Evolution of Pharmacologic Induction of Burst Suppression in Adult TBI: Barbiturate Coma Versus Modern Sedatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Data Extraction and Synthesis
3. Results
3.1. Sedative Mechanisms of Action and Burst Suppression Induction
3.2. Sedation Protocols and EEG Monitoring in TBI
3.3. Impact on Intracranial Pressure and Cerebral Physiology
3.4. Clinical Efficacy and Neurological Outcomes
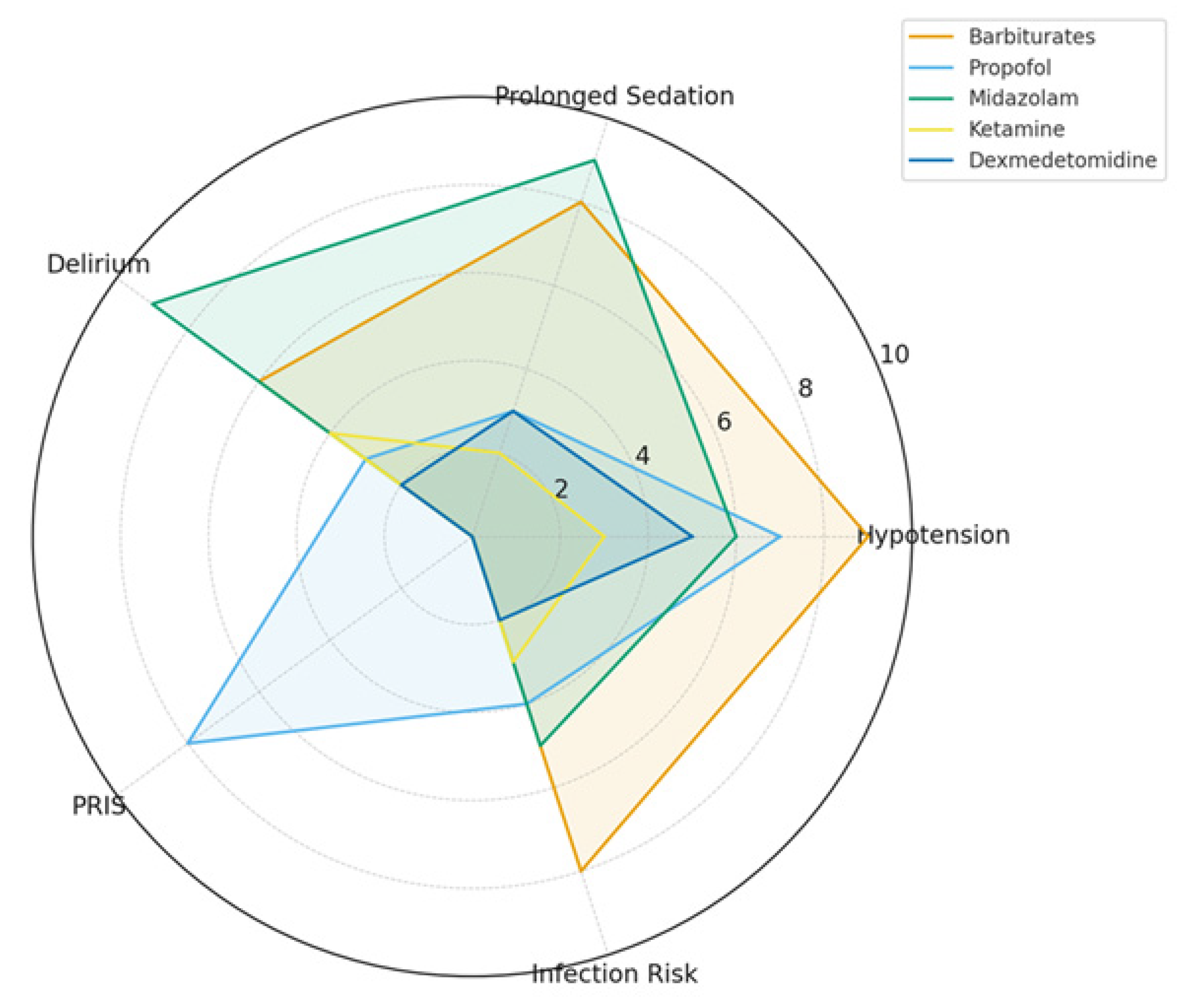
3.5. Safety Profiles and Complications
4. Discussion
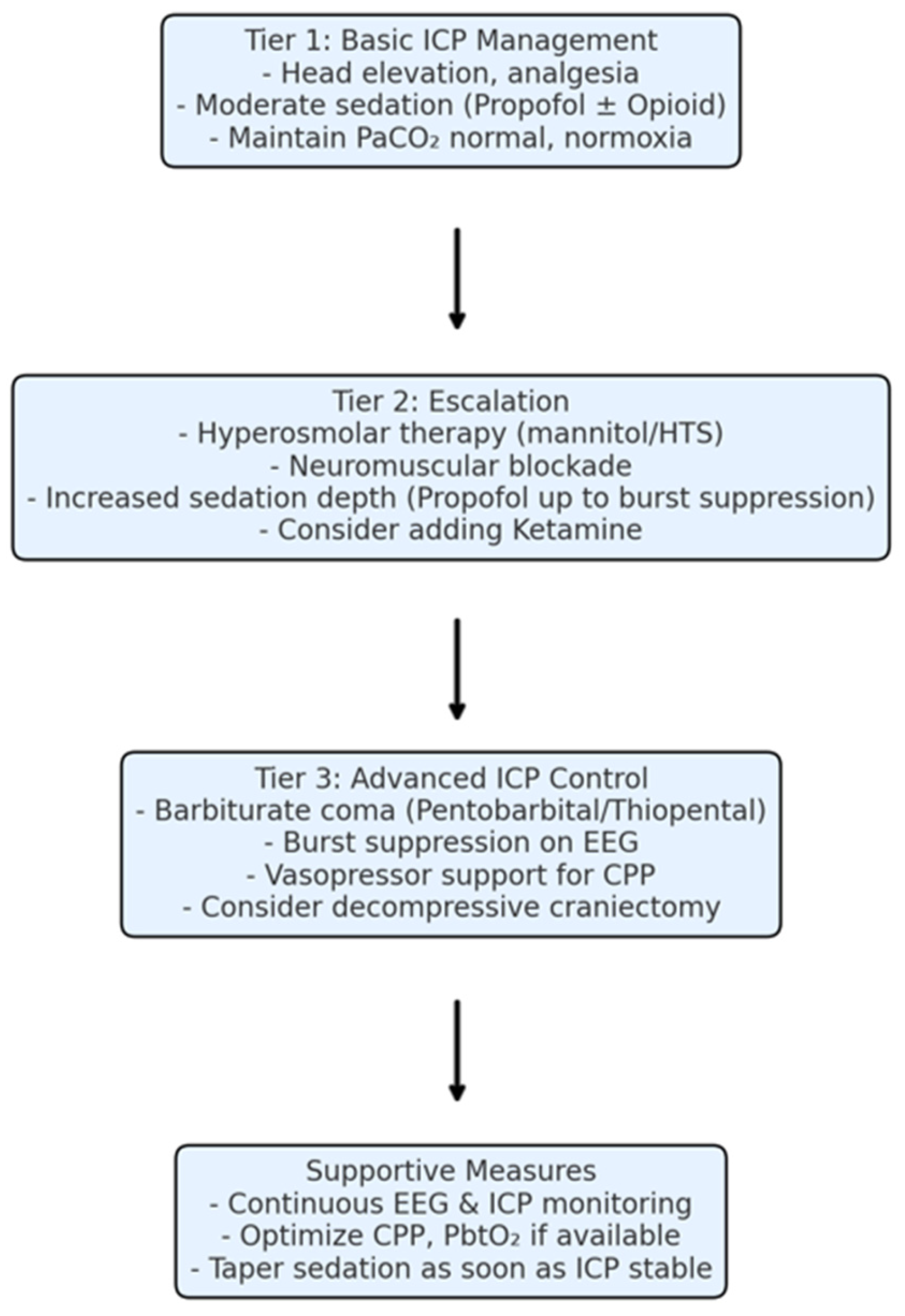
4.1. Integration of Sedation into Modern TBI Management
4.2. Clinical Implications and Recommendations
- Propofol is the workhorse for sedation in severe TBI due to its effective ICP control and ease of use. Use it as a first-line for ICP elevation and titrate as needed but be mindful of dose and duration limits. Monitor for propofol infusion syndrome beyond 48 h and prepare to adjust the regimen if metabolic disturbances appear.
- Reserve barbiturate coma for truly refractory ICP that is not controlled by optimized conventional measures (sedation, CSF drainage, osmotherapy, paralysis, mild hyperventilation). When used, ensure comprehensive monitoring. Before initiating, correct hypotension and have vasopressors ready, and place a continuous EEG if at all possible. Use a structured dosing protocol and avoid overshooting the necessary depth of burst suppression (more drug is not better once bursts are eliminated or minimal). Once ICP is controlled, start weaning as early as is safely possible to reduce exposure.
- Midazolam is useful as a supplemental or alternative sedative in certain circumstances: for instance, when prolonged deep sedation is needed and propofol must be lightened, or when treating concomitant status epilepticus. However, avoid relying on midazolam as the sole agent for refractory ICP-it is better suited for moderate sedation or seizure control. Watch for accumulation; if using >48–72 h, expect delayed awakening and plan accordingly (like no sudden expectation of awakening when the drip is turned off; it may take a day or more).
- Ketamine should be considered in TBI sedation protocols, especially for hypotensive patients or as an adjunct for analgesia. It can be started early to reduce the requirement for other sedatives that depress hemodynamics. In the setting of elevated ICP, do not hesitate to use ketamine (with ventilation controlled), as evidence indicates it will not harm and can often help. It is also beneficial during painful interventions and can reduce the use of opioids (which, in high doses, cause their own issues like hypotension and CO2 retention).
- Dexmedetomidine can improve the overall quality of sedation management, reduce delirium and facilitate neurological evaluations. Use it in the later phase of ICU care: for example, once ICP is stable or if the patient is difficult to wean off the ventilator due to agitation. It can be layered with other sedatives to achieve a more comfortable, cooperative sedation state. Avoid expecting ICP control from dexmedetomidine; instead, think of it as a means to an end: the end being a calmer emergence and possibly shorter vent time.
- Continuous EEG or BIS monitoring is strongly recommended whenever sedation is escalated to an intended burst suppression level. This not only guides dosing but also helps prognostication (for instance, if, despite deep sedation, the EEG shows no reactivity or a highly suppressed background even at modest doses, it may indicate very severe brain dysfunction-information valuable for family counseling). EEG can also catch non-convulsive seizures, which are common in severe TBI and could be contributing to ICP elevation if unnoticed.
- Incorporate sedation into a broader multimodal monitoring strategy: Use ICP monitoring to gauge the success of sedation (obviously) and also monitor CPP. Maintain CPP in the target range by fluid management and vasopressors as needed-sedation that lowers ICP at the cost of CPP is counterproductive. Utilize brain oxygen monitors (PbtO2) or microdialysis in some cases, if available, to ensure that deep metabolic suppression is not leading to ischemia. If brain oxygen drops when you deepen sedation, it might suggest overly aggressive vasoconstriction-perhaps a reason to moderate hyperventilation or raise blood pressure targets.
- Plan sedation withdrawal thoughtfully. When starting a barbiturate or heavy sedation, have an exit strategy: identify triggers for when to start weaning (e.g., ICP controlled for 24–48 h, or a maximum duration predetermined if possible). Taper slowly to prevent rebound ICP spikes. When coming off sedation, resume measures like analgesia and light sedation to keep the patient calm. Often, as mentioned, using dexmedetomidine or a low-dose midazolam during weaning can soften the transition.
- Safety protocols should be in place: for example, for pentobarbital use, a nursing protocol may involve q1h neuro checks (limited to brainstem reflexes), continuous blood pressure and ICP monitoring, and specified criteria for adjusting infusion or calling the physician (like if MAP < x or ICP > y for z minutes). For propofol, daily triglyceride checks after 2 days, and a metabolic panel for acidosis. For all sedated patients, elevate the head of the bed by 30°, protect pressure points (they cannot move, risk of bed sores), and provide prophylaxis for DVT and ulcers.
- Communication with neurosurgical colleagues is vital when using heavy sedation. For instance, if an exam is lost due to sedation, surgical decisions may rely more on imaging. Ensure serial CT scans are obtained if the patient’s exam cannot be followed. Neurosurgeons should be aware that “the ICP is controlled but exam is unavailable due to sedation”; in some cases, if prognosis is uncertain, families might be counseled that heavy sedation is being used as a temporizing measure and that the true neurologic status is unclear until sedation is off.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TBI | Traumatic Brain Injury |
| ICP | Intracranial Pressure |
| EEG | Electroencephalography |
| BIS | Bispectral Index (processed EEG depth of anesthesia monitor) |
| CMRO2 | Cerebral Metabolic Rate of Oxygen |
| CBF | Cerebral Blood Flow |
| CPP | Cerebral Perfusion Pressure |
| PRIS | Propofol-Related Infusion Syndrome |
| GCS | Glasgow Coma Scale |
| GOS | Glasgow Outcome Scale |
| RASS | Richmond Agitation–Sedation Scale |
| PbtO2 | Brain tissue partial oxygen tension (monitor of brain oxygenation) |
References
- Robba, C.; Citerio, G. How I Manage Intracranial Hypertension. Crit. Care 2019, 23, 243. [Google Scholar] [CrossRef]
- Flower, O.; Hellings, S. Sedation in Traumatic Brain Injury. Emerg. Med. Int. 2012, 2012, 637171. [Google Scholar] [CrossRef]
- Froese, L.; Hammarlund, E.U.; Åkerlund, C.; Tjerkaski, J.; Hong, E.; Lindblad, C.; Nelson, D.; Thelin, E.P.; Zeiler, F.A. The Impact of Sedative and Vasopressor Agents on Cerebrovascular Reactivity in Severe Traumatic Brain Injury. Intensive Care Med. Exp. 2023, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I.; Sydenham, E. Barbiturates for Acute Traumatic Brain Injury. Cochrane Database Syst. Rev. 1999, 3, CD000033. [Google Scholar]
- Marshall, L.F.; Smith, R.W.; Shapiro, H.M. The Outcome with Aggressive Treatment in Severe Head Injuries. J. Neurosurg. 1979, 50, 26. [Google Scholar] [CrossRef]
- Björk, S.; Hånell, A.; Ronne-Engström, E.; Stenwall, A.; Velle, F.; Lewén, A.; Enblad, P.; Wettervik, T.S. Thiopental and Decompressive Craniectomy as Last-Tier ICP-Treatments in Aneurysmal Subarachnoid Hemorrhage: Is Functional Recovery within Reach? Neurosurg. Rev. 2023, 46, 231. [Google Scholar] [CrossRef]
- Nattino, G.; Gamberini, L.; Brissy, O.; Carrara, G.; Chesnut, R.M.; Chiarini, V.; Chieregato, A.; Csomós, Á.; Fleming, J.M.; Gradišek, P.; et al. Comparative Effectiveness of Intracranial Pressure Monitoring on 6-Month Outcomes of Critically Ill Patients With Traumatic Brain Injury. JAMA Netw. Open 2023, 6, e2334214. [Google Scholar] [CrossRef]
- Bernstein, J.; Ghanchi, H.; Kashyap, S.; Podkovik, S.; Miulli, D.E.; Wacker, M.R.; Sweiss, R. Pentobarbital Coma With Therapeutic Hypothermia for Treatment of Refractory Intracranial Hypertension in Traumatic Brain Injury Patients: A Single Institution Experience. Cureus 2020, 12, e10591. [Google Scholar] [CrossRef]
- Moore, J.; Shehabi, Y.; Reade, M.C.; Bailey, M.; Fraser, J.F.; Murray, L.; Anstey, C.; Singer, M. Stress Response during Early Sedation with Dexmedetomidine Compared with Usual-Care in Ventilated Critically Ill Patients. Crit. Care 2022, 26, 359. [Google Scholar] [CrossRef]
- Ahmed, M.N.U.; Afifa, M.; Salim, M.; Asaduzzaman, M.; Rahman, A.F. Dexmedetomidine for Sedation and Analgesia in Mechanically Ventilated Patients. Sch. J. Appl. Med. Sci. 2021, 9, 149. [Google Scholar] [CrossRef]
- Chrysostomou, C.; Schmitt, C.G. Dexmedetomidine: Sedation, Analgesia and Beyond. Expert Opin. Drug Metab. Toxicol. 2008, 4, 619. [Google Scholar] [CrossRef]
- Gerlach, A.T.; Murphy, C.V. Sedation with Dexmedetomidine in the Intensive Care Setting. Open Access Emerg. Med. 2011, 3, 77–85. [Google Scholar] [CrossRef]
- Battaglini, D.; Anania, P.; Rocco, P.R.M.; Brunetti, I.; Prior, A.; Zona, G.; Pelosi, P.; Fiaschi, P. Escalate and De-Escalate Therapies for Intracranial Pressure Control in Traumatic Brain Injury. Front. Neurol. 2020, 11, 564751. [Google Scholar] [CrossRef]
- Wu, J.; Vogel, T.R.; Gao, X.; Lin, B.; Kulwin, C.; Chen, J. Neuroprotective Effect of Dexmedetomidine in a Murine Model of Traumatic Brain Injury. Sci. Rep. 2018, 8, 4935. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, E.; Agrawal, A.; Cincu, R.; Janjua, T.; Moscote-Salazar, L.R. Cooperative Sedation in Moderate Traumatic Brain Injury: A Tool for Neurocritical Care Management. J. Neurointensive Care 2023, 6, 67. [Google Scholar] [CrossRef]
- Yang, G. Research Progresses in Effects of Analgesics and Sedatives on Intracranial Pressure of Neurointensive Care Patients. World J. Neurosci. 2022, 12, 118. [Google Scholar] [CrossRef]
- Dabricot, E.; Seqat, I.; Dailler, F.; Rheims, S.; Boulogne, S.; Balança, B. How to Monitor Thiopental Administration in the Intensive Care Unit for Refectory Status Epilepticus or Intracranial Hypertension? Crit. Care 2021, 25, 439. [Google Scholar] [CrossRef] [PubMed]
- Majdán, M.; Mauritz, W.; Wilbacher, I.; Bražinová, A.; Rusňák, M.; Leitgeb, J. Barbiturates Use and Its Effects in Patients with Severe Traumatic Brain Injury in Five European Countries. J. Neurotrauma 2012, 30, 23. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, Y.; Morioka, M.; Negoto, T.; Orito, K.; Yoshitomi, M.; Nakamura, Y.; Takeshige, N.; Yamamoto, M.; Takeuchi, Y.; Oda, K.; et al. A Novel Step-down Infusion Method of Barbiturate Therapy: Its Safety and Effectiveness for Intracranial Pressure Control. Pharmacol. Res. Perspect. 2021, 9, e00719. [Google Scholar] [CrossRef]
- Adembri, C.; Venturi, L.; Pellegrini-Giampietro, D.E. Neuroprotective Effects of Propofol in Acute Cerebral Injury. CNS Drug Rev. 2007, 13, 333. [Google Scholar] [CrossRef]
- Murthy, T. Propofol in Neurotrauma. Indian J. Neurotrauma 2008, 5, 41. [Google Scholar] [CrossRef]
- Kotani, Y.; Shimazawa, M.; Yoshimura, S.; Iwama, T.; Hara, H. The Experimental and Clinical Pharmacology of Propofol, an Anesthetic Agent with Neuroprotective Properties. CNS Neurosci. Ther. 2008, 14, 95. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yue, W. Comparative Analysis of Dexmedetomidine, Midazolam, and Propofol Impact on Epilepsy-Related Mortality in the ICU: Insights from the MIMIC-IV Database. BMC Neurol. 2024, 24, 193. [Google Scholar] [CrossRef]
- Farrokh, S.; Tahsili-Fahadan, P.; Ritzl, E.K.; Lewin, J.J.; Mirski, M.A. Antiepileptic Drugs in Critically Ill Patients. Crit. Care 2018, 22, 153. [Google Scholar] [CrossRef]
- Rai, S.; Drislane, F.W. Treatment of Refractory and Super-Refractory Status Epilepticus. Neurotherapeutics 2018, 15, 697. [Google Scholar] [CrossRef]
- Tranung, M.; Solheim, T.S.; Løhre, E.T.; Marsaa, K.; Haugen, D.F.; Laird, B.; Thronæs, M.; Larsen, M.D. Midazolam Indications and Dosing in Palliative Medicine: Results from a Multinational Survey. Curr. Oncol. 2024, 31, 4093–4104. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, F.; Igneri, L.; Yilmaz, Y.A.; Burakgazi-Dalkilic, E. Anesthetic Use In Status Epilepticus: A Concise Review. Cooper Rowan Med. J. 2020, 20, 20–36. [Google Scholar] [CrossRef]
- Xu, D.; Wang, B.; Zhao, X.; Zheng, Y.; Du, J.; Wang, Y. General Anesthetics Protects against Cardiac Arrest-Induced Brain Injury by Inhibiting Calcium Wave Propagation in Zebrafish. Mol. Brain 2017, 10, 44. [Google Scholar] [CrossRef]
- Jędrzejczak, J.; Mazurkiewicz-Bełdzińska, M.; Szmuda, M.; Majkowska-Zwolińska, B.; Steinborn, B.; Ryglewicz, D.; Owczuk, R.; Bartkowska-Śniatkowska, A.; Emich-Widera, E.; Rejdak, K.; et al. Convulsive Status Epilepticus Management in Adults and Children: Report of the Working Group of the Polish Society of Epileptology. Neurol. I Neurochir. Pol. 2018, 52, 419. [Google Scholar] [CrossRef]
- Fisch, U.; Jünger, A.L.; Hert, L.; Rüegg, S.; Sutter, R. Therapeutically Induced EEG Burst-Suppression Pattern to Treat Refractory Status Epilepticus-What Is the Evidence? Z. Epileptol. 2022, 35, 303. [Google Scholar] [CrossRef]
- Simioli, F.; Annunziata, A.; Coppola, A.; Imitazione, P.; Mirizzi, A.I.; Marotta, A.; D’Angelo, R.; Fiorentino, G. The Role of Dexmedetomidine in ARDS: An Approach to Non-Intensive Care Sedation. Front. Med. 2023, 10, 1224242. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Dexmedetomidine: Present and Future Directions. Korean J. Anesthesiol. 2019, 72, 323. [Google Scholar] [CrossRef]
- Nelson, S.; Muzyk, A.J.; Bucklin, M.H.; Brudney, S.; Gagliardi, J.P. Defining the Role of Dexmedetomidine in the Prevention of Delirium in the Intensive Care Unit. BioMed Res. Int. 2015, 2015, 635737. [Google Scholar] [CrossRef]
- Ruijter, B.J.; van Putten, M.J.A.M.; van den Bergh, W.M.; Tromp, S.C.; Hofmeijer, J. Propofol Does Not Affect the Reliability of Early EEG for Outcome Prediction of Comatose Patients after Cardiac Arrest. Clin. Neurophysiol. 2019, 130, 1263. [Google Scholar] [CrossRef] [PubMed]
- Phabphal, K.; Chisurajinda, S.; Somboon, T.; Unwongse, K.; Geater, A. Does Burst-Suppression Achieve Seizure Control in Refractory Status Epilepticus? BMC Neurol. 2018, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Musialowicz, T.; Mervaala, E.; Kälviäinen, R.; Uusaro, A.; Ruokonen, E.; Parviainen, I. Can BIS Monitoring Be Used to Assess the Depth of Propofol Anesthesia in the Treatment of Refractory Status Epilepticus? Epilepsia 2010, 51, 1580. [Google Scholar] [CrossRef]
- Kratzer, S.; Schneider, M.; Obert, D.P.; Schneider, G.; García, P.S.; Kreuzer, M. Age-Related EEG Features of Bursting Activity During Anesthetic-Induced Burst Suppression. Front. Syst. Neurosci. 2020, 14, 599962. [Google Scholar] [CrossRef]
- Westover, M.B.; Kim, S.; Ching, S.; Purdon, P.L.; Brown, E.N. Robust Control of Burst Suppression for Medical Coma. J. Neural Eng. 2015, 12, 46004. [Google Scholar] [CrossRef]
- Greenwood, B.C.; Adams, C.; Khalpey, Z.; Hou, P.C. Barbiturate Coma: Rebound and Refractory Hyperkalemia. Case Rep. Clin. Med. 2014, 3, 304. [Google Scholar] [CrossRef]
- Sharma, R.; Tsikvadze, M.; Peel, J.B.; Howard, L.; Kapoor, N.; Freeman, W.D. Multimodal Monitoring: Practical Recommendations (Dos and Don’ts) in Challenging Situations and Uncertainty. Front. Neurol. 2023, 14, 1135406. [Google Scholar] [CrossRef]
- Dagod, G.; Laurens, M.; Roustan, J.; Deras, P.; Courvalin, E.; Girard, M.; Weber, H.; Capdevila, X.; Charbit, J. Impact of Lumbar Cerebrospinal Fluid Drainage to Control Intracranial Hypertension in Patients with Severe Traumatic Brain Injury: A Retrospective Monocentric Cohort. Res. Sq. 2024; preprint. [Google Scholar] [CrossRef]
- Kim, J.O.; Shim, Y.; Choo, Y.-H.; Kim, H.S.; Kim, Y.; Ha, E.J. Protein Requirement Changes According to the Treatment Application in Neurocritical Patients. J. Korean Neurosurg. Soc. 2023, 67, 451. [Google Scholar] [CrossRef]
- Freeman, W.D. How I Manage ICP-CPP: A Visual, yet Individualized Approach. Crit. Care 2019, 23, 287. [Google Scholar] [CrossRef]
- Abdelmonem, S.; Helmy, T.A.; Sayed, I.E.; Ghazal, S. A Prospective Study on Severe Hypotension in Critically Ill Patients Sedated with Propofol. Int. J. Crit. Care Emerg. Med. 2019, 5, 078. [Google Scholar] [CrossRef]
- Patel, S.; Maria-Rios, J.; Parikh, A.; Okorie, O.N. Diagnosis and Management of Elevated Intracranial Pressure in the Emergency Department. Int. J. Emerg. Med. 2023, 16, 72. [Google Scholar] [CrossRef]
- Bugedo, G.; Santis, C. Intracranial Hypertension and Deep Sedation. Crit. Care 2019, 23, 342. [Google Scholar] [CrossRef] [PubMed]
- Mirrakhimov, E.M.; Voore, P.; Halytskyy, O.; Khan, M.; Ali, A.M. Propofol Infusion Syndrome in Adults: A Clinical Update. Crit. Care Res. Pract. 2015, 2015, 260385. [Google Scholar]
- Ayele, T.T.; Ezeh, E.; Al-Qawasmi, L.; Ugonabo, O.S.; Saylor, J.R.; Dial, L. Diagnosed by Reversibility: Unmasking Propofol-Related Infusion Syndrome in a Critically-Ill Elderly Male. Cureus 2022, 14, e23504. [Google Scholar] [CrossRef]
- Singh, A.; Anjankar, A.P. Propofol-Related Infusion Syndrome: A Clinical Review. Cureus 2022, 14, e30383. [Google Scholar] [CrossRef]
- Spitzfaden, A.C.; Jimenez, D.F.; Tobias, J.D. Propofol for Sedation and Control of Intracranial Pressure in Children. Pediatr. Neurosurg. 1999, 31, 194. [Google Scholar] [CrossRef]
- Chang, L.C.; Raty, S.R.; Ortiz, J.; Bailard, N.S.; Mathew, S.J. The Emerging Use of Ketamine for Anesthesia and Sedation in Traumatic Brain Injuries. CNS Neurosci. Ther. 2013, 19, 390. [Google Scholar] [CrossRef]
- Peters, A.; Villasana, L.; Schnell, E. Ketamine Alters Hippocampal Cell Proliferation and Improves Learning in Mice after Traumatic Brain Injury. Anesthesiology 2018, 129, 278. [Google Scholar] [CrossRef]
- Vella, M.A.; Crandall, M.; Patel, M.B. Acute Management of Traumatic Brain Injury. Surg. Clin. N. Am. 2017, 97, 1015. [Google Scholar] [CrossRef]
- Cioccari, L.; Luethi, N.; Bailey, M.; Shehabi, Y.; Howe, B.; Messmer, A.S.; Proimos, H.; Peck, L.; Young, H.; Eastwood, G.M.; et al. The Effect of Dexmedetomidine on Vasopressor Requirements in Patients with Septic Shock: A Subgroup Analysis of the Sedation Practice in Intensive Care Evaluation [SPICE III] Trial. Crit. Care 2020, 24, 441. [Google Scholar] [CrossRef] [PubMed]
- Syrous, N.S.; Sundstrøm, T.; Søfteland, E.; Jammer, I. Effects of Intraoperative Dexmedetomidine Infusion on Postoperative Pain after Craniotomy: A Narrative Review. Brain Sci. 2021, 11, 1636. [Google Scholar] [CrossRef] [PubMed]
- Tadler, S.C.; Jones, K.; Lybbert, C.; Huang, J.C.; Jawish, R.; Solzbacher, D.; Kendrick, E.; Pierson, M.D.; Weischedel, K.; Rana, N.; et al. Propofol for Treatment Resistant Depression: A Randomized Controlled Trial. medRxiv, 2023; preprint. [Google Scholar] [CrossRef]
- Fleischmann, A.; Pilge, S.; Kiel, T.; Kratzer, S.; Schneider, G.; Kreuzer, M. Substance-Specific Differences in Human Electroencephalographic Burst Suppression Patterns. Front. Hum. Neurosci. 2018, 12, 368. [Google Scholar] [CrossRef]
- Krishnamurthy, K.B.; Drislane, F.W. Depth of EEG Suppression and Outcome in Barbiturate Anesthetic Treatment for Refractory Status Epilepticus. Epilepsia 1999, 40, 759. [Google Scholar] [CrossRef]
- Paul, L.; Greve, S.; Hegemann, J.; Gienger, S.; Löffelhardt, V.T.; Marina, A.D.; Felderhoff-Müser, U.; Dohna-Schwake, C.; Bruns, N. Association of Bilaterally Suppressed EEG Amplitudes and Outcomes in Critically Ill Children. Front. Neurosci. 2024, 18, 1411151. [Google Scholar] [CrossRef] [PubMed]
- Legriel, S. Burst Suppression as a Treatment Goal in Refractory Status Epilepticus. Neurocrit. Care 2023, 40, 847. [Google Scholar] [CrossRef]
- Woodcock, J.; Ropper, A.H.; Kennedy, S.K. High Dose Barbiturates in Non-Traumatic Brain Swelling: ICP Reduction and Effect on Outcome. Stroke 1982, 13, 785. [Google Scholar] [CrossRef]
- Huh, J.W.; Lang, S.-S.; Kilbaugh, T.J. Craniectomy for Traumatic Intracranial Hypertension. N. Engl. J. Med. 2016, 375, 2401. [Google Scholar]
- Herregods, L.; Verbeke, J.; Roily, G.; Colardyn, F. Effect of Propofol on Elevated Intracranial Pressure. Prelim. Results Anaesth. 1988, 43, 107. [Google Scholar] [CrossRef]
- Gaohua, L.; Kimura, H. Simulation of Propofol Anaesthesia for Intracranial Decompression Using Brain Hypothermia Treatment. Theor. Biol. Med. Model. 2007, 4, 46. [Google Scholar] [CrossRef]
- Wu, M.; Yin, X.; Chen, M.; Liu, Y.; Zhang, X.; Li, T.; Long, Y.; Wu, X.; Pu, L.; Zhang, M.; et al. Effects of Propofol on Intracranial Pressure and Prognosis in Patients with Severe Brain Diseases Undergoing Endotracheal Suctioning. BMC Neurol. 2020, 20, 394. [Google Scholar] [CrossRef]
- Cabrera-Arrocha, J.; Martín-Sánchez, E.; Farinas-Roman, O.; Ravelo-Hernandez, P.; Saavedra, P.; Santana, S.R. Risk Factors Associated to Morbid-Mortality Previous to Starting Hypothermia Treatment in ICU Patients after Cardiac Arrest. Intensive Care Med. 2012, 38, S39. [Google Scholar]
- Ali, M.A.; Hashmi, M.; Ahmed, W.; Raza, S.A.; Khan, M.F.; Salim, B. Incidence and Risk Factors of Delirium in Surgical Intensive Care Unit. Trauma Surg. Acute Care Open 2021, 6, e000564. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Arrocha, J.; Linares-Buitrago, D.; Ramírez-Cardozo, J.L.; Gómez-Lama, J.M.; Santana, S.R.; Saavedra, P. Risk Factors Associated to Morbid-Mortality in ICU Patients with Stibarachnoid Hemorrhage. Intensive Care Med. 2012, 38, S110. [Google Scholar]
- Amer, M.; Maghrabi, K.; Bawazeer, M.; Alshaikh, K.; Shaban, M.; Rizwan, M.; Amin, R.; Vol, E.D.; Baali, M.; Altewerki, M.; et al. Adjunctive Ketamine for Sedation in Critically Ill Mechanically Ventilated Patients: An Active-Controlled, Pilot, Feasibility Clinical Trial. J. Intensive Care 2021, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Madsen, F.A.; Andreasen, T.H.; Lindschou, J.; Gluud, C.; Møller, K. Ketamine for Critically Ill Patients with Severe Acute Brain Injury: Protocol for a Systematic Review with Meta-Analysis and Trial Sequential Analysis of Randomised Clinical Trials. PLoS ONE 2021, 16, e0259899. [Google Scholar] [CrossRef] [PubMed]
- Van der Kooi, A.; Tulen, J.H.; de Weerd, A.W.; Van Eijk, M.M.; Van Uitert, M.J.; De Rooij, S.E.; van Munster, B.C.; Slooter, A.J. Sleep Monitoring by Actigraphy in Short-Stay ICU Patients. Crit. Care 2012, 16, P320. [Google Scholar] [CrossRef][Green Version]
- Schomer, K.J.; Sebat, C.M.; Adams, J.Y.; Duby, J.J.; Shahlaie, K.; Louie, E.L. Dexmedetomidine for Refractory Intracranial Hypertension. J. Intensive Care Med. 2017, 34, 62. [Google Scholar] [CrossRef]
- Clark, J.; Ellens, N.; Figueroa, B. The Use of Barbiturate-Induced Coma during Cerebrovascular Neurosurgery Procedures: A Review of the Literature. Brain Circ. 2015, 1, 140. [Google Scholar] [CrossRef]
- Espinoza, A.; Vidaurre, J.; Torres, A. Scientific Evidence in the Management of Traumatic Brain Injury in Pediatrics: Ibero-American Academy of Pediatric Neurology. Bol. Méd. Hosp. Infant. Méx. 2024, 81, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Kassis, E.B.; Beitler, J.R.; Talmor, D. Lung-Protective Sedation: Moving toward a New Paradigm of Precision Sedation. Intensive Care Med. 2022, 49, 91. [Google Scholar] [CrossRef]
- Aragón, R.E.; Proaño, Á.; Mongilardi, N.; Ferrari, A.; Herrera, P.; Roldán, R.; Paz, E.; Jaymez, A.A.; Chirinos, E.; Portugal, J.L.; et al. Sedation Practices and Clinical Outcomes in Mechanically Ventilated Patients in a Prospective Multicenter Cohort. Crit. Care 2019, 23, 130. [Google Scholar] [CrossRef]
- Ziaka, M.; Exadaktylos, A.K. Brain–Lung Interactions and Mechanical Ventilation in Patients with Isolated Brain Injury. Crit. Care 2021, 25, 358. [Google Scholar] [CrossRef]
- Schizodimos, T.; Soulountsi, V.; Iasonidou, C.; Kapravelos, N. An Overview of Management of Intracranial Hypertension in the Intensive Care Unit. J. Anesth. 2020, 34, 741. [Google Scholar] [CrossRef]
- Anania, P.; Battaglini, D.; Miller, J.P.; Balestrino, A.; Prior, A.; D’Andrea, A.; Badaloni, F.; Pelosi, P.; Robba, C.; Zona, G.; et al. Escalation Therapy in Severe Traumatic Brain Injury: How Long Is Intracranial Pressure Monitoring Necessary? Neurosurg. Rev. 2020, 44, 2415. [Google Scholar] [CrossRef]
- Dolmans, R.G.F.; Russo, G.; Anstey, J.; Steyerberg, E.W.; Taccone, F.S.; Udy, A.; Citerio, G.; Ichai, C.; Badenes, R.; Prowle, J.; et al. Comparative Effectiveness of Midazolam-Based Sedation on the Need for Intracranial Pressure Lowering Therapies in Traumatic Brain Injury. Neurotrauma Rep. 2025, 6, 242–250. [Google Scholar]
- Bayliff, C.D.; Schwartz, M.L.; Hardy, B.G. Pharmacokinetics of High-Dose Pentobarbital in Severe Head Trauma. Clin. Pharmacol. Ther. 1985, 38, 457. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Heybati, K.; Yadav, H. Protocol for the Development and Validation of a Machine-Learning Based Tool for Predicting the Risk of Hypertriglyceridemia in Critically-Ill Patients Receiving Propofol Sedation. medRxiv, 2024; preprint. [Google Scholar] [CrossRef]
- Kovacevic, M.P.; Dube, K.M.; Lupi, K.; Szumita, P.M.; DeGrado, J.R. Evaluation of Hypertriglyceridemia in Critically Ill Patients With Coronavirus Disease 2019 Receiving Propofol. Crit. Care Explor. 2021, 3, e0330. [Google Scholar] [CrossRef] [PubMed]
- Hogan, J.; Sun, H.; Nour, H.A.; Jing, J.; Tabaeizadeh, M.; Shoukat, M.; Javed, F.; Kassa, S.; Edhi, M.M.; Bordbar, E.; et al. Burst Suppression: Causes and Effects on Mortality in Critical Illness. Neurocrit. Care 2020, 33, 565. [Google Scholar] [CrossRef]
- Nordt, S.P.; Clark, R.F. Midazolam: A Review of Therapeutic Uses and Toxicity. J. Emerg. Med. 1997, 15, 357. [Google Scholar] [CrossRef]
- Yang, R.; Zhao, R.; Chaudry, F.; Wang, T.; Brunton, P.; Khurshid, Z.; Ratnayake, J. Modern Sedative Agents and Techniques Used in Dentistry for Patients with Special Needs: A Review. J. Taibah Univ. Med. Sci. 2023, 19, 153. [Google Scholar] [CrossRef]
- Gałecki, P.; Bliźniewska-Kowalska, K.; Cubała, W.J.; Depukat, A.; Mosiołek, A.; Rybakowski, J.; Samochowiec, J.; Sobolewski, B.; Szulc, A.; Dudek, D. Polish Standard of Treatment with Racemic Ketamine for Patients with Depressive Disorders Developed by a Working Group Appointed by the National Consultant in the Field of Psychiatry. Psychiatr. Pol. 2024, 58, 377. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.Á.R.; Jambrina, C.C.; Vallhonrat, I.L.; Fernández, I. Indications of Dexmedetomidine in the Current Sedoanalgesia Trends in the Critical Patient. Med. Intensiv. (Engl. Ed.) 2014, 38, 41. [Google Scholar] [CrossRef]
- Helbok, R.; Kurtz, P.; Schmidt, J.M.; Stuart, M.; Fernandez, L.; Connolly, S.; Lee, K.; Schmutzhard, E.; Mayer, S.A.; Claassen, J.; et al. Effects of the Neurological Wake-up Test on Clinical Examination, Intracranial Pressure, Brain Metabolism and Brain Tissue Oxygenation in Severely Brain-Injured Patients. Crit. Care 2012, 16, R226. [Google Scholar] [CrossRef] [PubMed]
- Musick, S.; Alberico, A. Neurologic Assessment of the Neurocritical Care Patient. Front. Neurol. 2021, 12, 588989. [Google Scholar] [CrossRef]
- Gao, J.; Wei, L.; Xu, G.; Ren, C.; Zhang, Z.; Liu, Y. Effects of Dexmedetomidine vs Sufentanil during Percutaneous Tracheostomy for Traumatic Brain Injury Patients. Medicine 2019, 98, e17012. [Google Scholar] [CrossRef]
- Kurni, M.; Kaloria, N.; Hazarika, A.; Jain, K.; Gupta, S.; Walia, R. Comparison of Midazolam and Propofol Infusion to Suppress Stress Response in Patients With Severe Traumatic Brain Injury: A Prospective, Randomized Controlled Trial. Korean J. Neurotrauma 2023, 19, 70. [Google Scholar] [CrossRef]
- Rhoney, D.H.; Parker, D.W.L. Use of Sedative and Analgesic Agents in Neurotrauma Patients: Effects on Cerebral Physiology. Neurol. Res. 2001, 23, 237. [Google Scholar] [CrossRef]
- Lenell, S.; Lewén, A.; Howells, T.; Enblad, P. Neurointensive Care of Traumatic Brain Injury in the Elderly-Age-Specific Secondary Insult Levels and Optimal Physiological Levels to Target Need to Be Defined. Acta Neurochir. 2021, 164, 117. [Google Scholar] [CrossRef]
- Tasker, R.C. Analgesia, Sedation, and Intracranial Pressure. Crit. Care Med. 2016, 44, 851. [Google Scholar] [CrossRef] [PubMed]
- Dengler, B.A.; Karam, O.; Barthol, C.A.; Chance, A.; Snider, L.E.; Mundy, C.M.; Bounajem, M.T.; Johnson, W.C.; Maita, M.M.; Mendez-Gomez, P.M.; et al. Ketamine Boluses Are Associated with a Reduction in Intracranial Pressure and an Increase in Cerebral Perfusion Pressure: A Retrospective Observational Study of Patients with Severe Traumatic Brain Injury. Crit. Care Res. Pract. 2022, 2022, 3834165. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, C. Deciding Under Uncertainty: The Case of Refractory Intracranial Hypertension. Front. Neurol. 2020, 11, 908. [Google Scholar] [CrossRef]
- Schalén, W.; Messeter, K.; Nordström, C.-H. Complications and Side Effects during Thiopentone Therapy in Patients with Severe Head Injuries. Acta Anaesthesiol. Scand. 1992, 36, 369. [Google Scholar] [CrossRef]
- Hawkes, M.A.; Eliliwi, M.; Wijdicks, E.F.M. The Origin of the Burst-Suppression Paradigm in Treatment of Status Epilepticus. Neurocrit. Care 2023, 40, 849. [Google Scholar] [CrossRef]
- Amornyotin, S. Ketofol: A Combination of Ketamine and Propofol. J. Anesth. Crit. Care Open Access 2014, 1, 00031. [Google Scholar] [CrossRef][Green Version]
- Szántó, D.; Gál, J.; Tankó, B.; Síró, P.; Jakab, Z.; Luterán, P.; Fülesdi, B.; Molnár, C. Pediatric Neuroanesthesia-A Review of the Recent Literature. Curr. Anesthesiol. Rep. 2022, 12, 467. [Google Scholar] [CrossRef]
- Dolmans, R.G.F.; Nahed, B.V.; Robertson, F.C.; Peul, W.C.; Rosenthal, E.S.; Broekman, M.L.D. Practice-Pattern Variation in Sedation of Neurotrauma Patients in the Intensive Care Unit: An International Survey. J. Intensive Care Med. 2023, 38, 1143. [Google Scholar] [CrossRef]
- Kornilov, E.; Erdman, H.B.; Kahana, E.; Fireman, S.; Zarchi, O.; Israelashvili, M.; Reiner, J.; Glik, A.; Weiss, P.; Paz, R.; et al. Deep Brain Stimulation Surgery under Ketamine Induced Conscious Sedation: A Double Blind Randomized Controlled Trial. medRxiv, 2023; preprint. [Google Scholar] [CrossRef]
- Bekkevold, M.; Kvåle, R.; Brattebø, G. Relation of Reported Sedation and Ventilator Weaning Practices to Ventilator Time in Norwegian Intensive Care Units. J. Crit. Care Med. 2015, 2015, 173985. [Google Scholar] [CrossRef]
- Brasil, S.; Godoy, D.A.; Videtta, W.; Rubiano, A.M.; Solla, D.J.F.; Taccone, F.S.; Robba, C.; Rasulo, F.; Aries, M.; Smielewski, P.; et al. A Comprehensive Perspective on Intracranial Pressure Monitoring and Individualized Management in Neurocritical Care: Results of a Survey with Global Experts. Neurocrit. Care 2024, 41, 880. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.; Bhende, B.; Mathur, R.; Gonzalez, L.F.; Shah, V. Individualized Autoregulation-Guided Arterial Blood Pressure Management in Neurocritical Care. Neurotherapeutics 2025, 22, e00526. [Google Scholar] [CrossRef]
- Froese, L.; Gomez, A.; Sainbhi, A.S.; Batson, C.; Slack, T.; Stein, K.Y.; Mathieu, F.; Zeiler, F.A. Optimal Bispectral Index Level of Sedation and Cerebral Oximetry in Traumatic Brain Injury: A Non-Invasive Individualized Approach in Critical Care? Intensive Care Med. Exp. 2022, 10, 33. [Google Scholar] [CrossRef]
- Wettervik, T.S.; Lewén, A.; Enblad, P. Fine Tuning of Neurointensive Care in Aneurysmal Subarachnoid Hemorrhage: From One-Size-Fits-All towards Individualized Care. World Neurosurg. X 2023, 18, 100160. [Google Scholar] [CrossRef]
- Hawryluk, G.W.J.; Aguilera, S.; Büki, A.; Bulger, E.M.; Citerio, G.; Cooper, D.J.; Arrastia, R.D.; Diringer, M.N.; Figaji, A.; Gao, G.; et al. A Management Algorithm for Patients with Intracranial Pressure Monitoring: The Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2019, 45, 1783. [Google Scholar] [CrossRef]
- Frohlich, J.; Johnson, M.; McArthur, D.L.; Lutkenhoff, E.S.; Dell’Italia, J.; Real, C.; Shrestha, V.; Spivak, N.M.; Tejeda, J.E.R.; Vespa, P.; et al. Sedation-Induced Burst Suppression Predicts Positive Outcome Following Traumatic Brain Injury. Front. Neurol. 2021, 12, 750667. [Google Scholar] [CrossRef] [PubMed]
- Coronelli, M.M.; Coppi, F.; Mattioli, A.V. Inflammation, atherosclerosis and hypertension: The impact of depression and stress on their complex relationship. Future Cardiol. 2024, 20, 27–33. [Google Scholar] [CrossRef]
- Mattioli, A.V.; Coppi, F.; Bucciarelli, V.; Gallina, S. Cardiovascular risk stratification in young women: The pivotal role of pregnancy. J. Cardiovasc. Med. 2023, 24, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Coppi, F.; Bucciarelli, V.; Solodka, K.; Selleri, V.; Zanini, G.; Pinti, M.; Nasi, M.; Salvioli, B.; Nodari, S.; Gallina, S.; et al. The Impact of Stress and Social Determinants on Diet in Cardiovascular Prevention in Young Women. Nutrients 2024, 16, 1044. [Google Scholar] [CrossRef]
| Agent | Mechanism of Action | ICP Effect | Burst Suppression | Safety Profile/Adverse Effects |
|---|---|---|---|---|
| Barbiturates (Pentobarbital/Thiopental) | GABA_A agonist, glutamate inhibition | Strong decrease, effective in refractory ICP | Yes, potent and reliable | Hypotension, immunosuppression, prolonged coma |
| Propofol | GABA_A agonist, NMDA modulation | Strong decrease, titratable | Yes, achievable at high doses | Hypotension, risk of PRIS, hypertriglyceridemia |
| Midazolam | GABA_A potentiator (benzodiazepine site) | Moderate decrease | Not reliably, only at very high doses | Accumulation, delirium risk, prolonged sedation |
| Ketamine | NMDA antagonist, sympathomimetic | Neutral to mild decrease (ICP safe) | No (does not induce burst suppression) | Tachycardia, hypertension, increased secretions |
| Dexmedetomidine | α2-adrenergic agonist | Minimal effect, ICP-neutral | No | Bradycardia, hypotension, arousable sedation |
| Agent | Loading Dose | Maintenance Infusion | EEG Target |
|---|---|---|---|
| Pentobarbital | 10 mg/kg over 30 min, then 5 mg/kg q1h X3 | 1–3 mg/kg/h | Burst suppression, 2–5 bursts/min |
| Propofol | Bolus 1–2 mg/kg (optional) | 2–5 mg/kg/h (up to 10 for burst suppression) | Burst suppression, BIS < 20 |
| Midazolam | 0.05–0.2 mg/kg IV bolus | 0.1–0.4 mg/kg/h | Slowing, not reliable burst suppression |
| Ketamine | 0.5–1 mg/kg IV bolus | 0.5–3 mg/kg/h | No burst suppression, adjunct role |
| Dexmedetomidine | Avoid bolus (risk of brady/hypotension) | 0.2–0.7 mcg/kg/h | Light sedation, cooperative state |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Swiss Federation of Clinical Neuro-Societies. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đilvesi, Đ.; Tubić, T.; Maričić Prijić, S.; Golubović, J. Evolution of Pharmacologic Induction of Burst Suppression in Adult TBI: Barbiturate Coma Versus Modern Sedatives. Clin. Transl. Neurosci. 2025, 9, 53. https://doi.org/10.3390/ctn9040053
Đilvesi Đ, Tubić T, Maričić Prijić S, Golubović J. Evolution of Pharmacologic Induction of Burst Suppression in Adult TBI: Barbiturate Coma Versus Modern Sedatives. Clinical and Translational Neuroscience. 2025; 9(4):53. https://doi.org/10.3390/ctn9040053
Chicago/Turabian StyleĐilvesi, Đula, Teodora Tubić, Sanja Maričić Prijić, and Jagoš Golubović. 2025. "Evolution of Pharmacologic Induction of Burst Suppression in Adult TBI: Barbiturate Coma Versus Modern Sedatives" Clinical and Translational Neuroscience 9, no. 4: 53. https://doi.org/10.3390/ctn9040053
APA StyleĐilvesi, Đ., Tubić, T., Maričić Prijić, S., & Golubović, J. (2025). Evolution of Pharmacologic Induction of Burst Suppression in Adult TBI: Barbiturate Coma Versus Modern Sedatives. Clinical and Translational Neuroscience, 9(4), 53. https://doi.org/10.3390/ctn9040053






