population of the Americas in 1492; University of Wisconsin Press: Madison, WI, USA, 1987. [Google Scholar]
- Randell, D.H. The Indian Slave Trade and Population of Nicaragua during the Sixteenth Century. In The Native Population of the Americas in 1942; Denevan, W.M., Ed.; University of Wisconsin Press: Madison, WI, USA, 1976. [Google Scholar]
- Lovell, W.G.; Lutz, C.H. The Historical Demography of Colonial Central America. In Yearbook: Conference of Latin Americanist Geographers Conference of Latin Americanist Geographers; University of Texas Press: Austin, TX, USA, 1992; pp. 127–138. [Google Scholar]
- Kroeber, A.L. Cultural and Natural Areas of Native North America; University of California Publications in American Archaeology and Ethnology; University of California Press: Berkeley, CA, USA, 1939; Volume 38. [Google Scholar]
- MacLeod, M.J. Spanish Central America: A Socioeconomic History; University of California Press: Berkeley, CA, USA, 2010; pp. 1520–1720. [Google Scholar]
- Newson, L.A. The Cost of Conquest: Indian Decline in Honduras under Spanish Rule; Dellplain Latin American Studies; Westview Press: Boulder, CO, USA, 1986. [Google Scholar]
- Lange, F.W.; Haberland, W. The Archaeology of Pacific Nicaragua; University of New Mexico Press: Albuquerque, NM, USA, 1992. [Google Scholar]
- Healy, P.; Pohl, M. Archaeology of the Rivas Region, Nicaragua; Wilfrid Laurier University Press: Waterloo, ON, Canada, 1980. [Google Scholar]
- Vinogradov, A. Demography: The Population of the Countries of the World from Most Ancient Times to the Present Days; Statistical Tables, Russian Edition; CreateSpace Independent Publishing Platform: Scotts Valley, CA, USA; Volume 1, ISBN 1496106733.
- Worldometers. Nicaragua Population. 2014. Available online: http://www.worldometers.info/world-population/nicaragua-population/ (accessed on 25 April 2018).
- Janzen, D. Costa Rican Natural History; The University of Chicago Press: Chicago, IL, USA, 1983. [Google Scholar]
- Calvo-Alvarado, A.; McLennan, B.; Sanchez-Azofeifa, A.; Garvin, T. Deforestation and forest restoration in Guanacaste, Costa Rica: Putting conservation policies in context. J. For. Ecol. Manag. 2009, 258, 931–940. [Google Scholar] [CrossRef]
- Griscom, H.; Ashton, M.S. Restoration of dry tropical forests in Central America: A review of pattern and process. For. Ecol. Manag. 2011, 261, 1564–1579. [Google Scholar] [CrossRef]
- Murphy, G.; Lugo, A.E. Dry forests of Central America and the Caribbean. Seas. Dry Trop. For. 1995, 9–34. [Google Scholar] [CrossRef]
- Heckadon Moreno, S. Panama’s Expanding Cattle Front: The Santeno Campesinos and the Colonization of the Forests. Ph.D. Thesis, University of Essex, Colchester, UK, 1984. [Google Scholar]
- Sabogal, C. Regeneration of tropical dry forests in Central America, with examples from Nicaragua. J. Veg. Sci. 1992, 3, 407–414. [Google Scholar] [CrossRef]
- Roldan, H. Recursos Forestales y Cambio en el Uso de la Tierra; Republica de Nicaragua: Santiago, Chile, 2001; p. 73. [Google Scholar]
- Van Wyk de Vries, B.; Francis, W. Catastrophic collapse at stratovolcanoes induced by gradual volcano spreading. Nature 1997, 387, 387–390. [Google Scholar] [CrossRef]
- Stansell, N.D. Radiocarbon ages for the timing of debris avalanches at Mombacho Volcano, Nicaragua. Bull. Volcanol. 2013, 75, 1–4. [Google Scholar] [CrossRef]
- Montenegro-Guillén, S. Lake Cocibolca/Nicaragua: Lake Basin Management Initiative Experience and Lessons Learned Brief USAID; World Bank: Washington, DC, USA, 2003; p. 12. [Google Scholar]
- Beard, J.S. Montane vegetation in the Antilles. Caribb. For. 1942, 3, 61–74. [Google Scholar]
- Beard, J.S. Climax vegetation in tropical America. Ecology 1944, 25, 127–158. [Google Scholar] [CrossRef]
- Atwood, J.T. A floristic study of volcán Mombacho department of Granada, Nicaragua. Ann. MO Botanical Garden 1984, 71, 191–209. [Google Scholar] [CrossRef]
- Bellefontaine, R.; Petrucci, Y. Management of Natural Forests of Dry Tropical Zones: FAO Conservation Guide 32; Food and Agriculture Organization of the United Nations: Rome, Italy, 2000; Available online: http://www.fao.org/3/w4442e/w4442e00.htm (accessed on 16 July 2019).
- Biondi-Morra, B.N. Hungry Dreams: The Failure of Food Policy in Revolutionary Nicaragua, 1979–1990; Cornell University Press: Ithaca, NY, USA, 1993. [Google Scholar]
- UNIDA. Población Total 1993, Estimada al 30 de Junio del Año. Available online: http://www.inide.gob.ni/estadisticas/Cifras%20municipales%20a%C3%B1o%202012%20INIDE.pdf (accessed on 22 August 2018).
- Livingstone, D.A. A lightweight piston sampler for lake deposits. Ecology 1955, 36, 137–139. [Google Scholar] [CrossRef]
- Reimer, J.; Bard, E.; Bayliss, A.; Beck, J.W.; Blackwell, G.; Ramsey, C.B.; Brown, D.M.; Buck, C.E.; Edwards, R.L.; Friedrich, M.; et al. Selection and treatment of data for radiocarbon calibration: An update to the International Calibration (IntCal) criteria. Radiocarbon 2013, 55, 1923. [Google Scholar] [CrossRef]
- Berglund, B.E. Handbook of Holocene Palaeoecology and Palaeohydrology. J. Ecol. 1987, 75, 755. [Google Scholar]
- OxLEL, Diatom Preparation Procedure. Oxford Long-Term Ecology Lab. Protocols. Available online: https://oxlel.zoo.ox.ac.uk/wp-content/uploads/2014/04/OxLEL-diatom-protocol.pdf (accessed on 13 June 2018).
- Fleming, W.D. Naphrax: A synthetic mounting medium of high refractive Index new and improved methods of preparation. J. R. Microsc. Soc. 1954, 74, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Gasse, F. East African Diatoms: Taxonomy, Ecological Distribution; Cramer: Berlin, Germany, 1986; Volume 11. [Google Scholar]
- Round, F.E.; Crawford, R.M.; Mann, D.G. Diatoms: Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae: Teil 3: Centrales, Fragilariaceae, Eunotiaceae (Suesswasserflora von Mitteleuropa); Spektrum Akademischer Verlag: Berlin, Germany, 1991. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae: Teil 4: Achnanthaceae, Kritische Erganzungen Zu Achnanthes S.L., Navicula S.Str., Gomphonema (Suesswasserflora Von Mitteleuropa); Spektrum Akademischer Verlag: Berlin, Germany, 1991. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae: 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae (Suesswasserflora von Mitteleuropa); Spektrum Akademischer Verlag: Berlin, Germany, 1997. [Google Scholar]
- Lange-Bertalot, H.; Krammer, K. Süßwasserflora von Mitteleuropa, Bd. 02/1: Bacillariophyceae, 1. Teil: Naviculaceae, A: Text; B: Tafeln; Spektrum Akademischer Verlag: Berlin, Germany, 1997. [Google Scholar]
- Lange-Bertalot, H.; Krammer, K.; Bate, N. Süßwasserflora Von Mitteleuropa, Bd. 02/5: Bacillariophyceae: Teil 5: English And French Translation Of The Keys; Spektrum Akademischer Verlag: Berlin, Germany, 2000. [Google Scholar]
- OxLEL, Pollen Preparation Procedure. Oxford Long-Term Ecology Lab. Protocols. Available online: https://oxlel.zoo.ox.ac.uk/wp-content/uploads/2016/10/OxLEL-Fossil-pollen-extraction-protocol.pdf (accessed on 13 June 2018).
- Leyden, B.W. Pollen evidence for climatic variability and cultural disturbance in the Maya lowlands. Anc. Mesoam. 2002, 13, 85–101. [Google Scholar] [CrossRef]
- Bush, M.B.; Weng, C. Introducing a new (freeware) tool for palynology. J. Biogeogr. 2007, 34, 377–380. [Google Scholar] [CrossRef]
- APSA. The Australasian Pollen and Spore Atlas V1.0; Australian National University: Canberra, Australia, 2007; Available online: http://apsa.anu.edu.au/ (accessed on 13 June 2018).
- Martin, A.C.; Harvey, W.J. The Global Pollen Project: A new tool for pollen identification and the dissemination of physical reference collections. Methods Ecol. Evol. 2017, 8, 892–897. [Google Scholar] [CrossRef]
- Roubik, D.W.; Moreno, P. Pollen and spores of Barro Colorado Island (Panama). Pollen Spores Barro Colo. Isl. 1991, 4, 36. [Google Scholar]
- Willard, D.A.; Bernhardt, C.E.; Weimer, L.; Cooper, S.R.; Gamez, D.; Jensen, J. Atlas of pollen and spores of the Florida Everglades. Palynology 2004, 28, 175–227. [Google Scholar] [CrossRef]
- Bush, M.B. Neotropical plant reproductive strategies and fossil pollen representation. Am. Nat. 1995, 145, 594–609. [Google Scholar] [CrossRef]
- Bensch, C.N.; Horak, M.J.; Peterson, D. Interference of redroot pigweed (Amaranthus retroflexus L.), Palmer amaranth (A. palmeri), and common waterhemp (A. rudis) in soybean. Weed Sci. 2003, 51, 37–43. [Google Scholar] [CrossRef]
- Davis, O.K.; Shafer, D.S. Sporormiella fungal spores, a palynological means of detecting herbivore density. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 237, 40–50. [Google Scholar] [CrossRef]
- Baker, A.G.; Cornelissen, P.; Bhagwat, S.A.; Vera, F.W.; Willis, K.J. Quantification of population sizes of large herbivores and their long-term functional role in ecosystems using dung fungal spores. Methods Ecol. Evol. 2016, 7, 1273. [Google Scholar] [CrossRef]
- Gavin, D.G.; Brubaker, L.B.; Lertzman, K.P. An 1800-year record of the spatial and temporal distribution of fire from the west coast of Vancouver Island, Canada. Can. J. For. Res. 2003, 33, 573–586. [Google Scholar] [CrossRef]
- Lynch, J.A.; Clark, J.S.; Stocks, B.J. Charcoal production, dispersal, and deposition from the Fort Providence experimental fire: Interpreting fire regimes from charcoal records in boreal forests. Can. J. For. Res. 2004, 34, 1642. [Google Scholar] [CrossRef]
- Peters, M.E.; Higuera, P.E. Quantifying the source area of macroscopic charcoal with a particle dispersal model. Quat. Res. 2007, 67, 304–310. [Google Scholar] [CrossRef]
- Higuera, E.; Peters, M.E.; Brubaker, L.B.; Gavin, D.G. Understanding the origin and analysis of sediment-charcoal records with a simulation model. Quat. Sci. Rev. 2007, 26, 1790. [Google Scholar] [CrossRef]
- Higuera, E.; Gavin, D.G.; Bartlein, J.; Hallett, D.J. Peak detection in sediment–charcoal records: Impacts of alternative data analysis methods on fire-history interpretations. Int. J. Wildland Fire 2011, 19, 996. [Google Scholar] [CrossRef]
- Anderson, L.; Wahl, D. Two Holocene paleofire records from Peten, Guatemala: Implications for natural fire regime and prehispanic Maya land use. Glob. Planet. Chang. 2016, 138, 82–92. [Google Scholar] [CrossRef]
- Whitlock, C.; Anderson, R.S. Fire History Reconstructions Based on Sediment Records from Lakes and Wetlands. In Fire and Climatic Change in Temperate Ecosystems of the Western AMERICAS; Springer: New York, NY, USA, 2003; pp. 3–31. [Google Scholar]
- Maher, L.J. Statistics for microfossil concentration measurements employing samples spiked with marker grains. Rev. Palaeobot. Palynol. 1981, 32, 153–191. [Google Scholar] [CrossRef]
- Bennett, J.P. Concepts of mathematical modeling of sediment yield. Water Resour. Res. 1974, 10, 485–492. [Google Scholar] [CrossRef]
- Bennett, K.D.; Willis, K.J. Pollen. In Tracking Environmental Change Using Lake Sediments: Terrestrial, Algal, and Siliceous Indicators; Springer: Dordrecht, The Netherlands, 2001; Volume 3. [Google Scholar]
- Whitlock, C.; Larsen, C. Charcoal as a Fire Proxy. In Tracking Environmental Change Using Lake Sediments; Springer: Dordrecht, The Netherlands, 2002; pp. 75–97. [Google Scholar]
- Bennett, K.D. Determination of the number of zones in a biostratigraphical sequence. New Phytol. 1996, 132, 155–170. [Google Scholar] [CrossRef]
- Ter Braak, C.J.; Prentice, I.C. A Theory of Gradient Analysis. Adv. Ecol. Res. 1988, 18, 271–317. [Google Scholar]
- Murdoch, D.J.; Chow, E.D. A graphical display of large correlation matrices. Am. Stat. 1996, 50, 178–180. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H.; et al. Package ‘vegan’. Available online: https://cran.ism.ac.jp/web/packages/vegan/vegan.pdf (accessed on 25 April 2018).
- Juggins, S. Rioja: Analysis of Quaternary Science Data, R Package Version 0.9-5, The Comprehensive R Archive Network. 2015. Available online: https://cran.r-project.org/web/packages/rioja/index.html (accessed on 25 April 2018).
- Brown, K.J.; Hebda, R.J. Coastal rainforest connections disclosed through a Late Quaternary vegetation, climate, and fire history investigation from the Mountain Hemlock Zone on southern Vancouver Island, British Colombia, Canada. Rev. Palaeobot. Palynol. 2003, 123, 247–269. [Google Scholar] [CrossRef]
- Bush, M.B.; De Oliveira, E.; Colinvaux, A.; Miller, M.C.; Moreno, J.E. Amazonian paleoecological histories: One hill, three watersheds. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 214, 359–393. [Google Scholar] [CrossRef]
- Figueroa-Rangel, B.L.; Willis, K.J.; Olvera-Vargas, M. Late-Holocene successional dynamics in a transitional forest of west-central Mexico. Holocene 2012, 22, 143–153. [Google Scholar] [CrossRef]
- Wang, L.; Lu, H.; Liu, J.; Gu, Z.; Mingram, J.; Chu, G.; Li, J.; Rioual, P.; Negendank, J.F.; Han, J.; et al. Diatom-based inference of variations in the strength of Asian winter monsoon winds between 17,500 and 6000 calendar years BP. J. Geophys. Res. Atmos. 2008, 113. [Google Scholar] [CrossRef]
- McCafferty, G.G.; McCafferty, S.D. Bling Things: Ornamentation and Identity in Pacific Nicaragua. In Identity Crisis: Archaeological Perspectives on Social Identity; Chacmool Archaeological Association: Calgary, AB, Canada, 2011; pp. 243–252. [Google Scholar]
- Roman-Lacayo, M. Social and Environmental Risk and the Development of Social Complexity in Precolumbian Masaya, Nicaragua. Ph.D. Thesis, University of Pittsburgh, Pittsburgh, PA, USA, 2013. [Google Scholar]
- Avnery, S.; Dull, R.A.; Keitt, T.H. Human versus climatic influences on late-Holocene fire regimes in southwestern Nicaragua. Holocene 2011, 21, 699–706. [Google Scholar] [CrossRef]
- White, C.D. Reconstructing Ancient Maya Diet; University of Utah Press: Salt Lake City, UT, USA, 1999. [Google Scholar]
- Schnetzler, K.A. Food Uses and Amaranth Product Research: A Comprehensive Review. In Amaranth Biology, Chemistry, and Technology; CRC Press: Boca Raton, FL, USA, 2018; pp. 155–184. [Google Scholar]
- Rivera, D.; Inocencio, C.; Obon, C.; Carreno, E.; Reales, A.; Alcaraz, F. Archaeobotany of capers (Capparis)(Capparaceae). Veg. Hist. Archaeobot. 2002, 11, 295–314. [Google Scholar] [CrossRef]
- Kaimowitz, D. Policies Affecting Deforestation for Cattle in Central America. In Sustainable Agriculture in Central America; Palgrave Macmillan: London, UK, 1997; pp. 51–66. [Google Scholar]
- McNeil, C.L. Deforestation, agroforestry, and sustainable land management practices among the Classic period Maya. Quat. Int. 2012, 249, 19–30. [Google Scholar] [CrossRef]
- Burns, E.B. Patriarch and Folk: The Emergence of Nicaragua, 1798–1858; Harvard University Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Cole, L.E.; Bhagwat, S.A.; Willis, K.J. Recovery and resilience of tropical forests after disturbance. Nat. Commun. 2014, 5, 3906. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, S.; Liu, L.; Zhang, Y.; Li, S. Vulnerability of the global terrestrial ecosystems to climate change. Glob. Chang. Biol. 2018, 24, 4095. [Google Scholar] [CrossRef] [PubMed]
- Seddon, A.W.; Macias-Fauria, M.; Long, R.; Benz, D.; Willis, K.J. Sensitivity of global terrestrial ecosystems to climate variability. Nature 2016, 531, 229. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, R.; Good, P.; Martin, G.; Rowell, D.P. Large rainfall changes consistently projected over substantial areas of tropical land. Nat. Clim. Chang. 2015, 6, 177–181. [Google Scholar] [CrossRef]
- Feng, X.; Porporato, A.; Rodriguez-Iturbe, I. Changes in rainfall seasonality in the tropics. Nat. Clim. Chang. 2013, 3, 811. [Google Scholar] [CrossRef]
- Greve, P.; Orlowsky, B.; Mueller, B.; Sheffield, J.; Reichstein, M.; Seneviratne, S.I. Global assessment of trends in wetting and drying over land. Nat. Geosci. 2014, 7, 716. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Holbrook, N.M.; Gutierrez, M.V. Hydraulic and photosynthetic co-ordination in seasonally dry tropical forest trees. Plant Cell Environ. 2002, 25, 1435. [Google Scholar] [CrossRef]
- Scholz, G.; Liebner, F.; Koch, G.; Bues, C.T.; Günther, B.; Bäucker, E. Chemical, anatomical and technological properties of Snakewood (Brosimum guianense (Aubl.) Huber). Wood Sci. Technol. 2007, 41, 673. [Google Scholar] [CrossRef]
- Borchert, R. Soil and stem water storage determine phenology and distribution of tropical dry forest trees. Ecology 1994, 75, 1437. [Google Scholar] [CrossRef]
- David, T.S.; Pinto, C.A.; Nadezhdina, N.; David, J.S. Water and forests in the Mediterranean hot climate zone: A review based on a hydraulic interpretation of tree functioning. For. Syst. 2016, 25, 1–14. [Google Scholar] [CrossRef]

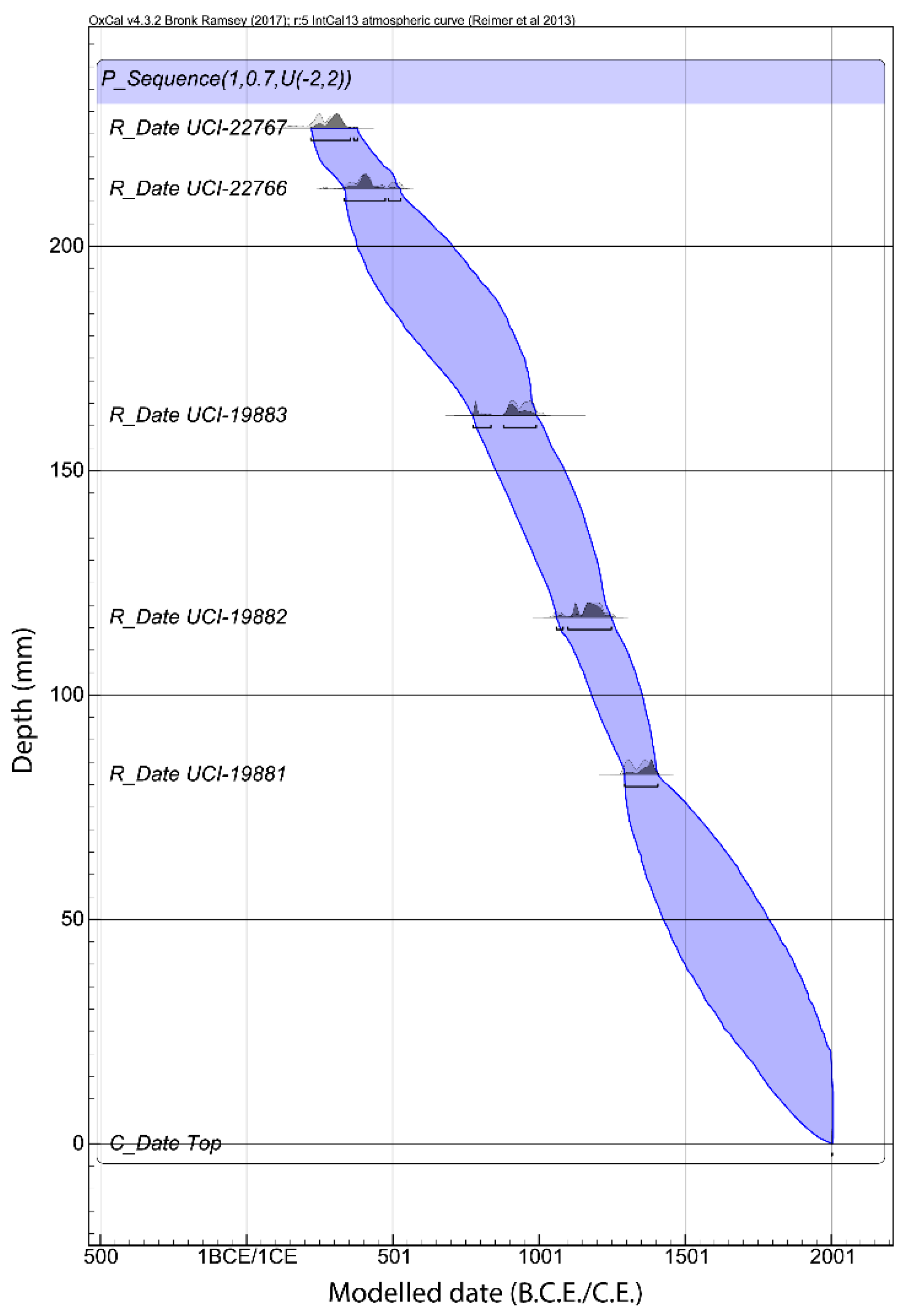
| Lab # | Measured Age (14C cal B.P.) | Depth (cm) | 2σ Calibrated Age Range (A.D.) | 2σ Median Calibrated Age | 2σ OxCal Modelled Age (A.D.) | Model Agreement Index | Median Modelled Age | |||
|---|---|---|---|---|---|---|---|---|---|---|
| UCI-19881 | 630 | ± 35 | 82.25 | 1286 | 1400 | 1343 | 1288 | 1405 | 99.1 | 1346.5 |
| UCI-19882 | 860 | ± 35 | 117.25 | 1046 | 1260 | 1153 | 1053 | 1253 | 105 | 1153 |
| UCI-19883 | 1100 | ± 30 | 162.25 | 887 | 1013 | 950 | 775 | 1012 | 90.7 | 893.5 |
| UCI-22766 | 1640 | ± 40 | 212.75 | 266 | 538 | 402 | 333 | 530 | 110.6 | 431.5 |
| UCI-22767 | 1770 | ± 30 | 226.25 | 123 | 345 | 234 | 186 | 385 | 103 | 285.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harvey, W.J.; Stansell, N.; Nogué, S.; Willis, K.J. The Apparent Resilience of the Dry Tropical Forests of the Nicaraguan Region of the Central American Dry Corridor to Variations in Climate Over the Last C. 1200 Years. Quaternary 2019, 2, 25. https://doi.org/10.3390/quat2030025
Harvey WJ, Stansell N, Nogué S, Willis KJ. The Apparent Resilience of the Dry Tropical Forests of the Nicaraguan Region of the Central American Dry Corridor to Variations in Climate Over the Last C. 1200 Years. Quaternary. 2019; 2(3):25. https://doi.org/10.3390/quat2030025
Chicago/Turabian StyleHarvey, William J., Nathan Stansell, Sandra Nogué, and Katherine J. Willis. 2019. "The Apparent Resilience of the Dry Tropical Forests of the Nicaraguan Region of the Central American Dry Corridor to Variations in Climate Over the Last C. 1200 Years" Quaternary 2, no. 3: 25. https://doi.org/10.3390/quat2030025
APA StyleHarvey, W. J., Stansell, N., Nogué, S., & Willis, K. J. (2019). The Apparent Resilience of the Dry Tropical Forests of the Nicaraguan Region of the Central American Dry Corridor to Variations in Climate Over the Last C. 1200 Years. Quaternary, 2(3), 25. https://doi.org/10.3390/quat2030025