Abstract
Recently, it has become increasingly clear that there is a bidirectional relationship between sleep/circadian rhythms and neurodegeneration. Knowledge about this topic further improved after the description of the glymphatic system, which is mainly active during sleep. Changes in sleep and circadian rhythms are present not only in overt neurodegenerative diseases but also in their early, prodromal, and preclinical phases, supporting that they precede (and contribute to) the development of neurodegeneration. This narrative review provides a brief overview of sleep and circadian rhythm disruption in neurodegeneration, highlights the bidirectional relationship between sleep changes and neurodegeneration, and addresses future perspectives, in particular, whether sleep changes are able to predict neurodegeneration and the potential sleep actionability to prevent or modulate the development of neurodegenerative diseases.
1. Introduction
Sleep has been long thought to be a passive state of inactivity and rest, not only of the body but also of the brain. However, it is now clear that sleep is a highly regulated function, and that several brain areas and circuits are actively interacting to promote and maintain sleep, as well as to ensure that different sleep stages occur in a cyclic pattern. Circadian rhythms are also highly regulated and interact with sleep circuits.
Sleep is classified into non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep. NREM sleep is divided into stages N1, N2, and N3 (slow-wave sleep). Each sleep stage in both NREM and REM sleep has unique characteristics of EEG pattern, eye movements, and muscle tone. NREM and REM sleep alternate cyclically [1].
We provide here a brief and simplified overview of the mechanisms regulating sleep and circadian rhythms. Waking is promoted by the activity of the serotonergic, noradrenergic, cholinergic, and orexin/hypocretin systems, among others. At sleep onset, the circadian clock localized in the suprachiasmatic nucleus activates sleep-active neurons through adenosine, a hypnogenic factor progressively accumulating in the brain during wakefulness. GABAergic neurons, localized not only in the ventrolateral preoptic nucleus, but also in the parafacial, accumbens, and reticular thalamic nuclei, inhibit the wake circuits, promoting and maintaining sleep. The switch between NREM and REM sleep is due to the interaction of multiple populations of glutamatergic and GABAergic neurons localized in the pontine reticular formation, in the sublaterodorsal nucleus, in the lateral hypothalamic area, in the dorsal part of the deep mesencephalic nucleus, and in the ventrolateral periaqueductal grey [2].
The regulation of circadian rhythm is modulated by light. Light information is detected by intrinsically photoreceptive retinal ganglion cells and delivered to the suprachiasmatic nucleus, which consists of thousands of neurons exhibiting self-sustaining and synchronous circadian rhythms. The suprachiasmatic nucleus encodes light information, synchronizes circadian oscillations, and projects signals to other brain regions [3].
In recent years, it has become increasingly clear that there is a bidirectional relationship between sleep/circadian rhythms and neurodegeneration. In fact, sleep and circadian rhythms are disturbed in neurodegenerative diseases. Additionally, it became evident that changes in sleep and circadian rhythms are also present in the early, prodromal, and preclinical phases of neurodegenerative diseases, raising the question of whether they are a consequence of a neurodegenerative process affecting key areas of the central nervous system, or if sleep and circadian rhythm disorders precede (and contribute to) the development of neurodegeneration.
This narrative review will provide an overview of sleep and circadian rhythm disruption in neurodegeneration, highlighting the bidirectional relationship between sleep changes and neurodegeneration while also focusing on sleep and circadian rhythm changes in prodromal and preclinical neurodegenerative diseases. Building on that, future perspectives will be addressed, in particular if sleep changes are able to predict neurodegeneration and the potential for sleep actionability to prevent or modulate the development of neurodegenerative diseases.
2. Sleep and Circadian Rhythms in Neurodegenerative Diseases
As sleep and circadian rhythms are highly regulated functions involving several central nervous system areas, nuclei, and neurotransmitters, it is not surprising that neurodegenerative processes affect at least some of these pathways, leading to the development of sleep disorders. For example, in Alzheimer’s disease, a loss of neurons in the suprachiasmatic nucleus [4] and altered synchronization of multiple circadian oscillators in the brain [5] have been reported, together with clinical symptoms such as daytime sleepiness, sleep apnea, insomnia, and sleep fragmentation [6]. Sleep and circadian rhythms are also affected in Parkinson’s disease, e.g., the expression of clock genes (i.e., genes serving as the basis of intracellular timekeeping and circadian rhythms) in peripheral blood cells is blunted [7,8], and sleep disorders are present in almost all patients with Parkinson’s disease, including rapid eye movement (REM) sleep behavior disorder, sleep apnea, insomnia, excessive daytime sleepiness, restless legs syndrome/periodic leg movements, or circadian rhythm disorders [9]. Sleep disorders are, however, not exclusive to the most common neurodegenerative diseases. In patients with dementia with Lewy bodies, sleep disorders such as REM sleep behavior disorder, insomnia, snoring, sleep apnea, hypersomnia, restless legs syndrome, and nocturnal visual hallucinations have been described [10]. Patients with multiple system atrophy report REM sleep behavior disorder, sleep fragmentation, sleep-related breathing disorders, and excessive daytime sleepiness [11,12]. In progressive supranuclear palsy, REM sleep behavior disorder can be present in 10–20% of patients, and insomnia is frequent [13]. In patients with Huntington’s’ disease, insomnia, frequent awakenings during the night, and excessive daytime sleepiness have been reported [14]. Different sleep disorders are present in the spinocerebellar ataxias, e.g., stridor (SCA1 and SCA3), REM sleep behavior disorder (SCA3), and restless legs syndrome (SCA1, SCA2, SCA3, and SCA6) [13]. In patients with amyotrophic lateral sclerosis, sleep-related breathing disorders, insomnia, and muscle cramps are present [15].
3. Bidirectional Relationship between Sleep/Circadian Rhythms and Dementia
The fact that centers regulating sleep and circadian rhythmicity are affected by neurodegeneration is not unexpected. However, several studies have shown a bidirectional relationship between sleep and neurodegenerative diseases.
The circadian rhythms affect sleep, glial, neuronal, and peripheral clocks, thus having an impact on amyloid β dynamics, glymphatic clearance, function of the blood–brain barrier, neuroinflammation, synaptic homeostasis, oxidative stress, and brain metabolism, among others [16,17]. All these factors may contribute to the development of neurodegeneration, and neurodegenerative processes lead to core clock disruptions and epigenetic changes on the one hand, and dysfunction/degeneration of the suprachiasmatic nucleus on the other hand, with subsequent impairment of circadian rhythms [18]. In addition to that, melatonin levels are known to drop with aging [19], potentially contributing to neurodegeneration. Few data are available on this topic; however, melatonin controls the mitotic activity of neural stem cells in the subventricular zone, suggesting its involvement in neuronal renewal, and in animal models of Alzheimer’s disease, melatonin supplementation defers amyloid-β accumulation and enhances its clearance from the central nervous system [20].
Besides circadian rhythms, several sleep changes showed a bidirectional relationship with neurodegenerative diseases. A clear example is the analysis of longitudinal changes in daytime napping inferred by actigraphy in 1401 participants of the Rush Memory and Aging Project, with up to a 14-year follow-up. In this cohort, longer or more frequent daytime napping correlated with worse cognition a year later. At the same time, worse cognition was correlated with more excessive naps a year later [21]. Another study investigated 41 older adults (mean age 83 years) with an actigraphy-assessed wake-after-sleep onset. Cognitive performance was assessed with memory recall (Rey–Osterrieth Complex Figure task), cognitive flexibility (Trail Making test), and verbal fluency (FAS word generation). Aβ was assessed with a global measure of the Pittsburgh Compound. This study showed that wake-after-sleep onset moderated the relationship between Aβ and memory, with a stronger positive association with Aβ and poorer cognitive performance in those with poorer sleep [22].
Not only wake-after-sleep onset but also sleep depth seems to influence neurodegenerative processes. In 104 individuals with mild–moderate Alzheimer’s disease, levels of neurofilament light chains (a protein found predominantly in the cytoplasm of neurons, particularly in large, myelinated axons, which serves as a biomarker for axonal damage) were higher in light sleepers and lower in deep sleepers [23].
A well-studied example of the bidirectional relationship between disturbed sleep and neurodegeneration is provided by the relationship between obstructive sleep apnea and Alzheimer’s disease. Obstructive sleep apnea causes fragmented sleep and hypoxic/inflammatory stress [24]. Fragmented sleep increases neuronal activity with the consequent excessive release of amyloid ß and amyloid deposition, which increases susceptibility to Alzheimer’s disease. The hypoxic/inflammatory stress, interacting with APOEε4, also contributes to increased susceptibility to Alzheimer’s disease [25]. In addition to that, amyloid deposition causes injury to sleep/wake centers, leading to sleep fragmentation. Moreover, increased susceptibility to Alzheimer’s disease is linked to decreased cognitive and physical activity, which also contributes to sleep fragmentation. Thus, there is a loop connecting sleep fragmentation, obstructive sleep apnea, and amyloid deposition/susceptibility to Alzheimer’s disease [26]. Although the bidirectional relationship between obstructive sleep apnea and Alzheimer’s disease has been more deeply investigated, the presence of obstructive sleep apnea seems to also interact with α-synuclein deposition. When comparing 46 controls with simple snoring and 42 age- and gender-matched patients with obstructive sleep apnea, plasma phosphorylated α-synuclein levels were higher in the sleep apnea group and correlated with the apnea-hypopnea index [27]. A recent analysis of data from the Korean National Health Information Database revealed a reciprocal relationship between obstructive sleep apnea (OSA) and Parkinson’s disease. The study found that patients with obstructive sleep apnea had a 1.54 times higher likelihood of developing Parkinson’s disease. Conversely, individuals diagnosed with Parkinson’s disease exhibited a 1.92 times greater incidence of obstructive sleep apnea, highlighting a bidirectional association between the two conditions [28].
Another relevant aspect to consider is sleep loss. Chronic sleep loss creates a proinflammatory environment at the synapse level, with impaired glial functionality and synapse loss [29]. As most of these effects are seen in the hippocampus or cortex, they likely impair learning and memory. In addition, microglia can release cytokines, contributing to the inflammatory environment. The loss of locus coeruleus neurons leads to the loss of the anti-inflammatory effects of noradrenaline and increased microglial activation [30]. At the same time, in astrocytes, cytokine production increases, and elevated ATP in the extracellular space amplifies the inflammatory response. The astrocytes’ ability to regulate glutamate levels in the synapse becomes impaired, with subsequent neuronal excitotoxicity [31]. Moreover, astrocytes release less brain-derived neurotrophic factor, which may also contribute to synapse loss. Thus, in the extracellular space, cytokines increase, noradrenaline decreases, likely due to locus coeruleus neuron loss, and ATP increases.
Further evidence comes from animal studies. Chronic short sleep exposure in young adult mice imparts late-in-life neurodegeneration and persistent derangements in amyloid and tau homeostasis. Of note, these findings occur in the absence of a genetic predisposition to neurodegeneration [32]. In humans, a cross-sectional genome-wide analysis of DNA methylation in relation to self-reported insufficient sleep in a community-based sample (mean age 39 years), and in relation to shift work disorder in an occupational cohort (mean age 45 years) revealed DNA methylation patterns associated with sleep loss, suggesting a possible modification of processes related to neuroplasticity and neurodegeneration [33].
Another study reported that lower intra-individual variability in both sleep duration and sleep efficiency and longer mean sleep duration are associated with lower levels of amyloid β pathology and better cognition. Of note, this effect differs by apolipoprotein E4 status, indicating that longer sleep duration and more consistent sleep efficiency may be protective against β-amyloid burden in apolipoprotein E4 carriers [34].
In summary, chronic sleep loss causes impaired glial functionality and synapse loss [35]. In line with this, in wild-type, mice the chronic sleep loss phenotype is similar to the Alzheimer’s disease phenotype, with hippocampal volume loss, hippocampal tau phosphorylation and hippocampal-dependent cognitive decline, locus coeruleus neuronal loss, increased microglia, and astrocyte activation [35]. In humans, a cross-over design study assessed the Aβ and tau concentrations in the cerebrospinal fluid of five cognitively normal individuals every two hours for 36 h during sleep-deprived and normal sleep control conditions. Of note, sleep loss altered plasma Alzheimer’s disease biomarkers by lowering brain clearance mechanisms [36]. The glymphatic system is a waste clearance system facilitating the removal of metabolic byproducts from the central nervous system via perivascular channels formed by astrocytes, which operate predominantly during sleep [37]. In fact, when the sleep–wake cycle is balanced, worn synapses are removed, activity-dependent amyloid β and tau release can be cleared, and astrocyte–neuron interaction is maintained so that amyloid β and tau do not aggregate. However, a disrupted sleep–wake cycle (e.g., due to sleep loss) leads to chronic glial activation. At the same time, there is an increased activity-induced release and accumulation of amyloid β and tau, which leads to an enlargement of the perivascular space. Moreover, due to increased waking, astrocytes and aquaporins are polarized, further contributing to decreases in glymphatic clearance [38]. The function of the glymphatic system is not only relevant in Alzheimer’s disease but also in alpha-synucleinopathies. Alpha-synuclein has been shown to cause aggressive neuroinflammatory insults and aquaporin 4 deficiency, which perpetuates glymphatic impairment, causing dopaminergic degeneration in the substantia nigra and in other brain regions [39].
Another interesting yet still understudied aspect is the role of mitochondria, which seem to interact with the glymphatic system [40]. In fact, sleep disorders may impair both the glymphatic system and the activity of mitochondria. At the same time, mitochondrial disturbances are potentially associated with the accumulation of aggregated proteins and impairment of glymphatic clearance. Of note, melatonin may link sleep disorders, mitochondrial function, and the glymphatic system, as it plays a key role in the control of proper mitochondrial functions and has a very powerful effect as a free radical scavenger and antioxidant [40]. Figure 1 summarizes the bi-directional relationship linking sleep and neurodegeneration.
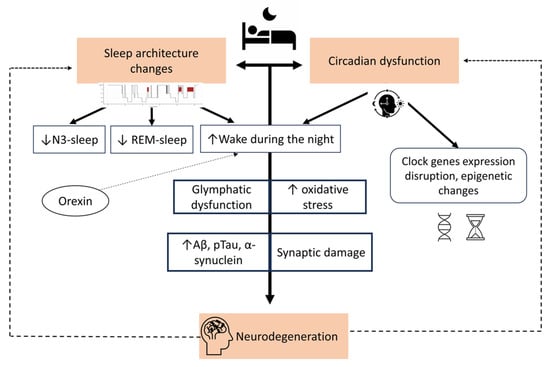
Figure 1.
The bidirectional relationship between sleep and neurodegeneration.
The figure illustrates the link between altered sleep and neurodegenerative processes. Changes in sleep architecture and circadian rhythms are both outcomes of and contributors to neurodegeneration. Sleep changes compromise glymphatic function with subsequent aggregation of misfolded proteins such as amyloid-beta (Aβ), phosphorylated tau (pTau), and alpha-synuclein. This leads to synaptic impairment, contributing to the neurodegenerative process.
4. Sleep and Circadian Rhythm in Early, Prodromal, and Preclinical Neurodegeneration
In the early stages of Alzheimer’s disease, sleep is already affected: melatonin release is dysregulated, sleep apnea and naps are frequent, sleep macroarchitecture is affected with sleep fragmentation, reduced sleep efficiency, and REM sleep dysregulation, and on the microarchitectural level, K-complexes are altered [41].
In early Parkinson’s disease, a circadian dysfunction is already present with altered variation in melatonin concentration over the 24 h and reduced melatonin nadir. Peripheral clock gene expression oscillations are also altered [8]. The prodromal stage of the alpha-synucleinopathies is represented by the isolated form of REM sleep behavior disorder, and a preclinical phase can be detected in the presence of isolated REM sleep without atonia or excessive movements during REM sleep [42,43]. Specific impairment of muscle atonia during REM sleep is well characterized in this population, whereas NREM sleep seems to be unaffected and changes in circadian rhythms have been scarcely investigated [44,45].
5. Future Directions
Current knowledge about the bidirectional relationship between sleep/circadian rhythms and neurodegeneration raises two important questions with potential relevant implications for the early detection of neurodegeneration and the neuromodulatory management approaches to neurodegenerative diseases.
The first question is whether sleep changes can predict or allow early detection of neurodegeneration.
The current literature investigating the link between altered circadian rhythm and the risk of neurodegeneration shows that habits of dysregulated circadian rhythm [46,47] and a decreased circadian amplitude [48,49] are associated with a higher risk of cognitive decline or dementia. Moreover, a lower amplitude of the 24 h activity rhythm and a higher intra-daily variability for hourly fragmentation of the activity rhythm are associated with a higher risk of Alzheimer’s disease [50]. In addition to that, sleep–wake rhythm fragmentation and medial temporal lobe atrophy are related, and noteworthy rhythm fragmentation accounts for individual differences in atrophy more than age [51].
Excessive daytime sleepiness seems to be another relevant predictor of neurodegeneration. In fact, excessive daytime sleepiness is associated with global cortical thinning in cognitively normal elderly [52], and the presence of excessive daytime sleepiness at baseline is longitudinally associated with increased amyloid β accumulation in the cingulate gyrus and precuneus regions [53].
Further evidence comes from mendelian randomization studies. Two independent studies showed that daytime sleepiness causes an increase in the risk of amyotrophic lateral sclerosis [54,55]. One of these mendelian randomization studies investigated the association also with other neurodegenerative diseases, pointing to a causal effect of both subjectively and objectively measured morning chronotype and later age at onset of Parkinson’s disease. A similar effect was present for reduced motor activity in the 10 most active hours of the day. The same study reported a causal association between increased sleep efficiency and a reduced risk of Alzheimer’s disease [55].
In line with this, a large cohort study investigating subjects undergoing video-polysomnography with up to 16.8 years of follow-up showed that each one-percentage decrease in sleep efficiency, N3 sleep, or REM sleep was associated with a 1.9%, 6.5%, and 5.2% increased risk, respectively, for the future diagnosis of a neurodegenerative disease. On the other hand, a percentage decrease in nocturnal wakefulness represented a 2.2% decreased risk of neurodegeneration [56]. Similarly, a machine-learning-based algorithm using features based on sleep architecture, frequency band powers, spindles, slow oscillation, and coherence in 10,784 sleep studies from 8044 participants allowed distinction between participants with dementia, those with mild cognitive impairment, and cognitively normal participants based on sleep features [57].
A study of 62 cognitively normal older adults combining Pittsburgh compound B positron emission tomography scanning with sleep electroencephalography to quantify NREM slow-wave activity and a hippocampal-dependent face–name learning task found that NREM SWA significantly moderates the effect of Aβ status on memory function. In particular, NREM slow-wave activity selectively supported superior memory function in individuals suffering high amyloid β burden, suggesting that NREM slow-wave activity may be a cognitive reserve factor providing resilience against the memory impairment otherwise caused by high Alzheimer’s disease pathology burden [58].
Additional evidence comes from studies investigating cognitively unimpaired adults. A study of 35 cognitively normal healthy controls, 23 participants with mild cognitive impairment, and 19 patients with Alzheimer’s disease investigated the slow-to-fast-activity ratio of entropy in REM sleep and found that it differentiated dementia from mild cognitive impairment and controls. Moreover, a higher slow-to-fast activity ratio entropy during REM sleep was associated with worse cognitive performance [59]. In 121 cognitively unimpaired older adults (mean age 69 years), a multimodal neuroimaging was used to investigate amyloid deposition, gray matter volume, and perfusion in relation to REM sleep integrity. A positive relationship was present between theta power and grey matter volume in widespread brain regions, whereas beta power was negatively associated with grey matter volume in the anterior cingulate cortex. Moreover, higher REM sleep delta power was associated with greater perfusion in fronto-cingulate areas, and higher REM sleep theta, alpha, and beta power was associated with lower perfusion in fronto-cingulate and parietal areas. Of note, theta power was negatively associated with amyloid deposition [60].
Recently, an artificial intelligence model was developed to detect Parkinson’s disease and track its progression through nocturnal breathing signals. It was evaluated on a large dataset based on several U.S. hospital recordings and multiple public datasets. The model achieved an area under the curve (AUC) of 0.90 for internal tests and 0.85 for external tests. Moreover, it could estimate Parkinson’s disease severity and progression according to the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MD-UPDRS) [61].
Based on this evidence, it seems that sleep can predict neurodegeneration, and, thus, early neurodegeneration may be identified through sleep changes. Different changes in sleep and circadian rhythms are observed in different neurodegenerative diseases, in particular Alzheimer’s disease and Parkinson’s disease. The specific impact of tau-related or synuclein-related pathology needs to be further disentangled in future studies.
The second question is whether sleep is actionable to reduce the risk or even prevent neurodegeneration. A clear and definite answer to this question requires long-term longitudinal studies. However, it is clear that sleep loss affects neurodegeneration. A small study of 20 cognitively normal, amyloid β-negative participants aged 30 to 60 years, randomized to 36 h sleep deprivation, increased sleep with sodium oxybate (to increase slow-wave sleep), or normal sleep (control group), showed that sleep loss affected tau phosphorylation [62]. One study showed that targeted slow-wave sleep disruption increased overnight cerebrospinal fluid Aβ content [63].
Based on this evidence, an easily actionable sleep feature with an impact on brain health seems to be sleep duration. According to the American Academy of Sleep Medicine position statement, most adults need at least 7 h of sleep [64]. Social jet-lag is one of the factors mostly impacting sleep duration, starting already during childhood and being present throughout adulthood and working life. Awareness of the importance of sleep should be increased, and both school times and working times, when possible, should take into account the individual sleep needs and chronotypes. Besides sleep duration, the number of specific sleep stages seems to play a role in maintaining brain health [63]. Interventions such as targeted acoustic stimuli are feasible and could increase slow-wave sleep in Alzheimer’s disease significantly [65]. Thus, interventions to enhance sleep in older adults and in those at risk for neurodegenerative disease may modulate neurodegenerative processes [65,66].
Despite limited data, the potential actionability of sleep in modulating neurodegeneration is further supported by data showing that adherence to positive airway pressure therapy may lower the odds of an incident diagnosis of Alzheimer’s disease or multiple cognitive impairment and slow cognitive impairment or its progression to Alzheimer’s disease [67,68].
Orexin is a hypothalamic neuropeptide that regulates arousal and wakefulness, influencing the transition from sleep to wakefulness. Dysfunctions in orexin signaling are associated with sleep pathologies, in particular narcolepsy [69]. Thus, another actionable pathway is represented by the orexin system, as agonists and antagonists are available [70]. In Alzheimer’s disease, high orexin levels may increase amyloid β and tau accumulation by promoting wakefulness (among other mechanisms), which in turn increases orexin levels in a positive feedback loop [71]. On the other hand, orexin may exert beneficial effects on Parkinson’s disease symptoms by neuroprotecting dopaminergic neurons within the substantia nigra and through other brain regions.
6. Conclusions
The bidirectional relationship between sleep/circadian rhythm disorders and neurodegenerative diseases provides the basis for early detection of neurodegeneration through sleep changes and highlights sleep health as an actionable pillar of brain health potentially able to modulate the development of neurodegeneration from the early, prodromal, and preclinical phases. Sleep actionability has potential even earlier, when the neurodegenerative process has not started yet but only risk factors are present. A better understanding of specific sleep and circadian rhythm changes leading to the development of neurodegeneration is needed in order to develop specific management strategies able to modulate neurodegeneration and promote brain health.
Author Contributions
Conceptualization, A.S.; writing—original draft preparation, A.I. and A.S.; writing—review and editing, A.S. and B.H.; supervision, A.S. and B.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable (no new data were created).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kryger, M.H.; Roth, T.; Goldstein, C.A. Principles and Practice of Sleep Medicine, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 1. [Google Scholar]
- Luppi, P.-H.; Fort, P. Sleep-Wake Physiology. Handb. Clin. Neurol. 2019, 160, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Lv, Q.; Xie, W.; Gong, S.; Zhuang, S.; Liu, J.; Mao, C.; Liu, C. Circadian Disruption and Sleep Disorders in Neurodegeneration. Transl. Neurodegener. 2023, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Lim, A.S.; Chiang, W.-Y.; Hsieh, W.-H.; Lo, M.-T.; Schneider, J.A.; Buchman, A.S.; Bennett, D.A.; Hu, K.; Saper, C.B. Suprachiasmatic Neuron Numbers and Rest-Activity Circadian Rhythms in Older Humans. Ann. Neurol. 2015, 78, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Cermakian, N.; Lamont, E.W.; Boudreau, P.; Boivin, D.B. Circadian Clock Gene Expression in Brain Regions of Alzheimer ’s Disease Patients and Control Subjects. J. Biol. Rhythms 2011, 26, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Holth, J.; Patel, T.; Holtzman, D.M. Sleep in Alzheimer’s Disease—Beyond Amyloid. Neurobiol. Sleep. Circadian Rhythm. 2017, 2, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Liu, S.; Sothern, R.B.; Xu, S.; Chan, P. Expression of Clock Genes Per1 and Bmal1 in Total Leukocytes in Health and Parkinson’s Disease. Eur. J. Neurol. 2010, 17, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Breen, D.P.; Vuono, R.; Nawarathna, U.; Fisher, K.; Shneerson, J.M.; Reddy, A.B.; Barker, R.A. Sleep and Circadian Rhythm Regulation in Early Parkinson Disease. JAMA Neurol. 2014, 71, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Stefani, A.; Högl, B. Sleep in Parkinson’s Disease. Neuropsychopharmacology 2020, 45, 121–128. [Google Scholar] [CrossRef]
- Fernández-Arcos, A.; Morenas-Rodríguez, E.; Santamaria, J.; Sánchez-Valle, R.; Lladó, A.; Gaig, C.; Lleó, A.; Iranzo, A. Clinical and Video-Polysomnographic Analysis of Rapid Eye Movement Sleep Behavior Disorder and Other Sleep Disturbances in Dementia with Lewy Bodies. Sleep 2019, 42, zsz086. [Google Scholar] [CrossRef]
- Videnovic, A. Management of Sleep Disorders in Parkinson’s Disease and Multiple System Atrophy. Mov. Disord. 2017, 32, 659–668. [Google Scholar] [CrossRef]
- Eckhardt, C.; Fanciulli, A.; Högl, B.; Heidbreder, A.; Eschlböck, S.; Raccagni, C.; Krismer, F.; Leys, F.; Kiechl, S.; Ransmayr, G.; et al. Analysis of Sleep, Daytime Sleepiness, and Autonomic Function in Multiple System Atrophy and Parkinson Disease: A Prospective Study. J. Clin. Sleep Med. 2023, 19, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Iranzo, A. Sleep in Neurodegenerative Diseases. Sleep Med. Clin. 2016, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Herzog-Krzywoszanska, R.; Krzywoszanski, L. Sleep Disorders in Huntington’s Disease. Front. Psychiatry 2019, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Boentert, M. Sleep and Sleep Disruption in Amyotrophic Lateral Sclerosis. Curr. Neurol. Neurosci. Rep. 2020, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, U.; Ripperger, J.A. Circadian Clocks and Sleep: Impact of Rhythmic Metabolism and Waste Clearance on the Brain. Trends Neurosci. 2018, 41, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Artiushin, G.; Sehgal, A. The Glial Perspective on Sleep and Circadian Rhythms. Annu. Rev. Neurosci. 2020, 43, 119–140. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Musiek, E.S.; Hu, K.; Cappuccio, F.P.; Yaffe, K. Association between Circadian Rhythms and Neurodegenerative Diseases. Lancet Neurol. 2019, 18, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Karasek, M. Melatonin, Human Aging, and Age-Related Diseases. Exp. Gerontol. 2004, 39, 1723–1729. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Cucielo, M.S.; Tan, D.X.; Rosales-Corral, S.; Gancitano, G.; de Almeida Chuffa, L.G. Brain Washing and Neural Health: Role of Age, Sleep, and the Cerebrospinal Fluid Melatonin Rhythm. Cell. Mol. Life Sci. 2023, 80, 88. [Google Scholar] [CrossRef]
- Li, P.; Gao, L.; Yu, L.; Zheng, X.; Ulsa, M.C.; Yang, H.-W.; Gaba, A.; Yaffe, K.; Bennett, D.A.; Buchman, A.S.; et al. Daytime Napping and Alzheimer’s Dementia: A Potential Bidirectional Relationship. Alzheimer’s Dement. 2023, 19, 158–168. [Google Scholar] [CrossRef]
- Wilckens, K.A.; Tudorascu, D.L.; Snitz, B.E.; Price, J.C.; Aizenstein, H.J.; Lopez, O.L.; Erickson, K.I.; Lopresti, B.J.; Laymon, C.M.; Minhas, D.; et al. Sleep Moderates the Relationship between Amyloid Beta and Memory Recall. Neurobiol. Aging 2018, 71, 142–148. [Google Scholar] [CrossRef]
- Targa, A.; Dakterzada, F.; Benítez, I.; López, R.; Pujol, M.; Dalmases, M.; Arias, A.; Sánchez-de-la-Torre, M.; Zetterberg, H.; Blennow, K.; et al. Decrease in Sleep Depth Is Associated with Higher Cerebrospinal Fluid Neurofilament Light Levels in Patients with Alzheimer’s Disease. Sleep 2021, 44, zsaa147. [Google Scholar] [CrossRef]
- Kimoff, R.J. Sleep Fragmentation in Obstructive Sleep Apnea. Sleep 1996, 19, S61–S66. [Google Scholar] [CrossRef]
- Sun, Y.-Y.; Wang, Z.; Huang, H.-C. Roles of ApoE4 on the Pathogenesis in Alzheimer’s Disease and the Potential Therapeutic Approaches. Cell. Mol. Neurobiol. 2023, 43, 3115–3136. [Google Scholar] [CrossRef]
- Ju, Y.-E.S.; McLeland, J.S.; Toedebusch, C.D.; Xiong, C.; Fagan, A.M.; Duntley, S.P.; Morris, J.C.; Holtzman, D.M. Sleep Quality and Preclinical Alzheimer Disease. JAMA Neurol. 2013, 70, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-L.; Sun, B.-L.; Chen, D.-W.; Chen, Y.; Li, W.-W.; Xu, M.-Y.; Shen, Y.-Y.; Xu, Z.-Q.; Wang, Y.-J.; Bu, X.-L. Plasma α-Synuclein Levels Are Increased in Patients with Obstructive Sleep Apnea Syndrome. Ann. Clin. Transl. Neurol. 2019, 6, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-H.; Hwang, Y.S.; Oh, S.-Y.; Shin, B.-S.; Kang, M.G.; Lee, M.G.; Yeom, S.W.; Lee, J.H.; Kang, H.G.; Kim, J.S. Bidirectional Association between Parkinson’s Disease and Obstructive Sleep Apnea: A Cohort Study. J. Clin. Sleep Med. 2023, 19, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, M.R.; Gibbons, A.J. Neuroinflammation, Sleep, and Circadian Rhythms. Front. Cell. Infect. Microbiol. 2022, 12, 853096. [Google Scholar] [CrossRef] [PubMed]
- Gargano, A.; Olabiyi, B.F.; Palmisano, M.; Zimmer, A.; Bilkei-Gorzo, A. Possible Role of Locus Coeruleus Neuronal Loss in Age-Related Memory and Attention Deficits. Front. Neurosci. 2023, 17, 1264253. [Google Scholar] [CrossRef] [PubMed]
- Cuellar-Santoyo, A.O.; Ruiz-Rodríguez, V.M.; Mares-Barbosa, T.B.; Patrón-Soberano, A.; Howe, A.G.; Portales-Pérez, D.P.; Miquelajáuregui Graf, A.; Estrada-Sánchez, A.M. Revealing the Contribution of Astrocytes to Glutamatergic Neuronal Transmission. Front. Cell. Neurosci. 2023, 16, 1037641. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.E.; Zhu, Y.; Fenik, P.; Zhan, G.; Bell, P.; Liu, C.; Veasey, S. Late-in-Life Neurodegeneration after Chronic Sleep Loss in Young Adult Mice. Sleep 2021, 44, zsab057. [Google Scholar] [CrossRef]
- Lahtinen, A.; Puttonen, S.; Vanttola, P.; Viitasalo, K.; Sulkava, S.; Pervjakova, N.; Joensuu, A.; Salo, P.; Toivola, A.; Härmä, M.; et al. A Distinctive DNA Methylation Pattern in Insufficient Sleep. Sci. Rep. 2019, 9, 1193. [Google Scholar] [CrossRef]
- Fenton, L.; Isenberg, A.L.; Aslanyan, V.; Albrecht, D.; Contreras, J.A.; Stradford, J.; Monreal, T.; Pa, J. Variability in Objective Sleep Is Associated with Alzheimer’s Pathology and Cognition. Brain Commun. 2023, 5, fcad031. [Google Scholar] [CrossRef]
- Zamore, Z.; Veasey, S.C. Neural Consequences of Chronic Sleep Disruption. Trends Neurosci. 2022, 45, 678–691. [Google Scholar] [CrossRef]
- Liu, H.; Barthélemy, N.R.; Ovod, V.; Bollinger, J.G.; He, Y.; Chahin, S.L.; Androff, B.; Bateman, R.J.; Lucey, B.P. Acute Sleep Loss Decreases CSF-to-Blood Clearance of Alzheimer’s Disease Biomarkers. Alzheimer’s Dement. 2023, 19, 3055–3064. [Google Scholar] [CrossRef]
- Jessen, N.A.; Munk, A.S.F.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef] [PubMed]
- Ahnaou, A.; Drinkenburg, W.H.I.M. Sleep, Neuronal Hyperexcitability, Inflammation and Neurodegeneration: Does Early Chronic Short Sleep Trigger and Is It the Key to Overcoming Alzheimer’s Disease? Neurosci. Biobehav. Rev. 2021, 129, 157–179. [Google Scholar] [CrossRef] [PubMed]
- Scott-Massey, A.; Boag, M.K.; Magnier, A.; Bispo, D.P.C.F.; Khoo, T.K.; Pountney, D.L. Glymphatic System Dysfunction and Sleep Disturbance May Contribute to the Pathogenesis and Progression of Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 12928. [Google Scholar] [CrossRef] [PubMed]
- Kopeć, K.; Szleszkowski, S.; Koziorowski, D.; Szlufik, S. Glymphatic System and Mitochondrial Dysfunction as Two Crucial Players in Pathophysiology of Neurodegenerative Disorders. Int. J. Mol. Sci. 2023, 24, 10366. [Google Scholar] [CrossRef] [PubMed]
- Rigat, L.; Ouk, K.; Kramer, A.; Priller, J. Dysfunction of Circadian and Sleep Rhythms in the Early Stages of Alzheimer’s Disease. Acta Physiol. 2023, 238, e13970. [Google Scholar] [CrossRef] [PubMed]
- Högl, B.; Stefani, A.; Videnovic, A. Idiopathic REM Sleep Behaviour Disorder and Neurodegeneration—An Update. Nat. Rev. Neurol. 2018, 14, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Heidbreder, A.; St Louis, E.K.; Sixel-Döring, F.; Bliwise, D.L.; Baldelli, L.; Bes, F.; Fantini, M.L.; Iranzo, A.; Knudsen-Heier, S.; et al. Video-Polysomnography Procedures for Diagnosis of Rapid Eye Movement Sleep Behavior Disorder (RBD) and the Identification of Its Prodromal Stages: Guidelines from the International RBD Study Group. Sleep 2022, 43, zsab257. [Google Scholar] [CrossRef] [PubMed]
- Kunz, D.; Oster, H.; Rawashdeh, O.; Neumann, W.-J.; Münte, T.; Berg, D. Sleep and Circadian Rhythms in α-Synucleinopathies-Perspectives for Disease Modification. Acta Physiol. 2023, 238, e13966. [Google Scholar] [CrossRef] [PubMed]
- Weissová, K.; Škrabalová, J.; Skálová, K.; Červená, K.; Bendová, Z.; Miletínová, E.; Kopřivová, J.; Šonka, K.; Dudysová, D.; Bartoš, A.; et al. Circadian Rhythms of Melatonin and Peripheral Clock Gene Expression in Idiopathic REM Sleep Behavior Disorder. Sleep Med. 2018, 52, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bokenberger, K.; Ström, P.; Dahl Aslan, A.K.; Johansson, A.L.V.; Gatz, M.; Pedersen, N.L.; Åkerstedt, T. Association Between Sleep Characteristics and Incident Dementia Accounting for Baseline Cognitive Status: A Prospective Population-Based Study. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Bokenberger, K.; Sjölander, A.; Dahl Aslan, A.K.; Karlsson, I.K.; Åkerstedt, T.; Pedersen, N.L. Shift Work and Risk of Incident Dementia: A Study of Two Population-Based Cohorts. Eur. J. Epidemiol. 2018, 33, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Tranah, G.J.; Blackwell, T.; Stone, K.L.; Ancoli-Israel, S.; Paudel, M.L.; Ensrud, K.E.; Cauley, J.A.; Redline, S.; Hillier, T.A.; Cummings, S.R.; et al. Circadian Activity Rhythms and Risk of Incident Dementia and Mild Cognitive Impairment in Older Women. Ann. Neurol. 2011, 70, 722–732. [Google Scholar] [CrossRef]
- Walsh, C.M.; Blackwell, T.; Tranah, G.J.; Stone, K.L.; Ancoli-Israel, S.; Redline, S.; Paudel, M.; Kramer, J.H.; Yaffe, K. Weaker Circadian Activity Rhythms Are Associated with Poorer Executive Function in Older Women. Sleep 2014, 37, 2009–2016. [Google Scholar] [CrossRef]
- Li, P.; Gao, L.; Gaba, A.; Yu, L.; Cui, L.; Fan, W.; Lim, A.S.P.; Bennett, D.A.; Buchman, A.S.; Hu, K. Circadian Disturbances in Alzheimer’s Disease Progression: A Prospective Observational Cohort Study of Community-Based Older Adults. Lancet Healthy Longev. 2020, 1, e96–e105. [Google Scholar] [CrossRef]
- Van Someren, E.J.W.; Oosterman, J.M.; Van Harten, B.; Vogels, R.L.; Gouw, A.A.; Weinstein, H.C.; Poggesi, A.; Scheltens, P.; Scherder, E.J.A. Medial Temporal Lobe Atrophy Relates More Strongly to Sleep-Wake Rhythm Fragmentation than to Age or Any Other Known Risk. Neurobiol. Learn. Mem. 2019, 160, 132–138. [Google Scholar] [CrossRef]
- Carvalho, D.Z.; St Louis, E.K.; Boeve, B.F.; Mielke, M.M.; Przybelski, S.A.; Knopman, D.S.; Machulda, M.M.; Roberts, R.O.; Geda, Y.E.; Petersen, R.C.; et al. Excessive Daytime Sleepiness and Fatigue May Indicate Accelerated Brain Aging in Cognitively Normal Late Middle-Aged and Older Adults. Sleep Med. 2017, 32, 236–243. [Google Scholar] [CrossRef]
- Carvalho, D.Z.; St Louis, E.K.; Knopman, D.S.; Boeve, B.F.; Lowe, V.J.; Roberts, R.O.; Mielke, M.M.; Przybelski, S.A.; Machulda, M.M.; Petersen, R.C.; et al. Association of Excessive Daytime Sleepiness with Longitudinal β-Amyloid Accumulation in Elderly Persons Without Dementia. JAMA Neurol. 2018, 75, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, L.; Xia, K.; Zhuang, Z.; Huang, T.; Fan, D. Daytime Sleepiness Might Increase the Risk of ALS: A 2-Sample Mendelian Randomization Study. J. Neurol. 2021, 268, 4332–4339. [Google Scholar] [CrossRef] [PubMed]
- Cullell, N.; Cárcel-Márquez, J.; Gallego-Fábrega, C.; Muiño, E.; Llucià-Carol, L.; Lledós, M.; Amaut, K.E.U.; Krupinski, J.; Fernández-Cadenas, I. Sleep/Wake Cycle Alterations as a Cause of Neurodegenerative Diseases: A Mendelian Randomization Study. Neurobiol. Aging 2021, 106, 320.e1–320.e12. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Cesari, M.; Heidbreder, A.; Defrancesco, M.; Brandauer, E.; Seppi, K.; Kiechl, S.; Högl, B.; Stefani, A. Sleep Features and Long-Term Incident Neurodegeneration: A Polysomnographic Study. Sleep 2023, zsad304. [Google Scholar] [CrossRef] [PubMed]
- Adra, N.; Sun, H.; Ganglberger, W.; Ye, E.M.; Dümmer, L.W.; Tesh, R.A.; Westmeijer, M.; Cardoso, M.D.S.; Kitchener, E.; Ouyang, A.; et al. Optimal Spindle Detection Parameters for Predicting Cognitive Performance. Sleep 2022, 45, zsac001. [Google Scholar] [CrossRef] [PubMed]
- Zavecz, Z.; Shah, V.D.; Murillo, O.G.; Vallat, R.; Mander, B.A.; Winer, J.R.; Jagust, W.J.; Walker, M.P. NREM Sleep as a Novel Protective Cognitive Reserve Factor in the Face of Alzheimer’s Disease Pathology. BMC Med. 2023, 21, 156. [Google Scholar] [CrossRef] [PubMed]
- Azami, H.; Moguilner, S.; Penagos, H.; Sarkis, R.A.; Arnold, S.E.; Gomperts, S.N.; Lam, A.D. EEG Entropy in REM Sleep as a Physiologic Biomarker in Early Clinical Stages of Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 91, 1557–1572. [Google Scholar] [CrossRef]
- André, C.; Champetier, P.; Rehel, S.; Kuhn, E.; Touron, E.; Ourry, V.; Landeau, B.; Le Du, G.; Mézenge, F.; Segobin, S.; et al. Rapid Eye Movement Sleep, Neurodegeneration, and Amyloid Deposition in Aging. Ann. Neurol. 2023, 93, 979–990. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, Y.; Zhang, G.; Wang, H.; Chen, Y.-C.; Liu, Y.; Tarolli, C.G.; Crepeau, D.; Bukartyk, J.; Junna, M.R.; et al. Artificial Intelligence-Enabled Detection and Assessment of Parkinson’s Disease Using Nocturnal Breathing Signals. Nat. Med. 2022, 28, 2207–2215. [Google Scholar] [CrossRef]
- Barthélemy, N.R.; Liu, H.; Lu, W.; Kotzbauer, P.T.; Bateman, R.J.; Lucey, B.P. Sleep Deprivation Affects Tau Phosphorylation in Human Cerebrospinal Fluid. Ann. Neurol. 2020, 87, 700–709. [Google Scholar] [CrossRef]
- Ju, Y.-E.S.; Ooms, S.J.; Sutphen, C.; Macauley, S.L.; Zangrilli, M.A.; Jerome, G.; Fagan, A.M.; Mignot, E.; Zempel, J.M.; Claassen, J.A.H.R.; et al. Slow Wave Sleep Disruption Increases Cerebrospinal Fluid Amyloid-β Levels. Brain 2017, 140, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- Ramar, K.; Malhotra, R.K.; Carden, K.A.; Martin, J.L.; Abbasi-Feinberg, F.; Aurora, R.N.; Kapur, V.K.; Olson, E.J.; Rosen, C.L.; Rowley, J.A.; et al. Sleep Is Essential to Health: An American Academy of Sleep Medicine Position Statement. J. Clin. Sleep Med. 2021, 17, 2115–2119. [Google Scholar] [CrossRef]
- Van den Bulcke, L.; Peeters, A.-M.; Heremans, E.; Davidoff, H.; Borzée, P.; De Vos, M.; Emsell, L.; Van den Stock, J.; De Roo, M.; Tournoy, J.; et al. Acoustic Stimulation as a Promising Technique to Enhance Slow-Wave Sleep in Alzheimer’s Disease: Results of a Pilot Study. J. Clin. Sleep Med. 2023, 19, 2107–2112. [Google Scholar] [CrossRef] [PubMed]
- Mander, B.A.; Winer, J.R.; Jagust, W.J.; Walker, M.P. Sleep: A Novel Mechanistic Pathway, Biomarker, and Treatment Target in the Pathology of Alzheimer’s Disease? Trends Neurosci. 2016, 39, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Shieu, M.M.; Zaheed, A.; Shannon, C.; Chervin, R.D.; Conceicao, A.; Paulson, H.L.; Braley, T.J.; Dunietz, G.L. Positive Airway Pressure and Cognitive Disorders in Adults With Obstructive Sleep Apnea: A Systematic Review of the Literature. Neurology 2022, 99, e334–e346. [Google Scholar] [CrossRef] [PubMed]
- Dunietz, G.L.; Chervin, R.D.; Burke, J.F.; Conceicao, A.S.; Braley, T.J. Obstructive Sleep Apnea Treatment and Dementia Risk in Older Adults. Sleep 2021, 44, zsab076. [Google Scholar] [CrossRef]
- Sakurai, T. The Role of Orexin in Motivated Behaviours. Nat. Rev. Neurosci. 2014, 15, 719–731. [Google Scholar] [CrossRef]
- Mieda, M.; Sakurai, T. Orexin (Hypocretin) Receptor Agonists and Antagonists for Treatment of Sleep Disorders. CNS Drugs 2013, 27, 83–90. [Google Scholar] [CrossRef]
- Ten-Blanco, M.; Flores, Á.; Cristino, L.; Pereda-Pérez, I.; Berrendero, F. Targeting the Orexin/Hypocretin System for the Treatment of Neuropsychiatric and Neurodegenerative Diseases: From Animal to Clinical Studies. Front. Neuroendocrinol. 2023, 69, 101066. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).