Incidence, Risk Factors, and Prevention of Deep Vein Thrombosis in Acute Ischemic Stroke Patients (IRIS-DVT Study): A Systematic Review and Meta-Analysis
Abstract
1. Introduction
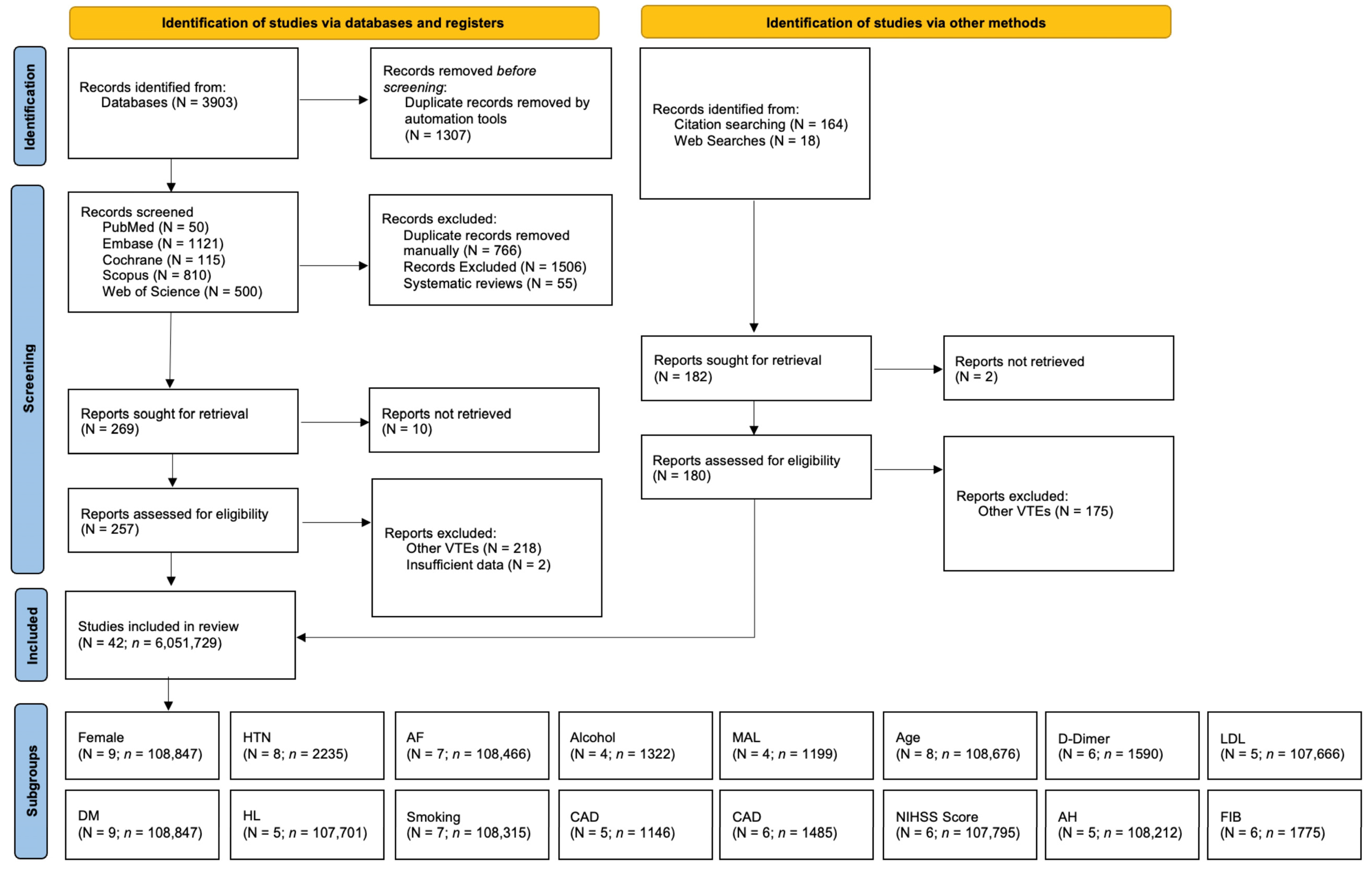
2. Materials and Methods
2.1. Literature Search and Study Selection
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Methodological Quality Assessment of Included Studies
2.5. Certainty of Evidence Assessment (Grading)
2.6. Statistical Analyses
3. Results
3.1. Description of Included Studies
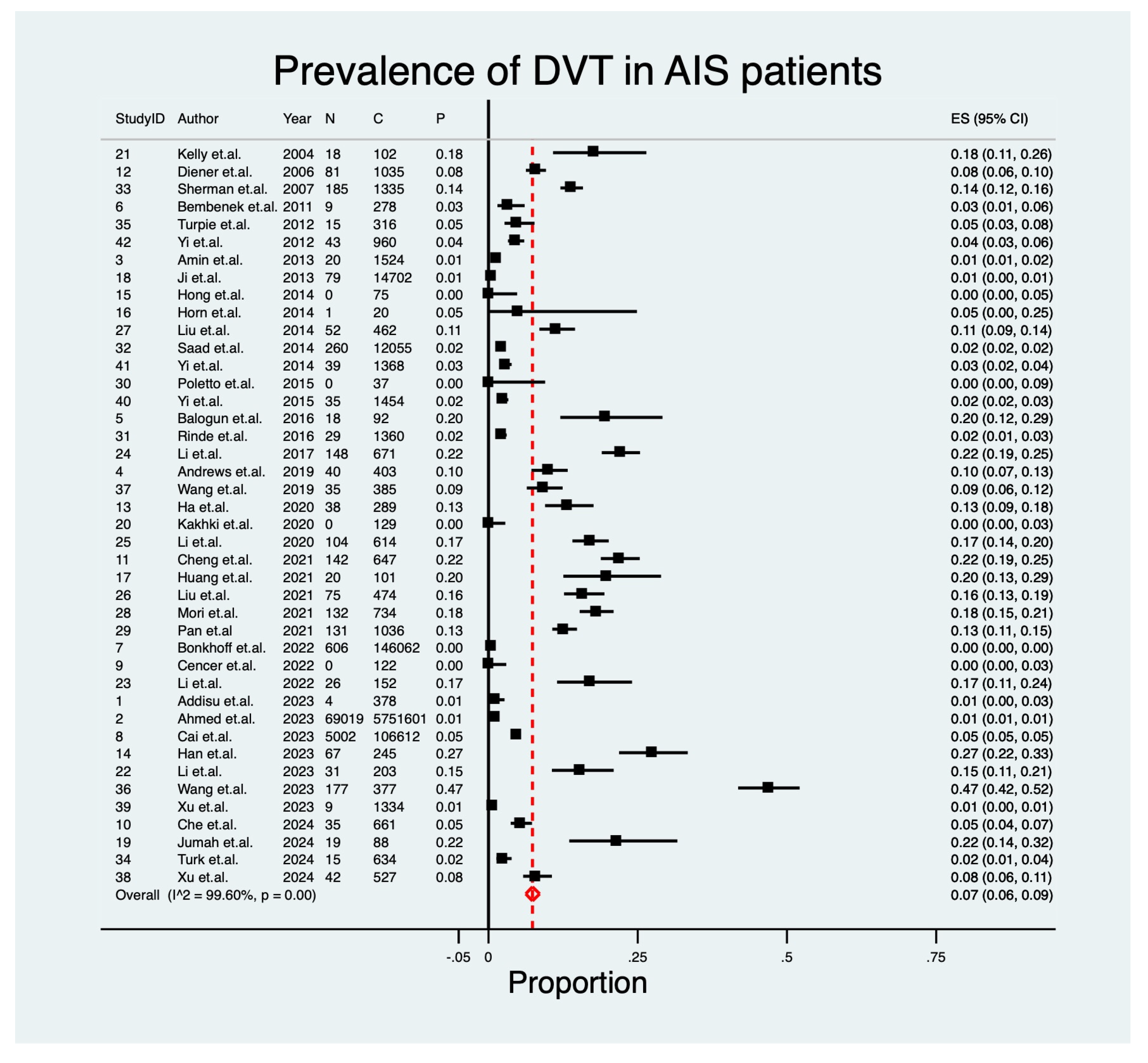
3.2. Pooled Prevalence of DVT in AIS
3.3. Predictive Indicators of DVT
| ID | Author | Year | Cohort Size | Crude Prevalence of DVT n (n%) | Country | Study Design | Primary Stroke Treatment | Immobilization Post Stroke? | DVT Diagnosis Modality | Diagnosis Days Post Thrombectomy (Median) | Chemical DVT Prophylaxis | Physical DVT Prophylaxis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Addisu et al. [32] | 2023 | 378 | 4 (1.1) | Ethiopia | Retrospective | No Acute Reperfusion | Unspecified | Medical Notes | During Hospitalization | Unspecified | Unspecified |
| 2 | Ahmed et al. [33] | 2023 | 5,751,601 | 69,019 (1.2) | United States | Retrospective | IV tPA or MT | Unspecified | Medical Notes | During Hospitalization | Unspecified | Unspecified |
| 3 | Amin et al. [34] | 2013 | 1524 | 20 (1.3) | United States | Retrospective | NA | Unspecified | Medical Notes | During Hospitalization | Variable | Variable |
| 4 | Andrews et al. [35] | 2019 | 403 | 40 (9.9) | United States | Retrospective | MT | Unspecified | Medical Notes | During Hospitalization | Unspecified | Unspecified |
| 5 | Balogun et al. [36] | 2016 | 92 | 18 (19.6) | United Kingdom | Retrospective | No Acute Reperfusion | Unspecified | CDU | Within 2 Weeks | Antiplatelet | Not Routine |
| 6 | Bembenek et al. [37] | 2011 | 269 | 9 (3.2) | Poland | Prospective | Unspecified | Unspecified | Color Doppler Ultrasound | Within 2 Weeks | Variable | Unspecified |
| 7 | Bonkhoff et al. [38] | 2022 | 146,062 | 606 (0.4) | Germany | Retrospective | IV tPA when possible | Unspecified | Medical Notes | During Hospitalization | Unspecified | Unspecified |
| 8 | Cai et al. [39] | 2023 | 106,612 | 5002 (4.7) | China | Retrospective | No Acute Reperfusion | Unspecified | Color Doppler Ultrasound | During Hospitalization | Variable | Variable |
| 9 | Cencer et al. [40] | 2022 | 122 | 0 (0.0) | United States | Retrospective | IV tPA | Unspecified | Medical Notes | During Hospitalization | Unspecified | Unspecified |
| 10 | Che et al. [41] | 2024 | 661 | 35 (5.3) | China | Retrospective | EVT | Unspecified | CDU | During Hospitalization | Unspecified | Unspecified |
| 11 | Cheng et al. [42] | 2021 | 431 | 142 (21.9) | China | Retrospective | Variable | Unspecified | CUS | Within 2 Weeks | Unspecified | Unspecified |
| 12 | Diener et al. [43] | 2006 | 1035 | 81 (7.8) | Multicenter | RCT | Variable | Unspecified | CDU | Within 2 Weeks | Antiplatelet or Anticoagulant | Unspecified |
| 13 | Ha et al. [44] | 2020 | 289 | 38 (13.1) | Korea | Retrospective | IV tPA when possible | Unspecified | CUS | Within 1 Week | Not Routine | Not Routine |
| 14 | Han et al. [5] | 2023 | 245 | 67 (27.3) | China | Retrospective | EVT | Yes | CDU | Within 2 Weeks | Antiplatelet | IPC |
| 15 | Hong et al. [45] | 2014 | 75 | 0 (0.0) | Korea | Prospective | Variable | Variable | CDU | During Hospitalization | Unspecified | Unspecified |
| 16 | Horn et al. [46] | 2014 | 20 | 1 (5.0) | United States | Prospective | MT | Yes | Medical Notes | During Hospitalization | Unspecified | Unspecified |
| 17 | Huang et al. [47] | 2021 | 101 | 20 (19.8) | China | Retrospective | NA | Unspecified | CDU | Within 2 Weeks | Not Routine | Unspecified |
| 18 | Ji et al. [48] | 2013 | 14,702 | 79 (0.5) | China | Retrospective | Unspecified | Unspecified | Medical Notes | During Hospitalization | Unspecified | Unspecified |
| 19 | Jumah et al. [49] | 2024 | 88 | 19 (21.6) | United States | Retrospective | Variable | Unspecified | Medical Notes | Within 72 h | IV Heparin | Unspecified |
| 20 | Kakhki et al. [50] | 2020 | 129 | 0 (0.0) | Iran | Retrospective | Unspecified | Unspecified | Medical Notes | During Hospitalization | Unspecified | Unspecified |
| 21 | Kelly et al. [75] | 2004 | 102 | 18 (17.6) | United Kingdom | Prospective | Unspecified | Unspecified | MRDTI | Within 1 Month | Aspirin | GCS |
| 22 | Li et al. [52] | 2023 | 234 | 31 (15.3) | China | Retrospective | Unspecified | Unspecified | CUS | Within 72 h | Unspecified | Unspecified |
| 23 | Li et al. [53] | 2022 | 152 | 26 (17.1) | China | Retrospective | IV tPA | Unspecified | Medical Notes | During Hospitalization | Unspecified | Unspecified |
| 24 | Li et al. [54] | 2017 | 671 | 148 (22.1) | United States | Retrospective | Unspecified | Unspecified | CDU | Within 2 Weeks | None Used | None Used |
| 25 | Li et al. [55] | 2020 | 614 | 104 (16.9) | China | Retrospective | MT | Unspecified | CDU | Within 2 Weeks | Unspecified | Unspecified |
| 26 | Liu et al. [57] | 2021 | 474 | 75 (15.8) | China | Retrospective | IV tPA | Yes | CDU | Within 72 h | Antiplatelet | Unspecified |
| 27 | Liu et al. [56] | 2014 | 462 | 52 (11.3) | China | Retrospective | Variable | Unspecified | CUS | Within 2 Weeks | Antiplatelet or Anticoagulant | Unspecified |
| 28 | Mori et al. [58] | 2021 | 734 | 132 (18.0) | Japan | Retrospective | Unspecified | Unspecified | CUS | Within 72 h | Unspecified | Unspecified |
| 29 | Pan et al. [59] | 2021 | 1036 | 131 (12.6) | China | Retrospective | Variable | Unspecified | CDU | Within 2 Weeks | Antiplatelet or Anticoagulant | Unspecified |
| 30 | Poletto et al. [60] | 2015 | 37 | 0 (0.0) | Brazil | RCT | IV tPA when possible | Unspecified | Medical Notes | Within 3 Months | Unspecified | Unspecified |
| 31 | Rinde et al. [61] | 2016 | 1360 | 29 (2.1) | Norway | Retrospective | Variable | Unspecified | CUS | During Hospitalization | Unspecified | Unspecified |
| 32 | Saad et al. [62] | 2014 | 12,055 | 260 (2.2) | United States | Retrospective | MT | Unspecified | Medical Notes | During Hospitalization | Unspecified | Unspecified |
| 33 | Sherman et al. [63] | 2007 | 1762 | 185 (13.9) | Multicenter | RCT | Variable | Yes | CUS | Within 2 Weeks | Antiplatelet or Anticoagulant | Unspecified |
| 34 | Turk et al. [64] | 2024 | 634 | 15 (2.4) | United States | Retrospective | Unspecified | Unspecified | Duplex Ultrasonography | Within 24 h | Variable | Unspecified |
| 35 | Turpie et al. [65] | 2012 | 316 | 15 (4.7) | Multicenter | Prospective | Unspecified | Unspecified | CDU | Within 1 Month | Not Routine | Unspecified |
| 36 | Wang et al. [66] | 2023 | 377 | 177 (46.9) | China | Retrospective | IV tPA | Unspecified | CUS | Within 72 h | Unspecified | Unspecified |
| 37 | Wang et al. [67] | 2019 | 385 | 35 (9.1) | NA | Prospective | Unspecified | Unspecified | CUS | During hospitalization | Unspecified | IPC |
| 38 | Xu et al. [68] | 2024 | 369 | 42 (8.0) | China | Retrospective | Unspecified | Unspecified | CDU | During hospitalization | Unspecified | Unspecified |
| 39 | Xu et al. [69] | 2023 | 1334 | 9 (0.7) | China | Retrospective | IV tPA | Unspecified | Medical Notes | During hospitalization | Unspecified | Unspecified |
| 40 | Yi et al. [70] | 2015 | 1454 | 35 (2.4) | China | RCT | Unspecified | Unspecified | Duplex Ultrasonography | Within 2 Weeks | Antiplatelet | Unspecified |
| 41 | Yi et al. [72] | 2014 | 1368 | 39 (2.9) | China | RCT | Unspecified | Unspecified | Duplex Ultrasonography | Within 2 Weeks | Antiplatelet or Anticoagulant | Unspecified |
| 42 | Yi et al. [71] | 2012 | 960 | 43 (4.5) | China | Prospective | Unspecified | Unspecified | Duplex Ultrasonography | Within 2 Weeks | Unspecified | Unspecified |
| ID | Author | DVT | Female | HTN | DM | HL | AF | Smoking | Alcohol | CAD | MAL | RI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (n%) | n (n%) | n (n%) | n (n%) | n (n%) | n (n%) | n (n%) | n (n%) | n (n%) | n (n%) | n (n%) | ||||||||||||
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |||
| 1 | Addisu et al. [32] | 4 (1.1) | - | - | - | - | - | - | - | - | 102 (27.0) | 276 (73.0) | - | - | - | - | - | - | - | - | - | - |
| 5 | Balogun et al. [36] | 18 (19.6) | 18 (19.6) | 74 (80.4) | 36 (39.1) | 56 (60.9) | 18 (19.6) | 74 (80.4) | 18 (19.6) | 74 (80.4) | 18 (19.6) | 74 (80.4) | 18 (19.6) | 74 (80.4) | - | - | 18 (19.6) | 74 (80.4) | - | - | - | - |
| 8 | Cai et al. [39] | 5002 (4.7) | 5002 (4.7) | 101,610 (95.3) | - | - | 5002 (4.7) | 101,610 (95.3) | 5002 (4.7) | 101,610 (95.3) | 5002 (4.7) | 101,610 (95.3) | 5002 (4.7) | 101,610 (95.3) | - | - | - | - | - | - | - | - |
| 11 | Cheng et al. [42] | 96 (22.3) | 96 (22.3) | 335 (77.7) | 96 (22.3) | 335 (77.7) | 96 (22.3) | 335 (77.7) | - | - | 96 (22.3) | 335 (77.7) | - | - | - | - | - | - | 96 (22.3) | 335 (77.7) | 96 (22.3) | 335 (77.7) |
| 14 | Ha et al. [44] | 38 (13.1) | 114 (39.4) | 175 (60.6) | 208 (72.0) | 81 (28.0) | 87 (30.1) | 202 (69.9) | 195 (67.5) | 94 (32.5) | - | - | 130 (45.0) | 159 (55.0) | - | - | - | - | 5 (1.7) | 284 (98.3) | - | - |
| 15 | Han et al. [5] | 67 (27.3) | 87 (35.5) | 158 (64.5) | 142 (58.0) | 103 (42.0) | 42 (17.1) | 203 (82.9) | - | - | 102 (41.6) | 143 (58.4) | 77 (31.4) | 168 (68.6) | 52 (21.2) | 193 (78.8) | 10 (4.1) | 235 (95.9) | 15 (6.1) | 230 (93.9) | 152 (62.0) | 93 (38.0) |
| 18 | Huang et al. [47] | 20 (19.8) | 29 (28.7) | 72 (71.3) | 83 (82.2) | 18 (17.8) | 15 (14.9) | 86 (85.1) | - | - | - | - | - | - | - | - | 23 (22.8) | 78 (77.2) | 0 (0.0) | 101 (100.0) | 66 (65.3) | 35 (34.7) |
| 23 | Li et al. [52] | 31 (15.3) | 31 (13.2) | 203 (86.8) | 31 (13.2) | 203 (86.8) | 31 (13.2) | 203 (86.8) | 31 (13.2) | 203 (86.8) | 31 (13.2) | 203 (86.8) | 31 (13.2) | 203 (86.8) | 31 (13.2) | 203 (86.8) | 31 (13.2) | 203 (86.8) | 31 (13.2) | 203 (86.8) | 31 (13.2) | 203 (86.8) |
| 27 | Liu et al. [57] | 75 (15.8) | 142 (30.0) | 332 (70.0) | 284 (59.9) | 190 (40.1) | 120 (25.3) | 354 (74.7) | 194 (40.9) | 280 (59.1) | 54 (11.4) | 420 (88.6) | 216 (45.6) | 258 (54.4) | 185 (39.0) | 289 (61.0) | 33 (7.0) | 441 (93.0) | - | - | 45 (9.5) | 429 (90.5) |
| 40 | Xu et al. [68] | 29 (7.9) | 217 (58.8) | 152 (41.2) | 269 (72.9) | 100 (27.1) | 164 (44.4) | 205 (55.6) | - | - | - | - | 73 (19.8) | 296 (80.2) | 105 (28.5) | 264 (71.5) | - | - | - | - | - | - |
| ID | Author | DVT | Age | NIHSS Score | D-Dimer | Admission Glucose | LDL | Fibrinogen | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (n%) | FR (Mean ± SD) | FR (Mean ± SD) | FR (Mean ± SD) | FR (Mean ± SD) | FR (Mean ± SD) | FR (Mean ± SD) | ||||||||
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |||
| 5 | Balogun et al. [36] | 18 (19.6) | 69.7 (13.4) | 69.1 (14.5) | 15.6 (7.3) | 12.8 (7.3) | 2.6 (1.9) | 1.4 (1.3) | - | - | - | - | 3.9 (1.4) | 3.9 (1.4) |
| 8 | Cai et al. [39] | 5002 (4.7) | 69.8 (11.7) | 67.2 (12.1) | 7.6 (7.1) | 5.3 (5.6) | - | - | 6.8 (3.2) | 6.6 (2.9) | 2.8 (1.5) | 2.8 (1.3) | - | - |
| 11 | Cheng et al. [42] | 96 (22.3) | 73.4 (8.4) | 68.9 (12.0) | - | - | 2.2 (1.9) | 1.6 (1.3) | 6.0 (2.4) | 6.0 (2.2) | - | - | 3.6 (1.6) | 3.6 (1.5) |
| 14 | Ha et al. [44] | 38 (13.1) | 71 (12.0) | 68.4 (11.2) | 7.4 (5.4) | 4.5 (3.8) | 12 (20.4) | 8.5 (13.4) | - | - | - | - | - | - |
| 15 | Han et al. [5] | 67 (27.3) | 72.1 (9.1) | 67.08 (11.7) | 16 (4.5) | 14.5 (5.7) | 2.8 (2.4) | 1.7 (1.7) | 6.6 (1.8) | 6.5 (1.7) | 2.1 (0.7) | 2.2 (0.8) | 0.032 (0.0) | 0.032 (0.0) |
| 18 | Huang et al. [47] | 20 (19.8) | 65 (16.4) | 66 (16.2) | 19.7 (10.6) | 16.9 (10.2) | 3.1 (5.3) | 1.6 (3.3) | 6.8 (3.2) | 6.6 (2.9) | 1.9 (1.2) | 2.0 (1.2) | 0.0025 (0.0) | 0.0029 (0.0) |
| 23 | Li et al. [52] | 31 (15.3) | 64.7 (11.7) | 60.2 (12.0) | - | - | 1.9 (1.7) | 0.8 (1.3) | 6.1 (1.9) | 6.3 (2.3) | 1.9 (0.6) | 1.7 (0.6) | 4.3 (1.2) | 4.1 (1.3) |
| 27 | Liu et al. [57] | 75 (15.8) | 69.8 (9.8) | 62.7 (11.6) | 9.7 (5.3) | 8.0 (4.7) | - | - | 8.0 (2.9) | 8.1 (3.1) | 2.92 (0.9) | 3.0 (0.8) | 3.15 (0.7) | 3.1 (0.7) |
3.4. Certainty of Evidence (GRADE)
| Subgroup | N | Pooled Prevalence Rate (from Meta-Analysis) | 95% CI | z-Score | p-Value | I2 | τ2≤ |
|---|---|---|---|---|---|---|---|
| Overall | 42 | 7% | 0.06–0.09 | 21.76. | p < 0.01 | 99.60% | 0.02 |
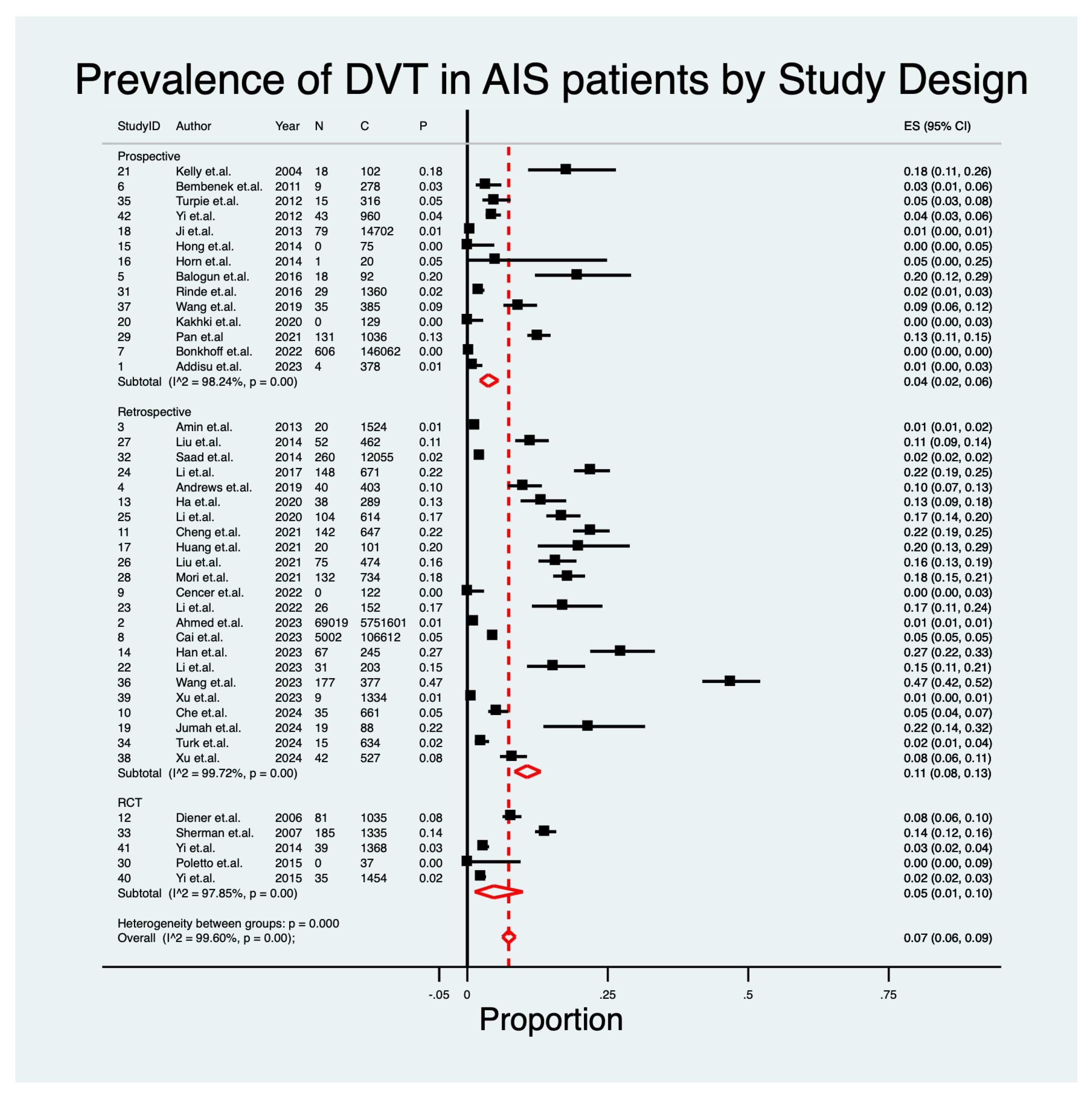
| Study Design | |||||||
| Retrospective | 23 | 11% | 0.08–0.13 | 15.96 | p < 0.01 | 99.72% | - |
| Prospective | 14 | 4% | 0.02–0.06 | 7.54 | p < 0.01 | 98.24% | - |
| RCT | 5 | 5% | 0.01–0.10 | 3.70 | p < 0.01 | 97.85% | - |
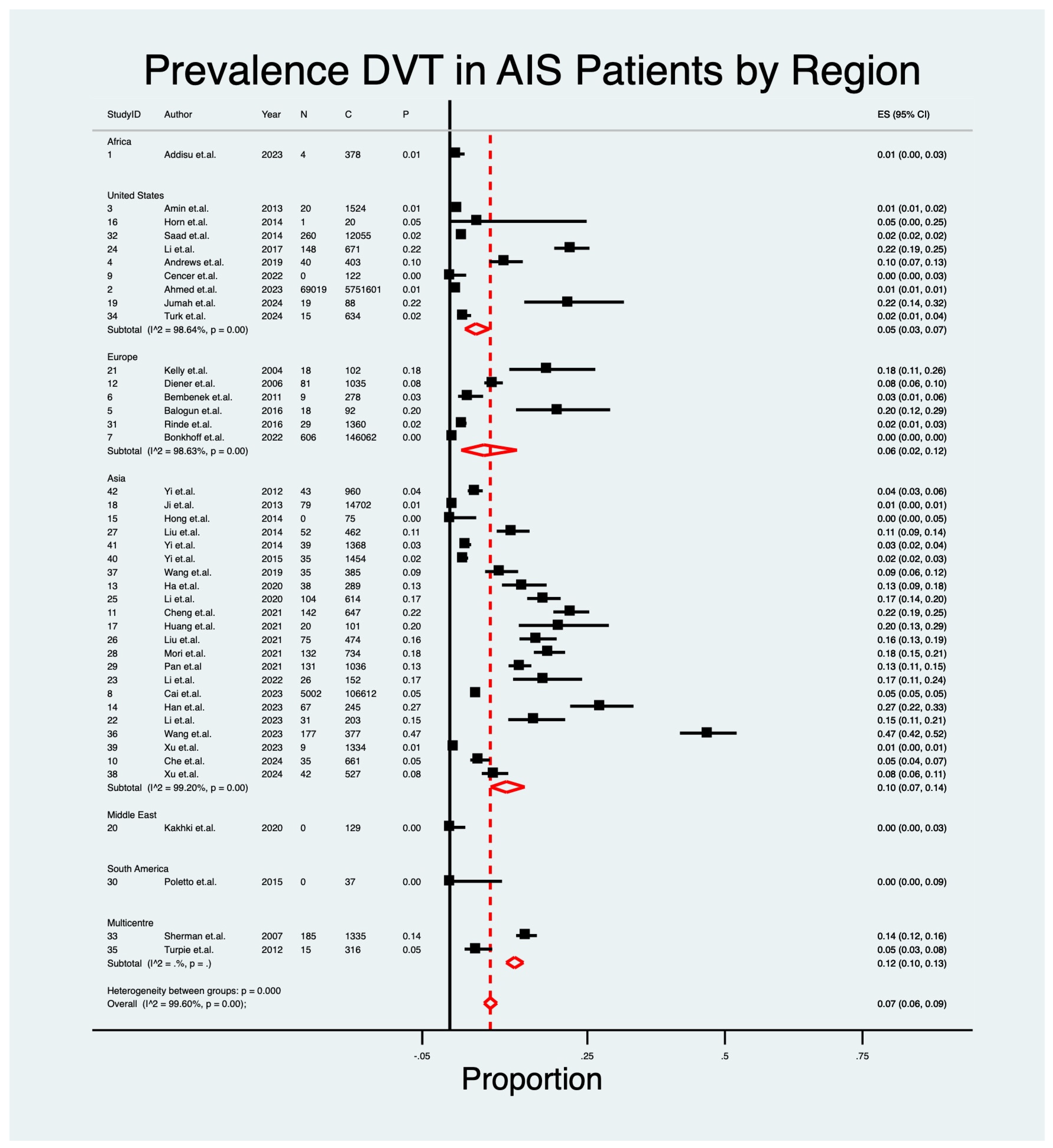
| Region | |||||||
| Asia | 22 | 10% | 0.07–0.14 | 3.24 | p < 0.01 | 99.20 | - |
| Europe | 6 | 6% | 0.02–0.12 | 4.20 | p < 0.01 | 98.63 | - |
| North America | 9 | 5% | 0.03–0.07 | 7.18 | p < 0.01 | 98.64 | - |
| Middle East | 1 | 0% | 0.00–0.03 | 0.00 | - | - | - |
| Africa | 1 | 1% | 0.00–0.03 | 3.24 | - | - | - |
| South America | 1 | 0% | 0.00–0.09 | 0.00 | - | - | - |
| Multiple | 2 | 12% | 0.10–0.13 | 26.77 | - | - | - |
| DVT Screening Post Stroke Screening | |||||||
| Within 24 h | 1 | 2% | 0.02–0.04 | 6.90 | - | - | - |
| Within 72 h | 5 | 23% | 0.12–0.35 | 6.66 | p < 0.01 | 96.97% | - |
| Within 1 Week | 1 | 13% | 0.09–0.18 | 11.69 | - | - | - |
| Within 2 Weeks | 14 | 12% | 0.08–0.17 | 9.40 | p < 0.01 | 97.89% | - |
| Within 1 Month | 2 | 7% | 0.05–10 | 9.63 | - | - | - |
| Within 3 Months | 1 | 0% | 0.00–0.09 | 0.00 | - | - | - |
| During hospitalization | 18 | 2% | 0.02–0.03 | 8.98 | p < 0.01 | 99.75% | - |
| Diagnosis Modality | |||||||
| MRDTI | 1 | 18% | 0.11–0.26 | 7.86 | - | - | - |
| CDU | 4 | 16% | 0.09–0.13 | 7.09 | p < 0.01 | 95.25% | - |
| CUS | 9 | 15% | 0.08–0.24 | 6.79 | p < 0.01 | 98.55% | - |
| Duplex Ultrasound | 4 | 3% | 0.02–0.04 | 12.07 | p < 0.01 | 65.85% | - |
| Color Doppler Ultrasound | 10 | 9% | 0.05–0.13 | 7.09 | p < 0.01 | 97.80% | - |
| Medical Notes | 14 | 2% | 0.01–0.02 | 8.49 | p < 0.01 | 99.14% | - |
| Temporal Trends | |||||||
| 2023 | 2 | 9% | 0.07–0.12 | 13.68 | - | - | |
| 2022 | 4 | 14% | 0.01–0.36 | 2.71 | p < 0.01 | - | |
| 2021 | 2 | 8% | 0.06–0.10 | 12.69 | - | - | |
| 2020 | 3 | 8% | 0.00–0.24 | 2.34 | - | - | |
| 2019 | 7 | 9% | 0.05–0.13 | 8.36 | p < 0.01 | - | |
| 2018 | 1 | 9% | 0.06–0.12 | 11.10 | - | - | |
| 2017 | 2 | 0% | 0.00–0.00 | 38.41 | - | - | |
| 2016 | 4 | 13% | 0.04–0.23 | 4.45 | p < 0.01 | - | |
| 2013 | 1 | 2% | 0.02–0.03 | 10.96 | - | - | |
| 2012 | 4 | 1% | 0.00–0.03 | 1.71 | p = 0.14 | - | |
| 2011 | 2 | 2% | 0.02–0.02 | 24.91 | - | - | |
| 2010 | 1 | 4% | 0.03–0.06 | 12.29 | - | - | |
| 2009 | 1 | 3% | 0.01–0.06 | 5.19 | - | - | |
| 2008 | 2 | 1% | 0.00–0.01 | 17.71 | - | - | |
| 2007 | 2 | 3% | 0.02–0.04 | 13.40 | - | - | |
| 2006 | 2 | 12% | 0.10–0.13 | 26.77 | - | - | |
| 2004 | 1 | 18% | 0.11–0.26 | 7.86 | - | - | |
| 2003 | 1 | 8% | 0.06–0.10 | 17.3 | - | - | |
| Summary Effects | Heterogeneity ¶ | Heterogeneity Variance Estimates | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| REDL | |||||||||||
| Outcome | N (Studies) | n (Cohort) | Effect Measure | Effect (OR/SMD) | [95% CI] | Tests of Overall Effect | z Score | Cochrane’s Q | H | I2 ≤ * | τ2 ≤ Φ |
| Female | 9 | 1,439,000 | OR | 1.332 | [1.185; 1.498] | p < 0.001 | 4.797 | 16.14 | 1.42 | 50.40% | 0.0077 |
| Respiratory Infection | 4 | 1054 | OR | 2.301 | [1.169; 4.529] | p = 0.016 | 2.411 | 5.85 | 1.396 | 48.70% | 0.1019 |
| Malignancy | 5 | 1,335,226 | OR | 2.69 | [1.557; 5.215] | p = 0.022 | 2.298 | 7.72 | 1.389 | 48.20% | 0.3959 |
| Atrial Fibrillation | 8 | 1,442,445 | OR | 1.684 | [0.930; 3.049] | p = 0.085 | 1.721 | 310.93 | 6.665 | 97.70% | 0.5145 |
| Coronary Artery Disease | 7 | 1,335,554 | OR | 1.164 | [0.871; 1.556] | p = 0.304 | 1.028 | 6.75 | 1.061 | 11.10% | 0.0286 |
| Peripheral Vascular Disease | 4 | 1,437,577 | OR | 1.477 | [0.665; 3.283] | p = 0.339 | 0.957 | 186.79 | 7.891 | 98.40% | 0.5098 |
| Diabetes Mellitus | 10 | 1,442,824 | OR | 1.06 | [0.898; 1.250] | p = 0.493 | 0.685 | 26.02 | 1.7 | 65.40% | 0.0188 |
| Hyperlipidemia | 5 | 107,701 | OR | 0.989 | [0.653; 1.497] | p = 0.957 | −0.054 | 11.49 | 1.695 | 65.20% | 0.125 |
| Hypertension | 9 | 1,336,167 | OR | 0.791 | [0.515; 1.214] | p = 0.283 | −1.074 | 35.11 | 2.095 | 77.20% | 0.2682 |
| Alcohol Use | 4 | 1322 | OR | 0.789 | [0.517; 1.203] | p = 0.271 | −1.102 | 4.49 | 1.223 | 33.10% | 0.0618 |
| Tobacco Use | 7 | 108,315 | OR | 0.767 | [0.618; 0.952 | p = 0.016 | −2.402 | 7.91 | 1.148 | 24.20% | 0.0216 |
| NIHSS Score | 6 | 113,033 | SMD | 0.405 | [0.377; 0.433] | p < 0.001 | 28.446 | 4.59 | 0.958 | 0% | 0 |
| D-Dimer | 6 | 1662 | SMD | 0.551 | [0.378; 0.723] | p < 0.001 | 6.24 | 7.66 | 1.238 | 34.80% | 0.0157 |
| LDL | 5 | 112,861 | SMD | −0.34 | [−0.126; 0.088] | p = 0.734 | 5.48 | 5.48 | 1.17 | 27% | 0.0047 |
| Admission Glucose | 5 | 113,267 | SMD | 0.066 | [0.039; 0.094] | p < 0.001 | 4.687 | 1.3 | 0.569 | 0% | 0 |
| Age | 8 | 113,825 | SMD | 0.32 | [0.181; 0.460] | p < 0.001 | 4.494 | 17.84 | 1.596 | 60.80% | 0.0197 |
| Fibrinogen | 6 | 1884 | SMD | 0.01 | [−0.112; 0.133] | p = 0.869 | 0.165 | 2.43 | 0.697 | 0% | 0 |
| A. Incidence/Prevalence | ||||||||||
| Outcome | No. of Studies (N) | Patient Number (n) | Effect Estimate (95% CI) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Certainty of Evidence (GRADE) | Reasons for Downgrade/Upgrade |
| Prevalence of DVT in AIS | 42 | 6,051,729 | Pooled prevalence: 7% (95% CI 5–9%) | Low | Moderate (regional and temporal heterogeneity) | Low | Minimal | Possible | ⬤⬤⬤◯ Moderate | Downgraded: heterogeneity; Upgraded: large sample size, precise estimates |
| B. Risk Factors | ||||||||||
| Predictor | No. of Studies (N) | Patient Number (n) | Effect Estimate (OR/SMD, 95% CI) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Certainty of Evidence (GRADE) | Reasons for Downgrade/Upgrade |
| Stroke severity (NIHSS) | 6 | 107,795 | SMD 0.41 (0.38–0.43) | Low | Very low (I2 = 0%) | Low | Minimal | Unlikely | ⬤⬤⬤◯ Moderate | Downgraded: observational designs; Upgraded: strong, consistent effect |
| Age | 8 | 108,676 | SMD 0.32 (0.18–0.46) | Low | Moderate (I2 ≈ 61%) | Low | Minimal | Unlikely | ⬤⬤⬤◯ Moderate | Downgraded: inconsistency; Upgraded: large sample size |
| Female sex | 9 | 108,847 | OR 1.33 (1.19–1.50) | Low | Moderate (I2 ≈ 50%) | Low | Adequate | Possible | ⬤⬤⬤◯ Moderate | Downgraded: inconsistency; Upgraded: robust effect |
| D-dimer elevation | 6 | 1590 | SMD 0.55 (0.38–0.72) | Low | Low–moderate (I2 ≈ 35%) | Low | Minimal | Possible | ⬤⬤⬤◯ Moderate | Downgraded: possible bias; Upgraded: strong effect |
| Malignancy | 5 | 1199 | OR 2.69 (1.56–5.22) | Low | Moderate (I2 ≈ 48%) | Low | Somewhat wide CI | Possible | ⬤⬤⬤◯ Moderate | Downgraded: inconsistency; Upgraded: large effect |
| Respiratory infection | 5 | 1485 | OR 2.30 (1.17–4.53) | Moderate | Moderate (I2 ≈ 49%) | Low | Wide CI | Likely | ⬤⬤◯◯ Low | Downgraded: inconsistency, imprecision, bias |
| Admission hyperglycemia | 5 | 108,212 | SMD 0.07 (0.04–0.09) | Low | Low (I2 = 0%) | Low | Small effect | Possible | ⬤⬤◯◯ Low | Downgraded: trivial effect size, possible bias |
| Tobacco use (inverse) | 7 | 108,315 | OR 0.77 (0.62–0.95) | High | Low (I2 ≈ 24%) | High | CI near null | Likely | ⬤◯◯◯ Very Low | Downgraded: confounding, indirectness, bias |
| LDL cholesterol | 5 | 107,666 | SMD −0.03 (−0.12–0.09) | Moderate | Low (I2 ≈ 27%) | Moderate | Null effect, small n | Likely | ⬤◯◯◯ Very Low | Downgraded: imprecision, indirectness |
| Fibrinogen | 6 | 1775 | SMD 0.01 (−0.11–0.13) | Moderate | Low (I2 = 0%) | Moderate | Wide CI incl. null | Possible | ⬤◯◯◯ Very Low | Downgraded: imprecision, indirectness |
| C. Prophylaxis | ||||||||||
| Intervention | No. of Studies (N) | Patient Number (n) | Effect Estimate (OR/SMD, 95% CI) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Certainty of Evidence (GRADE) | Reasons for Downgrade/Upgrade |
| Pharmacological prophylaxis (Anticoagulants) | 4 | 1066 | Heterogeneous, no stable pooled estimate | High (small, observational) | Very low (I2 = 0%) | Low | Wide CI | Likely | ⬤◯◯◯ Very Low | Downgraded: high risk of bias, small observational |
| Pharmacological prophylaxis (Antiplatelets) | 5 | 1531 | Heterogeneous, no stable pooled estimate | High (small, observational) | Very low (I2 = 0%) | Low | Wide CI | Likely | ⬤◯◯◯ Very Low | Downgraded: high risk of bias, small observational |
| IPC (intermittent pneumatic compression) | 3 | 732 | Trend toward reduced DVT; effect inconsistent | Moderate | Moderate–high | Low | Moderate | Possible | ⬤◯◯◯ Very Low | Downgraded: inconsistency, imprecision, small observational |
3.5. Prophylactic Interventions
3.6. Sensitivity and Bias Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.T.; Ikram, A.; Saeed, O.; Afridi, T.; Sila, C.A.; Smith, M.S.; Irshad, K.; Shuaib, A. Deep Vein Thrombosis in Acute Stroke—A Systemic Review of the Literature. Cureus 2017, 9, e1982. [Google Scholar] [CrossRef]
- Chen, D.; Bhaskar, S.M.M. Pulmonary Embolism in Acute Ischaemic Stroke: Evolving Evidence, Diagnostic Challenges, and a Novel Thromboinflammatory Axis Hypothesis. Int. J. Mol. Sci. 2025, 26, 6733. [Google Scholar] [CrossRef]
- Wenger, N.; Sebastian, T.; Engelberger, R.P.; Kucher, N.; Spirk, D. Pulmonary embolism and deep vein thrombosis: Similar but different. Thromb. Res. 2021, 206, 88–98. [Google Scholar] [CrossRef]
- Hayssen, H.; Cires-Drouet, R.; Englum, B.; Nguyen, P.; Sahoo, S.; Mayorga-Carlin, M.; Siddiqui, T.; Turner, D.; Yesha, Y.; Sorkin, J.D.; et al. Systematic review of venous thromboembolism risk categories derived from Caprini score. J. Vasc. Surg. Venous Lymphat. Disord. 2022, 10, 1401–1409.e1407. [Google Scholar] [CrossRef]
- Han, L.; Yang, J.M.; Qian, W.Y.; Xu, X.P.; Tung, T.H.; Liu, Y.; Wang, F. Risk factors for lower extremity deep vein thrombosis in acute stroke patients following endovascular thrombectomy: A retrospective cohort study. Front. Neurol. 2023, 14, 1249365. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, S.; Stanwell, P.; Cordato, D.; Attia, J.; Levi, C. Reperfusion therapy in acute ischemic stroke: Dawn of a new era? BMC Neurol. 2018, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, M.G.; Paciaroni, M. Treatments in Ischemic Stroke: Current and Future. Eur. Neurol. 2022, 85, 349–366. [Google Scholar] [CrossRef]
- Kushner, A.; West, W.P.; Khan Suheb, M.Z.; Pillarisetty, L.S. Virchow Triad. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Tondel, B.G.; Morelli, V.M.; Hansen, J.B.; Braekkan, S.K. Risk factors and predictors for venous thromboembolism in people with ischemic stroke: A systematic review. J. Thromb. Haemost. 2022, 20, 2173–2186. [Google Scholar] [CrossRef]
- Abramowitz, H.B.; Gertz, S.D. Venous stasis, deep venous thrombosis and airline flight: Can the seat be fixed? Ann. Vasc. Surg. 2007, 21, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Nesheim, M. Thrombin and fibrinolysis. Chest 2003, 124, 33S–39S. [Google Scholar] [CrossRef]
- Samama, M.M.; Cohen, A.T.; Darmon, J.Y.; Desjardins, L.; Eldor, A.; Janbon, C.; Leizorovicz, A.; Nguyen, H.; Olsson, C.G.; Turpie, A.G.; et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N. Engl. J. Med. 1999, 341, 793–800. [Google Scholar] [CrossRef]
- Lieberman, J.S.; Borrero, J.; Urdaneta, E.; Wright, I.S. Thrombophlebitis and cancer. JAMA 1961, 177, 542–545. [Google Scholar] [CrossRef]
- Stevens, S.M.; Woller, S.C.; Baumann Kreuziger, L.; Doerschug, K.; Geersing, G.J.; Klok, F.A.; King, C.S.; Murin, S.; Vintch, J.R.E.; Wells, P.S.; et al. Antithrombotic Therapy for VTE Disease: Compendium and Review of CHEST Guidelines 2012–2021. Chest 2024, 166, 388–404. [Google Scholar] [CrossRef]
- Linnemann, B.; Beyer-Westendorf, J.; Espinola-Klein, C.; Mühlberg, K.S.; Müller, O.J.; Klamroth, R. Management of Deep Vein Thrombosis: An Update Based on the Revised AWMF S2k Guideline. Hamostaseologie 2024, 44, 97–110. [Google Scholar] [CrossRef]
- Onwuzo, C.; Olukorode, J.; Sange, W.; Tanna, S.J.; Osaghae, O.W.; Hassan, A.; Kristilere, H.; Orimoloye, D.A.; Omokore, O.; Ganiyu, B.; et al. A Review of the Preventive Strategies for Venous Thromboembolism in Hospitalized Patients. Cureus 2023, 15, e48421. [Google Scholar] [CrossRef]
- Wang, M.; Zeraatkar, D.; Obeda, M.; Lee, M.; Garcia, C.; Nguyen, L.; Agarwal, A.; Al-Shalabi, F.; Benipal, H.; Ahmad, A.; et al. Drug-drug interactions with warfarin: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2021, 87, 4051–4100. [Google Scholar] [CrossRef]
- Horlocker, T.T. Low molecular weight heparin and neuraxial anesthesia. Thromb. Res. 2001, 101, V141–V154. [Google Scholar] [CrossRef] [PubMed]
- Pernod, G.; Joly, M.; Sonnet, B. Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for the treatment of cancer-associated thrombosis (which agent for which patient). J. Med. Vasc. 2020, 45, 6S17–6S23. [Google Scholar] [CrossRef]
- Sachdeva, A.; Dalton, M.; Lees, T. Graduated compression stockings for prevention of deep vein thrombosis. Cochrane Database Syst. Rev. 2018, 11, CD001484. [Google Scholar] [CrossRef] [PubMed]
- Makedonov, I.; Kahn, S.R.; Galanaud, J.P. Prevention and Management of the Post-Thrombotic Syndrome. J. Clin. Med. 2020, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Mol, G.C.; Dronkers, C.E.A.; van de Ree, M.A.; van der Pas, S.L.; Tegelberg-Stassen, M.; Sanders, F.B.M.; Koppen, S.; de Weerdt, O.; Koster, T.; Hovens, M.M.C.; et al. Elastic compression stockings one year after DVT diagnosis: Who might discontinue? Thromb. Res. 2019, 173, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Asmar, S.; Michael, G.; Gallo, V.; Weinberg, M.D. The Role of IVC Filters in the Management of Acute Pulmonary Embolism. J. Clin. Med. 2024, 13, 1494. [Google Scholar] [CrossRef]
- Li, X.; Haddadin, I.; McLennan, G.; Farivar, B.; Staub, D.; Beck, A.; Thompson, D.; Partovi, S. Inferior vena cava filter––Comprehensive overview of current indications, techniques, complications and retrieval rates. Vasa 2020, 49, 449–462. [Google Scholar] [CrossRef]
- Speth, J. Guidelines in Practice: Prevention of Venous Thromboembolism. Aorn J. 2023, 118, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Kakkos, S.K.; Caprini, J.A.; Geroulakos, G.; Nicolaides, A.N.; Stansby, G.; Reddy, D.J.; Ntouvas, I. Combined intermittent pneumatic leg compression and pharmacological prophylaxis for prevention of venous thromboembolism. Cochrane Database Syst. Rev. 2016, 9, Cd005258. [Google Scholar] [CrossRef] [PubMed]
- Badireddy, M.; Mudipalli, V.R. Deep Venous Thrombosis Prophylaxis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Chen, Z.J.; Li, X.F.; Liang, C.Y.; Cui, L.; Yang, L.Q.; Xia, Y.M.; Cao, W.; Gao, B.L. Comparison of Prior Bridging Intravenous Thrombolysis With Direct Endovascular Thrombectomy for Anterior Circulation Large Vessel Occlusion: Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 602370. [Google Scholar] [CrossRef]
- Dennis, M.; Sandercock, P.; Graham, C.; Forbes, J.; Collaboration, C.T.; Smith, J. The Clots in Legs Or sTockings after Stroke (CLOTS) 3 trial: A randomised controlled trial to determine whether or not intermittent pneumatic compression reduces the risk of post-stroke deep vein thrombosis and to estimate its cost-effectiveness. Health Technol. Assess. 2015, 19, 1–90. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Addisu, Z.D.; Mega, T.A. Clinical Characteristics and Treatment Outcomes of Acute Ischemic Stroke with Atrial Fibrillation Among Patients Admitted to Tertiary Care Hospitals in Amhara Regional State: Retrospective-Cohort Study. Vasc. Health Risk Manag. 2023, 19, 837–853. [Google Scholar] [CrossRef]
- Ahmed, R.; Mhina, C.; Philip, K.; Patel, S.D.; Aneni, E.; Osondu, C.; Lamikanra, O.; Akano, E.O.; Anikpezie, N.; Albright, K.C.; et al. Age- and Sex-Specific Trends in Medical Complications After Acute Ischemic Stroke in the United States. Neurology 2023, 100, e1282–e1295. [Google Scholar] [CrossRef]
- Amin, A.N.; Lin, J.; Thompson, S.; Wiederkehr, D. Rate of deep-vein thrombosis and pulmonary embolism during the care continuum in patients with acute ischemic stroke in the United States. BMC Neurol. 2013, 13, 17. [Google Scholar] [CrossRef]
- Andrews, C.E.; Mouchtouris, N.; Fitchett, E.M.; Al Saiegh, F.; Lang, M.J.; Romo, V.M.; Herial, N.; Jabbour, P.; Tjoumakaris, S.I.; Rosenwasser, R.H.; et al. Revascularization and functional outcomes after mechanical thrombectomy for acute ischemic stroke in elderly patients. J. Neurosurg. 2020, 132, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Balogun, I.O.; Roberts, L.N.; Patel, R.; Pathansali, R.; Kalra, L.; Arya, R. Clinical and laboratory predictors of deep vein thrombosis after acute stroke. Thromb. Res. 2016, 142, 33–39. [Google Scholar] [CrossRef]
- Bembenek, J.; Karlinski, M.; Kobayashi, A.; Czlonkowska, A. Early stroke-related deep venous thrombosis: Risk factors and influence on outcome. J. Thromb. Thrombolysis 2011, 32, 96–102. [Google Scholar] [CrossRef]
- Bonkhoff, A.K.; Rubsamen, N.; Grefkes, C.; Rost, N.S.; Berger, K.; Karch, A. Development and Validation of Prediction Models for Severe Complications After Acute Ischemic Stroke: A Study Based on the Stroke Registry of Northwestern Germany. J. Am. Heart Assoc. 2022, 11, e023175. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, R.; Wang, Y.; Li, Z.; Liu, L.; Gu, H.; Yang, K.; Yang, X.; Wang, C.; Wang, A.; et al. Predictors and outcomes of deep venous thrombosis in patients with acute ischemic stroke: Results from the Chinese Stroke Center Alliance. Int. Angiol. 2023, 42, 503–511. [Google Scholar] [CrossRef]
- Cencer, S.; Tubergen, T.; Packard, L.; Gritters, D.; LaCroix, H.; Frye, A.; Wills, N.; Zachariah, J.; Wees, N.; Khan, N.; et al. Shorter Intensive Care Unit Stay (12 Hours) Post Thrombolysis Is Safe and Reduces Length of Stay for Minor Stroke Patients. Neurohospitalist 2022, 12, 504–507. [Google Scholar] [CrossRef]
- Che, F.; Wang, A.; Ju, Y.; Liu, L.; Ma, N.; Cheng, Z.; Duan, H.; Zhao, X.; Geng, X. Prevalence and Impact of Medical Complications on Clinical Outcomes in Acute Ischemic Stroke Patients After Endovascular Therapy––Data From a Comprehensive Stroke Unit in China. World Neurosurg. 2024, 182, e386–e399. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.R.; Huang, G.Q.; Wu, Z.Q.; Wu, Y.M.; Lin, G.Q.; Song, J.Y.; Liu, Y.T.; Luan, X.Q.; Yuan, Z.Z.; Zhu, W.Z.; et al. Individualized predictions of early isolated distal deep vein thrombosis in patients with acute ischemic stroke: A retrospective study. BMC Geriatr. 2021, 21, 140. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.C.; Ringelstein, E.B.; von Kummer, R.; Landgraf, H.; Koppenhagen, K.; Harenberg, J.; Rektor, I.; Csanyi, A.; Schneider, D.; Klingelhofer, J.; et al. Prophylaxis of thrombotic and embolic events in acute ischemic stroke with the low-molecular-weight heparin certoparin: Results of the PROTECT Trial. Stroke 2006, 37, 139–144. [Google Scholar] [CrossRef]
- Ha, S.H.; Kim, Y.J.; Heo, S.H.; Chang, D.I.; Kim, B.J. Prediction of deep vein thrombosis by ultrasonography and D-dimer in Asian patients with ischemic stroke. BMC Neurol. 2020, 20, 257. [Google Scholar] [CrossRef]
- Hong, J.M.; Lee, J.S.; Song, H.J.; Jeong, H.S.; Choi, H.A.; Lee, K. Therapeutic hypothermia after recanalization in patients with acute ischemic stroke. Stroke 2014, 45, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Horn, C.M.; Sun, C.H.; Nogueira, R.G.; Patel, V.N.; Krishnan, A.; Glenn, B.A.; Belagaje, S.R.; Thomas, T.T.; Anderson, A.M.; Frankel, M.R.; et al. Endovascular Reperfusion and Cooling in Cerebral Acute Ischemia (ReCCLAIM I). J. Neurointerv. Surg. 2014, 6, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Guo, C.; Song, K.; Li, C.; Ding, N. Association of clinical and laboratory variables with in-hospital incidence of deep vein thrombosis in patients after acute ischemic stroke: A retrospective study. Medicine 2021, 100, e24601. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Wang, D.; Shen, H.; Pan, Y.; Liu, G.; Wang, P.; Wang, Y.; Li, H.; Wang, Y.; China National Stroke Registry (CNSR) Investigators. Interrelationship among common medical complications after acute stroke: Pneumonia plays an important role. Stroke 2013, 44, 3436–3444. [Google Scholar] [CrossRef]
- Jumah, A.; Fu, S.; Albanna, A.J.; Agarwal, U.; Fana, M.; Choudhury, O.; Idris, A.; Elfaham, A.; Iqbal, Z.; Schultz, L.; et al. Early vs late anticoagulation in acute ischemic stroke with indications outside atrial fibrillation. J. Stroke Cerebrovasc. Dis. 2024, 33, 107757. [Google Scholar] [CrossRef]
- Kakhki, R.D.; Dehghanei, M.; ArefNezhad, R.; Motedayyen, H. The Predicting Role of Neutrophil- Lymphocyte Ratio in Patients with Acute Ischemic and Hemorrhagic Stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 105233. [Google Scholar] [CrossRef]
- Kelly, J.; Rudd, A.; Lewis, R.R.; Coshall, C.; Moody, A.; Hunt, B.J. Venous thromboembolism after acute ischemic stroke: A prospective study using magnetic resonance direct thrombus imaging. Stroke 2004, 35, 2320–2325. [Google Scholar] [CrossRef]
- Li, F.; Wei, C.; Huo, S.; Liu, X.; Du, J. Predictors of deep-vein thrombosis for acute stroke at admission to a rehabilitation unit: A retrospective study. Front. Neurol. 2023, 14, 1137485. [Google Scholar] [CrossRef]
- Li, G.; Wang, C.; Wang, S.; Hao, Y.; Xiong, Y.; Zhao, X. Clinical Significance and Dynamic Change of Coagulation Parameters in Ischemic Stroke Patients Treated with Intravenous Thrombolysis. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221121287. [Google Scholar] [CrossRef]
- Li, S.Y.; Feng, L.; Xiao, M.J.; Chen, S.Y.; He, J.C.; Wang, Z. Derivation and Validation of a Clinical Prediction Scale for Isolated Distal Deep Venous Thrombosis in Patients after Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2017, 26, 2087–2092. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ding, J.; Sui, X.; Qi, Z.; Wu, L.; Sun, C.; Ji, K.; Ma, Q.; Ji, X.; Liu, K.J. Prognosis and risk factors for reocclusion after mechanical thrombectomy. Ann. Clin. Transl. Neurol. 2020, 7, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.P.; Zheng, H.G.; Wang, D.Z.; Wang, Y.L.; Hussain, M.; Sun, H.X.; Wang, A.X.; Zhao, X.Q.; Dong, K.H.; Wang, C.X.; et al. Risk assessment of deep-vein thrombosis after acute stroke: A prospective study using clinical factors. CNS Neurosci. Ther. 2014, 20, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, D.; Guo, Z.N.; Jin, H.; Sun, T.; Ni, C.; Yan, X. Incidence and Risk Factors of Lower-Extremity Deep Vein Thrombosis After Thrombolysis Among Patients with Acute Ischemic Stroke. Pharmgenomics Pers. Med. 2021, 14, 1107–1114. [Google Scholar] [CrossRef]
- Mori, T.; Yoshioka, K.; Tanno, Y. Frequency of deep vein thrombosis at admission for acute stroke and associated factors: A cross-sectional study. Thromb. J. 2021, 19, 62. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Z.; Chen, Q.; Xu, L.; Fang, Q. Development and Validation of a Nomogram for Lower Extremity Deep Venous Thrombosis in Patients after Acute Stroke. J. Stroke Cerebrovasc. Dis. 2021, 30, 105683. [Google Scholar] [CrossRef]
- Poletto, S.R.; Rebello, L.C.; Valenca, M.J.; Rossato, D.; Almeida, A.G.; Brondani, R.; Chaves, M.L.; Nasi, L.A.; Martins, S.C. Early mobilization in ischemic stroke: A pilot randomized trial of safety and feasibility in a public hospital in Brazil. Cerebrovasc. Dis. Extra 2015, 5, 31–40. [Google Scholar] [CrossRef]
- Rinde, L.B.; Smabrekke, B.; Mathiesen, E.B.; Lochen, M.L.; Njolstad, I.; Hald, E.M.; Wilsgaard, T.; Braekkan, S.K.; Hansen, J.B. Ischemic Stroke and Risk of Venous Thromboembolism in the General Population: The Tromso Study. J. Am. Heart Assoc. 2016, 5, e004311. [Google Scholar] [CrossRef]
- Saad, A.; Adil, M.M.; Patel, V.; Owada, K.; Winningham, M.J.; Nahab, F. Clinical outcomes after thrombectomy for acute ischemic stroke on weekends versus weekdays. J. Stroke Cerebrovasc. Dis. 2014, 23, 2708–2713. [Google Scholar] [CrossRef]
- Sherman, D.G.; Albers, G.W.; Bladin, C.; Fieschi, C.; Gabbai, A.A.; Kase, C.S.; O’Riordan, W.; Pineo, G.F.; Investigators, P. The efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of venous thromboembolism after acute ischaemic stroke (PREVAIL Study): An open-label randomised comparison. Lancet 2007, 369, 1347–1355. [Google Scholar] [CrossRef]
- Al Turk, M.; Abraham, M. Incidence of Symptomatic Venous Thromboembolisms in Stroke Patients. J. Intensive Care Med. 2024, 39, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Turpie, A.G.; Hull, R.D.; Schellong, S.M.; Tapson, V.F.; Monreal, M.; Samama, M.M.; Chen, M.; Yusen, R.D.; Investigators, E. Venous thromboembolism risk in ischemic stroke patients receiving extended-duration enoxaparin prophylaxis: Results from the EXCLAIM study. Stroke 2013, 44, 249–251. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, M.; Liu, X.; Sun, Y.; Wang, Y.; Jin, R.; Zhang, W.; Shao, B. Nomogram Prediction for Lower Extremity Deep Vein Thrombosis in Acute Ischemic Stroke Patients Receiving Thrombolytic Therapy. Clin. Appl. Thromb. Hemost. 2023, 29, 10760296231171603. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Y.; Dong, Y.; Dong, Q.; Ye, T.; Fang, K. Clinical Risk Factors of Asymptomatic Deep Venous Thrombosis in Patients With Acute Stroke. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029619868534. [Google Scholar] [CrossRef]
- Xu, H.; Yin, Q. Construction and validation of a prediction model for acute ischemic stroke patients with concomitant deep vein thrombosis. Medicine 2024, 103, e40754. [Google Scholar] [CrossRef]
- Xu, J.; Chen, X.; Xie, Y.; Wang, Y.; Chen, S.; Dong, Q.; Dong, Y.; Fang, K. Low-dose vs. standard-dose alteplase for Chinese patients with acute ischemic stroke: A propensity score analysis. Front. Neurol. 2023, 14, 1120547. [Google Scholar] [CrossRef]
- Yi, X.; Chi, W.; Wang, C.; Zhang, B.; Lin, J. Low-molecular-weight heparin or dual antiplatelet therapy is more effective than aspirin alone in preventing early neurological deterioration and improving the 6-month outcome in ischemic stroke patients. J. Clin. Neurol. 2015, 11, 57–65. [Google Scholar] [CrossRef]
- Yi, X.; Lin, J.; Han, Z.; Zhou, X.; Wang, X.; Lin, J. The incidence of venous thromboembolism following stroke and its risk factors in eastern China. J. Thromb. Thrombolysis 2012, 34, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Lin, J.; Wang, C.; Zhang, B.; Chi, W. Low-molecular-weight heparin is more effective than aspirin in preventing early neurologic deterioration and improving six-month outcome. J. Stroke Cerebrovasc. Dis. 2014, 23, 1537–1544. [Google Scholar] [CrossRef]
- Li, Z.; Ni, J. Role of microRNA-26a in the diagnosis of lower extremity deep vein thrombosis in patients with bone trauma. Exp. Ther. Med. 2017, 14, 5069–5074. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, T.; Zhou, L.; Yin, X.; Dong, Q. Stratification of venous thromboembolism risk in stroke patients by Caprini score. Ann. Palliat. Med. 2020, 9, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Kinoda, A.; Macznik, A.; Kimura, T.; Muramoto, Y.; Katsumata, Y.; Sato, K. 1-Year Prevalence and Factors Related to Injuries and Illnesses in Japanese Judo Collegiate Athletes. J. Funct. Morphol. Kinesiol. 2024, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Barco, S.; Di Nisio, M.; Mahan, C.E.; Christodoulou, K.C.; Ter Haar, S.; Konstantinides, S.; Kucher, N.; Klok, F.A.; Cannegieter, S.C.; et al. Epidemiology of deep vein thrombosis. Vasa 2024, 53, 298–307. [Google Scholar] [CrossRef]
- Hirsh, J.; Hoak, J. Management of Deep Vein Thrombosis and Pulmonary Embolism. Circulation 1996, 93, 2212–2245. [Google Scholar] [CrossRef]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef]
- Dennis, M.; Caso, V.; Kappelle, L.J.; Pavlovic, A.; Sandercock, P.; For the European Stroke Organisation. European Stroke Organisation (ESO) guidelines for prophylaxis for venous thromboembolism in immobile patients with acute ischaemic stroke. Eur. Stroke J. 2016, 1, 6–19. [Google Scholar] [CrossRef]
- De Stefano, V.; Chiusolo, P.; Paciaroni, K.; Leone, G. Epidemiology of factor V Leiden: Clinical implications. Semin. Thromb. Hemost. 1998, 24, 367–379. [Google Scholar] [CrossRef]
- Krishnan, R.; Mays, W.; Elijovich, L. Complications of Mechanical Thrombectomy in Acute Ischemic Stroke. Neurology 2021, 97, S115–S125. [Google Scholar] [CrossRef] [PubMed]
- Gladstone, D.J.; Black, S.E. Update on intravenous tissue plasminogen activator for acute stroke: From clinical trials to clinical practice. Cmaj 2001, 165, 311–317. [Google Scholar]
- Jadhav, A.P.; Jovin, T.G. Endovascular therapy for acute ischemic stroke: The standard of care. Brain Circ. 2016, 2, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Douds, G.L.; Hellkamp, A.S.; Olson, D.M.; Fonarow, G.C.; Smith, E.E.; Schwamm, L.H.; Cockroft, K.M. Venous thromboembolism in the Get With The Guidelines-Stroke acute ischemic stroke population: Incidence and patterns of prophylaxis. J. Stroke Cerebrovasc. Dis. 2014, 23, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Sartori, M.; Favaretto, E.; Cosmi, B. Relevance of immobility as a risk factor for symptomatic proximal and isolated distal deep vein thrombosis in acutely ill medical inpatients. Vasc. Med. 2021, 26, 542–548. [Google Scholar] [CrossRef]
- Navarrete, S.; Solar, C.; Tapia, R.; Pereira, J.; Fuentes, E.; Palomo, I. Pathophysiology of deep vein thrombosis. Clin. Exp. Med. 2023, 23, 645–654. [Google Scholar] [CrossRef]
- Anderson, F.A., Jr.; Spencer, F.A. Risk factors for venous thromboembolism. Circulation 2003, 107, I9–I16. [Google Scholar] [CrossRef]
- Huang, S.; Xu, J.; Kang, H.; Guo, W.; Ren, C.; Wehbe, A.; Song, H.; Ma, Q.; Zhao, W.; Ding, Y.; et al. A Comprehensive Prediction Model for Futile Recanalization in AIS Patients Post-Endovascular Therapy: Integrating Clinical, Imaging, and No-Reflow Biomarkers. Aging Dis. 2024, 15, 2852–2862. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Kobayashi, H.; Kario, K.; Suzuki, S. Fibrin D-dimer in thrombogenic disorders. Semin. Thromb. Hemost. 2000, 26, 101–107. [Google Scholar] [CrossRef]
- Wang, J.; Feng, A.; Xu, J.; Liu, Y.; Li, F.; Sun, Y.; Sun, H.; Yang, F.; Zhao, J.; Tang, Y. D-dimer and its Combination with Blood Lipid on Prognosis of Patients with Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 105394. [Google Scholar] [CrossRef]
- Collaboration, C.T.; Dennis, M.; Sandercock, P.; Reid, J.; Graham, C.; Forbes, J.; Murray, G. Effectiveness of intermittent pneumatic compression in reduction of risk of deep vein thrombosis in patients who have had a stroke (CLOTS 3): A multicentre randomised controlled trial. Lancet 2013, 382, 516–524. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Swiss Federation of Clinical Neuro-Societies. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Chen, D.; Bhaskar, S.M.M. Incidence, Risk Factors, and Prevention of Deep Vein Thrombosis in Acute Ischemic Stroke Patients (IRIS-DVT Study): A Systematic Review and Meta-Analysis. Clin. Transl. Neurosci. 2025, 9, 49. https://doi.org/10.3390/ctn9040049
Yang Y, Chen D, Bhaskar SMM. Incidence, Risk Factors, and Prevention of Deep Vein Thrombosis in Acute Ischemic Stroke Patients (IRIS-DVT Study): A Systematic Review and Meta-Analysis. Clinical and Translational Neuroscience. 2025; 9(4):49. https://doi.org/10.3390/ctn9040049
Chicago/Turabian StyleYang, Yuxiang, Darryl Chen, and Sonu M. M. Bhaskar. 2025. "Incidence, Risk Factors, and Prevention of Deep Vein Thrombosis in Acute Ischemic Stroke Patients (IRIS-DVT Study): A Systematic Review and Meta-Analysis" Clinical and Translational Neuroscience 9, no. 4: 49. https://doi.org/10.3390/ctn9040049
APA StyleYang, Y., Chen, D., & Bhaskar, S. M. M. (2025). Incidence, Risk Factors, and Prevention of Deep Vein Thrombosis in Acute Ischemic Stroke Patients (IRIS-DVT Study): A Systematic Review and Meta-Analysis. Clinical and Translational Neuroscience, 9(4), 49. https://doi.org/10.3390/ctn9040049







