Approaching Headaches—A Guide to Differential-Diagnostic Considerations and Causal Claims
Abstract
1. Introduction
2. Identification of Potential Causes of Headache
| Diagnosis | Epidemiology | Proportion with Headache |
|---|---|---|
| Acute rhinosinusitis | I: 17540 per 100,000 persons per year [27] | 29% [28] |
| Bacterial meningitis | I: 1.49 per 100,000 persons per year [29] | 84% to 90% [30,31,32] |
| Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) | P: 1.32 to 1.98 definite cases per 100,000 adults [33,34] | 45% to 55% [35,36] |
| Cerebral ischaemic event | I: 156 per 100,000 persons per year [37] | 7% to 34% [38] |
| Cerebral venous thrombosis | I: 1.32 to 1.75 per 100,000 persons per year [39,40] | 76% to 77% [41,42] |
| Cervical vertebral artery dissection | I: 0.97 per 100,000 persons per year [43] | 69% [44] |
| Chiari malformation type I | P: 96 per 100,000 persons [45] | 43% to 81% [46,47,48,49] |
| Giant cell arteritis | P: 51.74 per 100,000 persons over 50 years [50] | 86% to 87% [51,52] |
| Hypothyroidism | I: 226.2 per 100,000 persons per year [53] | 30% to 34% [54,55] |
| Idiopathic intracranial hypertension | I: 0.9 per 100,000 persons per year [56,57] | 75% to 92% [56,58,59,60] |
| Internal carotid artery dissection | I: 1.72 per 100,000 persons per year [43] | 68% [44] |
| Intracranial neoplasia | I: 14.8 per 100,000 persons per year [61] | 48% to 60% [62,63,64,65] |
| Mitochondrial Encephalopathy, Lactic Acidosis and Stroke-like episodes (MELAS) | P: 0.18 per 100,000 persons in Japan [66] | 69% to 86% [67,68] |
| Moyamoya angiopathy | P: 1.01, 16.1, and 6.03 per 100,000 in China [69] Korea [70], and Japan [71], respectively | 20% to 67% [72,73,74,75] |
| Neurosarcoidosis | I: 11.5 per 100,000 persons per year are affected by Sarcoidosis [76], 0.2 per 100,000 persons per year for isolated Neurosarcoidosis [77] | 32% [78] |
| Non-traumatic intracranial haemorrhage | I: 29.9 per 100,000 persons per year [79] | 26% [80] |
| Pituitary apoplexy | I: 4.0 per 100,000 persons per year [37] | 82% to 100% [81,82,83] |
| Retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations | Unknown, probably exceedingly rare | 27% to 59% [84,85] |
| Reversible cerebral vasoconstriction syndrome (RCVS) | I: 0.3 per 100,000 persons per year [86] | 95% to 100% [87] |
| Sleep apnoea | P: 3420 per 100,000 persons [88] | 12% to 18% [89,90,91] |
| Spontaneous intracranial hypotension | I: 3.7 per 100,000 persons per year [92] | 90% to 100% [92,93] |
| Transient ischemic attack | I: 83 per 100,000 persons per year [94] | 26% to 36% [38] |
| Unruptured vascular malformation | P: 18 per 100,000 adults for arterio-venous malformations [95], 500 per 100,000 [96] for cavernous malformations | 49% to 54% [97] |
| Viral meningitis | I: 0.26 to 17 per 100,000 persons per year [98] | 99% [32] |
- Several secondary headaches, including traumatic injuries to the head, whiplash, and craniotomy, likely result from the activation of nociceptors by tissue damage or distension. A similar mechanism may apply to the reversible cerebral vasoconstriction syndrome and subarachnoid haemorrhage. The latter could also lead to pain through a mechanism such as that of mass lesions (see below).
- Mass lesions, e.g., brain tumours, might cause pain through pressure-induced traction of pain-sensitive structures due to their size, accompanying oedema, or hydrocephalus. Subsequently, sensitisation could increase the pain intensity [101].
- Similarly, strokes could lead to pain by exerting pressure on pain-sensitive structures. However, pain also occurs in smaller ischemia suggesting additional mechanisms. The release of pro-inflammatory substances and perhaps the occurrence of a cortical spreading depression could contribute to the pain [38,102].
- Different hypotheses attempt to explain headaches in patients affected by CADASIL. One is that they are more prone to pain induced by cortical spreading depression. Hypoperfusion might also be a mediating factor [103]. Another hypothesis is that damage to the periaqueductal grey could increase the likelihood of headaches [103].
- Milhorat and co-workers suggest that the cause of headaches in patients with Chiari Type 1 malformation may be a reduced CSF volume that results in difficulty mitigating pressure changes [46,104]. Additionally, Williams observed a “craniospinal pressure dissociation” that comprised a steeply increased pressure in the cranial but not the spinal CSF [105]. In addition to the elevated pressure distending the meninges, the pressure gradient might result in a further herniation of the tonsils with subsequent straining of pain-sensitive structures, which might contribute to the pain [104].
- Headache due to pituitary gland apoplexy may result from increased intrasellar pressure, which activates nociceptors [106].
- Focal demyelination of the trigeminal nerve leads to hyperexcitable afferents, which can result in synchronised after-discharge activity [108]. The latter results in pain perception in trigeminal neuralgia.
3. Searching for Potential Causes of Headaches
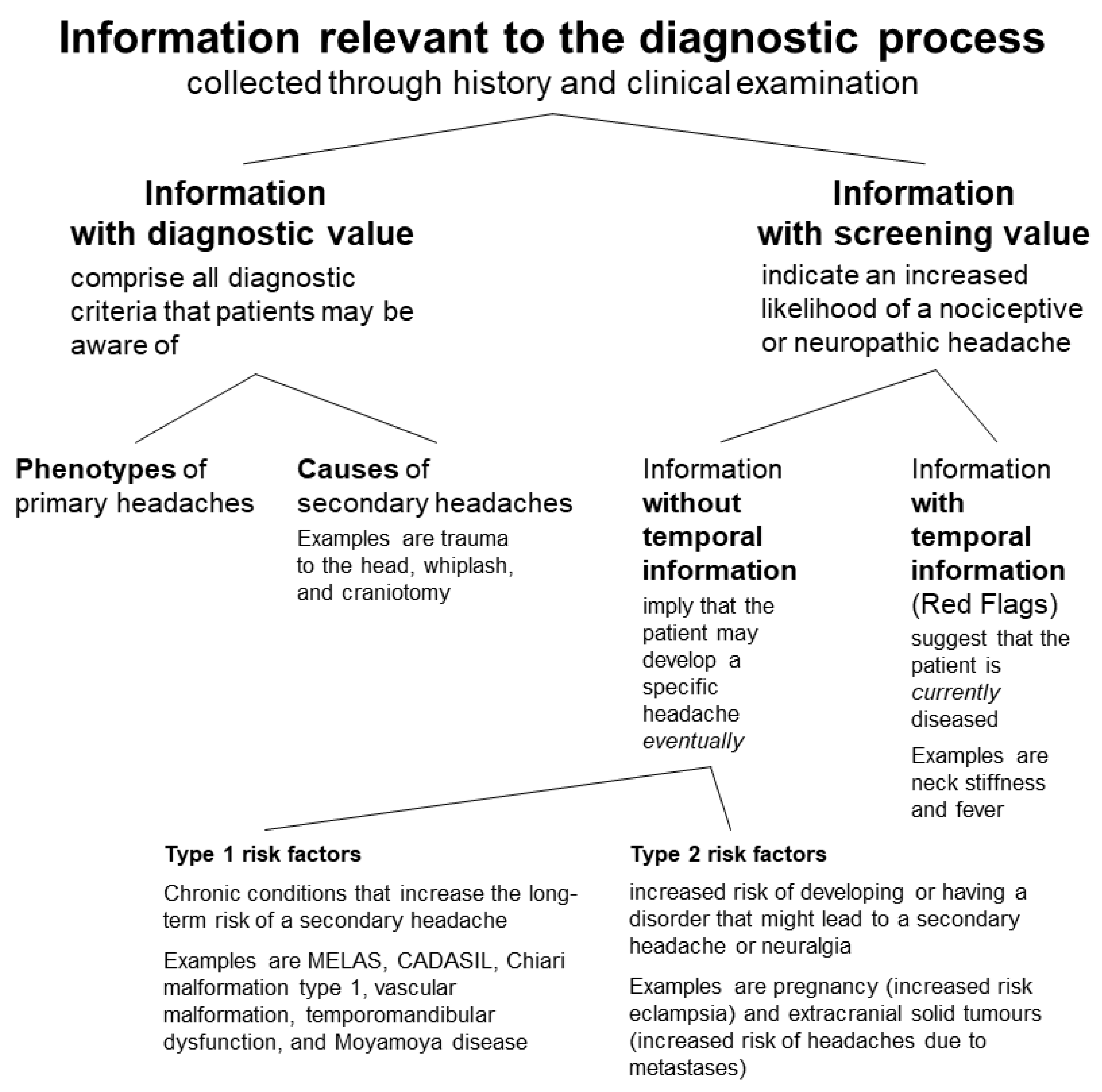
- Information with diagnostic value comprises all diagnostic criteria that patients may be aware of, i.e., phenotypes and several specific causes of headaches.
- Phenotypes are relevant for the diagnosis of primary headaches. The causes that patients can be inquired about are trauma to the head, whiplash, craniotomy, medication overuse, exposure to a substance (nitric oxide [NO] donor, phosphodiesterase [PDE] inhibitor, alcohol, and cocaine), withdrawal (pain killers in the case of medication overuse headache and caffeine), high altitude, aeroplane travel, diving, dialysis, and fasting [5]. Moreover, many patients will know if they have a systemic or localised inflammation.
- There are two types of information with screening value: One comprises symptoms and signs typical of a headache, whose diagnosis requires additional examinations [111]. The other includes risk factors indicating an increased likelihood of a secondary headache.
- The difference between these two types lies in the provided temporal information: A risk factor implies that the patient may develop a specific headache eventually; however, it allows no conclusions about the pathophysiology of the current headache. On the other hand, a symptom of another disorder suggests that the patient is currently diseased [111]. The former does not provide temporal information; the latter does.
3.1. Screening Factors with Temporal Information
3.2. Screening Factors without Temporal Information (Risk Factors)
- Type 1 risk factors are chronic conditions that increase the long-term risk of a secondary headache. Examples listed in the ICHD-3 are MELAS, CADASIL, Chiari malformation type 1, vascular malformation, temporomandibular dysfunction, and Moyamoya disease [5].
- Type 2 risk factors indicate an increased risk of developing or having a disorder that might lead to a secondary headache or neuralgia.
- Examples are pregnancy (increased risk of hypertensive disorders, e.g., eclampsia) [143] and extracranial solid tumours (increased risk of headaches due to metastases) [144]. Furthermore, overweight females have an increased risk of idiopathic intracranial hypertension [145], and multiple sclerosis increases the risk of trigeminal neuralgia [146]. Moreover, polymyalgia rheumatica increases the risk of giant cell arteritis [147].
- Additionally, exposure to several substances is a type 2 risk factor. Several chemotherapeutic agents, CHOP/R-CHOP regimens in particular, increase the risk of a posterior reversible encephalopathy syndrome (PRES) [150]. Cannabis and perhaps cocaine consumption could precipitate a reversible cerebral vasoconstriction syndrome (RCVS) [151]. Treatment with doxycycline and retinoids can increase intracranial pressure [152]; and eculizumab treatment predisposes meningitis [153]. Furthermore, treatment with oral contraception increases the risk of cerebral thromboses [154].
- Cerebral imaging is helpful when a haematoma, haemorrhage, ischemia, blood vessel malformation, tumour, hydrocephalus, and other causes of increased intracranial pressure or inflammation are suspected [155].
- A spinal tap allows measuring the intracranial pressure and searching for inflammation, haemorrhage, and tumour cells.
- An ophthalmic examination may help detect signs of raised intracranial or intraocular pressure, inflammation, including keratitis, and refractive errors [156].
- An ear, nose, and throat specialist should be consulted when local inflammation (e.g., otitis or mastoiditis) and craniomandibular dysfunction are suspected [157].
- Monitoring the blood oxygen levels during sleep can detect sleep apnoea.
- Occasionally, myelography can help to detect a cerebrospinal fluid leak [158].
4. Causal Claims
- Temporal sequence: An essential requirement is that a cause must appear before its effect. However, in practice, that sequence may be difficult to evidence. Accordingly, the ICHD-3 relaxes that criterion for headaches due to acute disorders. It stipulates merely that the condition “has been diagnosed” [5]. Nevertheless, for every symptom supposedly due to an underlying disease, one should attempt to clarify the temporal sequence and note the onset time of each symptom.
- For non-acute disorders that permanently increase the likelihood of a headache (see above, type 1 risk factors), it is sufficient to make it plausible that the disorder was present before the headaches.
- Correlation: A spurious relationship seems unlikely when a disorder known to cause pain appears in close temporal association with pain. However, given how unspecific and prevalent headaches are, it is reasonable to consider the possibility of a random co-occurrence, especially when dealing with stimuli that weakly correlate with pain (see Table 1).
- Evidence supporting the assumption of a non-spurious relationship can be mechanistic, manipulative, and probabilistic (see below).
- Elimination of alternate causes: It is impossible to unequivocally eliminate all alternate causes of a headache, as there are no diagnostic tests for primary headaches, and the process of collecting information, as outlined above, does not necessarily detect all underlying conditions. Yet, for practical reasons, it makes sense to assume that this prerequisite is satisfied if clinical evidence does not suggest a yet-to-be-discovered secondary headache or neuralgia—provided that data were collected meticulously.
4.1. Mechanistic Evidence
4.2. Manipulative Evidence
4.3. Probabilistic Evidence
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Federspil, G.; Giaretta, P.; Rguarli, C.; Scandarelli, C.; Serra, P. Filosofia Della Medicina; Rafaello Cortina Editore: Milano, Italy, 2008. [Google Scholar]
- Isler, H. Headache classification prior to the Ad Hoc criteria. Cephalalgia 1993, 13 (Suppl. S12), 9–10. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.P.; Finley, K.H.; Graham, J.R.; Kunkle, E.C.; Ostfeld, A.M.; Wolff, H.G. A classification of headache: Ad Hoc Committee on Classification of Headache. Neurology 1962, 12, 378. [Google Scholar] [CrossRef]
- Anonymous. Classification. Cephalalgia 1988, 8, 13–17. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS). Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef] [PubMed]
- Granella, F.; D’Alessandro, R.; Manzoni, G.C.; Cerbo, R.; Colucci D’Amato, C.; Pini, L.A.; Savi, L.; Zanferrari, C.; Nappi, G. International Headache Society Classification: Interobserver Reliability in the Diagnosis of Primary Headaches. Cephalalgia 1994, 14, 16–20. [Google Scholar] [CrossRef]
- Neumeier, M.S.; Stattmann, M.; Wegener, S.; Gantenbein, A.R.; Pohl, H. Interrater agreement in headache diagnoses. Cephalalgia Rep. 2022, 5, 1–6. [Google Scholar] [CrossRef]
- Tsushima, Y.; Endo, K. MR Imaging in the Evaluation of Chronic or Recurrent Headache. Radiology 2005, 235, 575–579. [Google Scholar] [CrossRef]
- Sciascia, A.D.; Jacobs, C.A.; Morris, B.J.; Kibler, W.B. The degree of tissue injury in the shoulder does not correlate with pain perception. J. Shoulder Elb. Surg. 2017, 26, e151–e152. [Google Scholar] [CrossRef]
- Linn, F.H.; Rinkel, G.J.; Algra, A.; van Gijn, J. Headache characteristics in subarachnoid haemorrhage and benign thunderclap headache. J. Neurol. Neurosurg. Psychiatry 1998, 65, 791–793. [Google Scholar] [CrossRef]
- Zhu, Q.; Liang, Y.; Fan, Z.; Liu, Y.; Zhou, C.; Zhang, H.; Li, T.; Zhou, Y.; Yang, J.; Wang, Y.; et al. Ischemic Infarction of Pituitary Apoplexy: A Retrospective Study of 46 Cases From a Single Tertiary Center. Front. Neurosci. 2021, 15, 808111. [Google Scholar] [CrossRef]
- International Association for the Study of Pain IASP Taxonomy. Pain Terms. Neuropathic Pain. Available online: www.iasp-pain.org/Taxonomy#Neuropathicpain (accessed on 5 July 2023).
- Steinberg, D.F.; Argoff, C. Understanding Pain. Pract. Neurol. 2021. [Google Scholar]
- Nijs, J.; Lahousse, A.; Kapreli, E.; Bilika, P.; Saraçoğlu, I.; Malfliet, A.; Coppieters, I.; De Baets, L.; Leysen, L.; Roose, E.; et al. Nociplastic Pain Criteria or Recognition of Central Sensitization? Pain Phenotyping in the Past, Present and Future. J. Clin. Med. 2021, 10, 3203. [Google Scholar] [CrossRef]
- Wasner, G. Central Pain Syndromes. Curr. Pain Headache Rep. 2010, 14, 489–496. [Google Scholar] [CrossRef]
- Chabriat, H.; Danchot, J.; Michel, P.; Joire, J.E.; Henry, P. Precipitating Factors of Headache. A Prospective Study in a National Control-Matched Survey in Migraineurs and Nonmigraineurs. Headache 1999, 39, 335–338. [Google Scholar] [CrossRef]
- Peroutka, S.J. What Turns on a Migraine? A Systematic Review of Migraine Precipitating Factors. Curr. Pain Headache Rep. 2014, 18, 454. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Chu, M.K.; Kim, J.-M.; Park, S.-G.; Cho, S.-J. Analysis of Trigger Factors in Episodic Migraineurs Using a Smartphone Headache Diary Applications. PLoS ONE 2016, 11, e0149577. [Google Scholar] [CrossRef] [PubMed]
- Kundermann, B.; Krieg, J.-C.; Schreiber, W.; Lautenbacher, S. The Effect of Sleep Deprivation on Pain. Pain Res. Manag. 2004, 9, 25–32. [Google Scholar] [CrossRef]
- Schuh-Hofer, S.; Wodarski, R.; Pfau, D.B.; Caspani, O.; Magerl, W.; Kennedy, J.D.; Treede, R.-D. One night of total sleep deprivation promotes a state of generalized hyperalgesia: A surrogate pain model to study the relationship of insomnia and pain. Pain 2013, 154, 1613–1621. [Google Scholar] [CrossRef]
- Ma, B.; Yu, L.-H.; Fan, J.; Cong, B.; He, P.; Ni, X.; Burnstock, G. Estrogen modulation of peripheral pain signal transduction: Involvement of P2X3 receptors. Purinergic Signal. 2011, 7, 73–83. [Google Scholar] [CrossRef]
- Kumar, M.; Saxena, I.; Verma, A. Pain response during fasting and postprandial conditions in healthy young Indian males. Int. J. Clin. Exp. Physiol. 2014, 1, 262. [Google Scholar] [CrossRef]
- Horn-Hofmann, C.; Büscher, P.; Lautenbacher, S.; Wolstein, J. The effect of nonrecurring alcohol administration on pain perception in humans: A systematic review. J. Pain Res. 2015, 8, 175–187. [Google Scholar] [CrossRef]
- Russo, F.; Williamson, J. Interpreting Causality in the Health Sciences. Int. Stud. Philos. Sci. 2007, 21, 157–170. [Google Scholar] [CrossRef]
- Williamson, J. Establishing Causal Claims in Medicine. Int. Stud. Philos. Sci. 2019, 32, 33–61. [Google Scholar] [CrossRef]
- Stovner, L.J.; Hagen, K.; Linde, M.; Steiner, T.J. The global prevalence of headache: An update, with analysis of the influences of methodological factors on prevalence estimates. J. Headache Pain 2022, 23, 34. [Google Scholar] [CrossRef]
- Hoffmans, R.; Wagemakers, A.; van Drunen, C.; Hellings, P.; Fokkens, W. Acute and chronic rhinosinusitis and allergic rhinitis in relation to comorbidity, ethnicity and environment. PLoS ONE 2018, 13, e0192330. [Google Scholar] [CrossRef] [PubMed]
- Clifton, N.J.; Jones, N.S. Prevalence of facial pain in 108 consecutive patients with paranasal mucopurulent discharge at endoscopy. J. Laryngol. Otol. 2007, 121, 345–348. [Google Scholar] [CrossRef]
- Subbarao, S.; Ribeiro, S.; Campbell, H.; Okike, I.; Ramsay, M.E.; Ladhani, S. Trends in Laboratory-Confirmed Bacterial Meningitis (2012–2019): National Observational Study, England. SSRN Electron. J. 2021. [Google Scholar] [CrossRef]
- Hasbun, R. Progress and Challenges in Bacterial Meningitis: A Review. JAMA 2022, 328, 2147–2154. [Google Scholar] [CrossRef]
- Sharifi-Mood, B.; Khajeh, A.; Metanat, M.; Rasouli, A. Epidemiology of Meningitis Studied at a University Hospital in Zahedan, South-Eastern Iran. Int. J. Infect. 2015, 2. [Google Scholar] [CrossRef]
- McGill, F.; Griffiths, M.J.; Bonnett, L.J.; Geretti, A.M.; Michael, B.D.; Beeching, N.J.; McKee, D.; Scarlett, P.; Hart, I.J.; Mutton, K.J.; et al. Incidence, aetiology, and sequelae of viral meningitis in UK adults: A multicentre prospective observational cohort study. Lancet Infect. Dis. 2018, 18, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Razvi, S.S.; Davidson, R.; Bone, I.; Muir, K.W. The prevalence of cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL) in the west of Scotland. J. Neurol. Neurosurg. Psychiatry 2005, 76, 739–741. [Google Scholar] [CrossRef]
- Narayan, S.K.; Gorman, G.; Kalaria, R.N.; Ford, G.A.; Chinnery, P.F. The minimum prevalence of CADASIL in northeast England. Neurology 2012, 78, 1025–1027. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.C.; Song, S.-K.; Lee, J.S.; Kang, S.-Y.; Kang, J.-H. Headache among CADASIL patients with R544C mutation: Prevalence, characteristics, and associations. Cephalalgia 2014, 34, 22–28. [Google Scholar] [CrossRef]
- Guey, S.; Mawet, J.; Hervé, D.; Duering, M.; Godin, O.; Jouvent, E.; Opherk, C.; Alili, N.; Dichgans, M.; Chabriat, H. Prevalence and characteristics of migraine in CADASIL. Cephalalgia 2016, 36, 1038–1047. [Google Scholar] [CrossRef]
- Saini, V.; Guada, L.; Yavagal, D.R. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology 2021, 97, S6–S16. [Google Scholar] [CrossRef]
- Oliveira, F.A.A.; Sampaio Rocha-Filho, P.A. Headaches Attributed to Ischemic Stroke and Transient Ischemic Attack. Headache 2019, 59, 469–476. [Google Scholar] [CrossRef]
- Coutinho, J.M.; Zuurbier, S.M.; Aramideh, M.; Stam, J. The Incidence of Cerebral Venous Thrombosis: A cross-sectional study. Stroke 2012, 43, 3375–3377. [Google Scholar] [CrossRef]
- Kristoffersen, E.S.; Harper, C.E.; Vetvik, K.G.; Zarnovicky, S.; Hansen, J.M.; Faiz, K.W. Incidence and Mortality of Cerebral Venous Thrombosis in a Norwegian Population. Stroke 2020, 51, 3023–3029. [Google Scholar] [CrossRef]
- Wasay, M.; Bakshi, R.; Bobustuc, G.; Kojan, S.; Sheikh, Z.; Dai, A.; Cheema, Z. Cerebral Venous Thrombosis: Analysis of a Multicenter Cohort From the United States. J. Stroke Cerebrovasc. Dis. 2008, 17, 49–54. [Google Scholar] [CrossRef]
- Iurlaro, S.; Beghi, E.; Massetto, N.; Guccione, A.; Autunno, M.; Colombo, B.; Di Monda, T.; Gionco, M.; Cortelli, P.; Perini, F.; et al. Does headache represent a clinical marker in early diagnosis of cerebral venous thrombosis? A prospective multicentric study. Neurol. Sci. 2004, 25 (Suppl. S3), s298–s299. [Google Scholar] [CrossRef]
- Lee, V.H.; Brown, R.D., Jr.; Mandrekar, J.N.; Mokri, B. Incidence and outcome of cervical artery dissection: A population-based study. Neurology 2006, 67, 1809–1812. [Google Scholar] [CrossRef] [PubMed]
- Silbert, P.L.; Mokri, B.; Schievink, W.I. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology 1995, 45, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Passias, P.G.; Pyne, A.; Horn, S.R.; Poorman, G.W.; Janjua, M.B.; Vasquez-Montes, D.; Bortz, C.A.; Segreto, F.A.; Frangella, N.J.; Siow, M.Y.; et al. Developments in the treatment of Chiari type 1 malformations over the past decade. J. Spine Surg. 2018, 4, 45–54. [Google Scholar] [CrossRef]
- Milhorat, T.H.; Chou, M.W.; Trinidad, E.M.; Kula, R.W.; Mandell, M.; Wolpert, C.; Speer, M.C. Chiari I Malformation Redefined: Clinical and Radiographic Findings for 364 Symptomatic Patients. Neurosurgery 1999, 44, 1005–1017. [Google Scholar] [CrossRef]
- Stovner, L.J. Headache Associated With the Chiari Type I Malformation. Headache 1993, 33, 175–181. [Google Scholar] [CrossRef]
- Wu, Y.W.; Chin, C.T.; Chan, K.M.; Barkovich, A.J.; Ferriero, D.M. Pediatric Chiari I malformations: Do clinical and radiologic features correlate? Neurology 1999, 53, 1271–1276. [Google Scholar] [CrossRef]
- Aitken, L.A.; Lindan, C.E.; Sidney, S.; Gupta, N.; Barkovich, A.J.; Sorel, M.; Wu, Y.W. Chiari Type I Malformation in a Pediatric Population. Pediatr. Neurol. 2009, 40, 449–454. [Google Scholar] [CrossRef]
- Li, K.J.; Semenov, D.; Turk, M.; Pope, J. A meta-analysis of the epidemiology of giant cell arteritis across time and space. Arthritis Res. Ther. 2021, 23, 82. [Google Scholar] [CrossRef]
- Vincenten, S.C.C.; Mulleners, W.M. The quest for a headache pattern in giant cell arteritis: A cohort study. Cephalalgia Rep. 2021, 4. [Google Scholar] [CrossRef]
- Gonzalez-Gay, M.A.; Barros, S.; Lopez-Diaz, M.J.; Garcia-Porrua, C.; Sanchez-Andrade, A.; Llorca, J. Giant Cell Arteritis: Disease patterns of clinical presentation in a series of 240 patients. Medicine 2005, 84, 269–276. [Google Scholar] [CrossRef]
- Garmendia Madariaga, A.; Santos Palacios, S.; Guillén-Grima, F.; Galofré, J.C. The Incidence and Prevalence of Thyroid Dysfunction in Europe: A Meta-Analysis. J. Clin. Endocrinol. Metab. 2014, 99, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Moreau, T.; Manceau, E.; Giroud-Baleydier, F.; Dumas, R.; Giroud, M. Headache in hypothyroidism. Prevalence and outcome under thyroid hormone therapy. Cephalalgia 1998, 18, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Lima Carvalho, M.F.; de Medeiros, J.S.; Valença, M.M. Headache in recent onset hypothyroidism: Prevalence, characteristics and outcome after treatment with levothyroxine. Cephalalgia 2017, 37, 938–946. [Google Scholar] [CrossRef]
- Durcan, F.J.; Corbett, J.J.; Wall, M. The Incidence of Pseudotumor Cerebri. Population studies in Iowa and Louisiana. Arch. Neurol. 1988, 45, 875–877. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Ahlskog, J.E.; Cross, S.A.; Kurland, L.T.; O’Fallon, W.M. Idiopathic Intracranial Hypertension (Pseudotumor Cerebri). Descriptive epidemiology in Rochester, Minn, 1976 to 1990. Arch. Neurol. 1993, 50, 78–80. [Google Scholar] [CrossRef]
- Wall, M.; George, D. Idiopathic intracranial hypertension: A prospective study of 50 patients. Brain 1991, 114, 155–180. [Google Scholar] [CrossRef]
- Friedman, D.I.; Quiros, P.A.; Subramanian, P.S.; Mejico, L.J.; Gao, S.; McDermott, M.; Wall, M.; the NORDIC IIHTT Study Group. Headache in Idiopathic Intracranial Hypertension: Findings From the Idiopathic Intracranial Hypertension Treatment Trial. Headache 2017, 57, 1195–1205. [Google Scholar] [CrossRef]
- D′Amico, D.A.; Curone, M.; Ciasca, P.; Cammarata, G.; Melzi, L.; Bussone, G.; Bianchi Marzoli, S. Headache prevalence and clinical features in patients with idiopathic intracranial hypertension (IIH). Neurol. Sci. 2013, 34 (Suppl. S1), S147–S149. [Google Scholar] [CrossRef]
- Bondy, M.L.; Scheurer, M.E.; Malmer, B.; Barnholtz-Sloan, J.S.; Davis, F.G.; Il’Yasova, D.; Kruchko, C.; McCarthy, B.J.; Rajaraman, P.; Schwartzbaum, J.A.; et al. Brain tumor epidemiology: Consensus from the Brain Tumor Epidemiology Consortium. Cancer 2008, 113, 1953–1968. [Google Scholar] [CrossRef]
- Pfund, Z.; Szapáry, L.; Jászberényi, O.; Nagy, F.; Czopf, J. Headache in Intracranial Tumors. Cephalalgia 1999, 19, 787–790, discussion 765. [Google Scholar] [CrossRef]
- Forsyth, P.A.; Posner, J.B. Headaches in patients with brain tumors: A study of 111 patients. Neurology 1993, 43, 1678–1683. [Google Scholar] [CrossRef] [PubMed]
- Valentinis, L.; Tuniz, F.; Valent, F.; Mucchiut, M.; Little, D.; Skrap, M.; Bergonzi, P.; Zanchin, G. Headache attributed to intracranial tumours: A prospective cohort study. Cephalalgia 2010, 30, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Schankin, C.J.; Ferrari, U.; Reinisch, V.M.; Birnbaum, T.; Goldbrunner, R.; Straube, A. Characteristics of Brain Tumour-Associated Headache. Cephalalgia 2007, 27, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Yatsuga, S.; Povalko, N.; Nishioka, J.; Katayama, K.; Kakimoto, N.; Matsuishi, T.; Kakuma, T.; Koga, Y.; Taro Matsuoka for MELAS Study Group in Japan. MELAS: A nationwide prospective cohort study of 96 patients in Japan. Biochim. Biophys. Acta 2012, 1820, 619–624. [Google Scholar] [CrossRef]
- Tiehuis, L.H.; Koene, S.; Saris, C.G.J.; Janssen, M.C.H. Mitochondrial migraine; a prevalence, impact and treatment efficacy cohort study. Mitochondrion 2020, 53, 128–132. [Google Scholar] [CrossRef]
- Kraya, T.; Deschauer, M.; Joshi, P.R.; Zierz, S.; Gaul, C. Prevalence of Headache in Patients With Mitochondrial Disease: A Cross-Sectional Study. Headache J. Head Face Pain 2018, 58, 45–52. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, G.; Feng, J.; Chen, L.; Liu, G.; Wang, J.; Wang, Q.; Yu, J.; Yang, X.; Yang, Z.; et al. Incidence and prevalence of moyamoya disease in urban China: A nationwide retrospective cohort study. Stroke Vasc. Neurol. 2021, 6, 615–623. [Google Scholar] [CrossRef]
- Ahn, I.M.; Park, D.-H.; Hann, H.J.; Kim, K.H.; Kim, H.J.; Ahn, H.S. Incidence, Prevalence, and Survival of Moyamoya Disease in Korea: A nationwide, population-based study. Stroke 2014, 45, 1090–1095. [Google Scholar] [CrossRef]
- Kuriyama, S.; Kusaka, Y.; Fujimura, M.; Wakai, K.; Tamakoshi, A.; Hashimoto, S.; Tsuji, I.; Inaba, Y.; Yoshimoto, T. Prevalence and Clinicoepidemiological Features of Moyamoya Disease in Japan: Findings from a nationwide epidemiological survey. Stroke 2008, 39, 42–47. [Google Scholar] [CrossRef]
- Seol, H.J.; Wang, K.-C.; Kim, S.-K.; Hwang, Y.-S.; Kim, K.J.; Cho, B.-K. Headache in pediatric moyamoya disease: Review of 204 consecutive cases. J. Neurosurg. 2005, 103, 439–442. [Google Scholar] [CrossRef]
- Battistella, P.A.; Carollo, C. Clinical and neuroradiological findings of Moyamoya disease in Italy. Clin. Neurol. Neurosurg. 1997, 99 (Suppl. S2), S54–S57. [Google Scholar] [CrossRef]
- Kraemer, M.; Lee, S.-I.; Ayzenberg, I.; Schwitalla, J.C.; Diehl, R.R.; Berlit, P.; Bosche, B.; Katsarava, Z.; Obermann, M. Headache in Caucasian patients with Moyamoya angiopathy—A systematic cohort study. Cephalalgia 2017, 37, 496–500. [Google Scholar] [CrossRef]
- Hishikawa, T.; Sugiu, K.; Date, I. Moyamoya Disease: A Review of Clinical Research. Acta Med. Okayama 2016, 70, 229–236. [Google Scholar] [CrossRef]
- Arkema, E.V.; Grunewald, J.; Kullberg, S.; Eklund, A.; Askling, J. Sarcoidosis incidence and prevalence: A nationwide register-based assessment in Sweden. Eur. Respir. J. 2016, 48, 1690–1699. [Google Scholar] [CrossRef]
- Nowak, D.A.; Widenka, D.C. Neurosarcoidosis: A review of its intracranial manifestation. J. Neurol. 2001, 248, 363–372. [Google Scholar] [CrossRef]
- Fritz, D.; van de Beek, D.; Brouwer, M.C. Clinical features, treatment and outcome in neurosarcoidosis: Systematic review and meta-analysis. BMC Neurol. 2016, 16, 220. [Google Scholar] [CrossRef]
- Wang, S.; Zou, X.-L.; Wu, L.-X.; Zhou, H.-F.; Xiao, L.; Yao, T.; Zhang, Y.; Ma, J.; Zeng, Y.; Zhang, L. Epidemiology of intracerebral hemorrhage: A systematic review and meta-analysis. Front. Neurol. 2022, 13, 915813. [Google Scholar] [CrossRef]
- Ljubisavljevic, S.; Ignjatovic, A.; Ljubisavljevic, M. Headache secondary to nontraumatic brain hemorrhage: A single-center, retrospective clinical study. Arch. Med. Sci. 2019. [Google Scholar] [CrossRef]
- Falhammar, H.; Tornvall, S.; Höybye, C. Pituitary Apoplexy: A Retrospective Study of 33 Cases From a Single Center. Front. Endocrinol. 2021, 12, 656950. [Google Scholar] [CrossRef]
- Biousse, V.; Newman, N.J.; Oyesiku, N.M. Precipitating factors in pituitary apoplexy. J. Neurol. Neurosurg. Psychiatry 2001, 71, 542–545. [Google Scholar] [CrossRef]
- Briet, C.; Salenave, S.; Bonneville, J.-F.; Laws, E.R.; Chanson, P. Pituitary Apoplexy. Endocr. Rev. 2015, 36, 622–645. [Google Scholar] [CrossRef] [PubMed]
- Stam, A.H.; Kothari, P.H.; Shaikh, A.; Gschwendter, A.; Jen, J.C.; Hodgkinson, S.; Hardy, T.A.; Hayes, M.; Kempster, P.A.; Kotschet, K.E.; et al. Retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations. Brain 2016, 139, 2909–2922. [Google Scholar] [CrossRef] [PubMed]
- Pelzer, N.; Hoogeveen, E.S.; Haan, J.; Bunnik, R.; Poot, C.C.; Van Zwet, E.W.; Inderson, A.; Fogteloo, A.J.; Reinders, M.E.J.; Middelkoop, H.A.M.; et al. Systemic features of retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations: A monogenic small vessel disease. J. Intern. Med. 2019, 285, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Magid-Bernstein, J.; Omran, S.S.; Parikh, N.S.; Merkler, A.E.; Navi, B.; Kamel, H. RCVS: Symptoms, Incidence, and Resource Utilization in a Population-Based US Cohort. Neurology 2021, 97, e248–e253. [Google Scholar] [CrossRef] [PubMed]
- Wolff, V.; Ducros, A. Reversible Cerebral Vasoconstriction Syndrome Without Typical Thunderclap Headache. Headache 2016, 56, 674–687. [Google Scholar] [CrossRef]
- Acquavella, J.; Mehra, R.; Bron, M.; Suomi, J.M.; Hess, G.P. Prevalence of narcolepsy and other sleep disorders and frequency of diagnostic tests from 2013–2016 in insured patients actively seeking care. J. Clin. Sleep Med. 2020, 16, 1255–1263. [Google Scholar] [CrossRef]
- Russell, M.B.; Kristiansen, H.A.; Kværner, K.J. Headache in sleep apnea syndrome: Epidemiology and pathophysiology. Cephalalgia 2014, 34, 752–755. [Google Scholar] [CrossRef]
- Kristiansen, H.A.; Kværner, K.J.; Akre, H.; Øverland, B.; Sandvik, L.; Russell, M.B. Sleep apnoea headache in the general population. Cephalalgia 2012, 32, 451–458. [Google Scholar] [CrossRef]
- Suzuki, K.; Miyamoto, M.; Miyamoto, T.; Numao, A.; Suzuki, S.; Sakuta, H.; Iwasaki, A.; Watanabe, Y.; Fujita, H.; Hirata, K. Sleep apnoea headache in obstructive sleep apnoea syndrome patients presenting with morning headache: Comparison of the ICHD-2 and ICHD-3 beta criteria. J. Headache Pain 2015, 16, 56. [Google Scholar] [CrossRef]
- Schievink, W.I.; Maya, M.M.; Moser, F.G.; Simon, P.; Nuño, M. Incidence of spontaneous intracranial hypotension in a community: Beverly Hills, California, 2006–2020. Cephalalgia 2022, 42, 312–316. [Google Scholar] [CrossRef]
- Schievink, W.I.; Maya, M.M.; Moser, F.; Tourje, J.; Torbati, S. Frequency of spontaneous intracranial hypotension in the emergency department. J. Headache Pain 2007, 8, 325–328. [Google Scholar] [CrossRef]
- Kleindorfer, D.; Panagos, P.; Pancioli, A.; Khoury, J.; Kissela, B.; Woo, D.; Schneider, A.; Alwell, K.; Jauch, E.; Miller, R.; et al. Incidence and Short-Term Prognosis of Transient Ischemic Attack in a Population-Based Study. Stroke 2005, 36, 720–723. [Google Scholar] [CrossRef]
- Al-Shahi, R.; Fang, J.S.; Lewis, S.C.; Warlow, C.P. Prevalence of adults with brain arteriovenous malformations: A community based study in Scotland using capture-recapture analysis. J. Neurol. Neurosurg. Psychiatry 2002, 73, 547–551. [Google Scholar] [CrossRef]
- Flemming, K.D. Incidence, Prevalence, and Clinical Presentation of Cerebral Cavernous Malformations. Methods Mol. Biol. 2020, 2152, 27–33. [Google Scholar] [CrossRef]
- Mohr, J.P.; Parides, M.K.; Stapf, C.; Moquete, E.; Moy, C.S.; Overbey, J.R.; Al-Shahi Salman, R.; Vicaut, E.; Young, W.L.; Houdart, E.; et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): A multicentre, non-blinded, randomised trial. Lancet 2014, 383, 614–621. [Google Scholar] [CrossRef]
- McGill, F.; Griffiths, M.J.; Solomon, T. Viral meningitis: Current issues in diagnosis and treatment. Curr. Opin. Infect. Dis. 2017, 30, 248–256. [Google Scholar] [CrossRef]
- Trigo, J.; García-Azorín, D.; Sierra-Mencía, A.; Tamayo-Velasco, A.; Martínez-Paz, P.; Tamayo, E.; Guerrero, A.L.; Gonzalo-Benito, H. Cytokine and interleukin profile in patients with headache and COVID-19: A pilot, CASE-control, study on 104 patients. J. Headache Pain 2021, 22, 51. [Google Scholar] [CrossRef]
- Bobker, S.M.; Robbins, M.S. COVID-19 and Headache: A Primer for Trainees. Headache 2020, 60, 1806–1811. [Google Scholar] [CrossRef]
- Goffaux, P.; Fortin, D. Brain Tumor Headaches: From bedside to bench. Neurosurgery 2010, 67, 459–466. [Google Scholar] [CrossRef]
- Leira, R.; Dávalos, A.; Aneiros, A.; Serena, J.; Pumar, J.M.; Castillo, J. Headache as A Surrogate Marker of the Molecular Mechanisms Implicated in Progressing Stroke. Cephalalgia 2002, 22, 303–308. [Google Scholar] [CrossRef]
- Liem, M.K.; Oberstein, S.A.; Van Der Grond, J.; Ferrari, M.D.; Haan, J. CADASIL and migraine: A narrative review. Cephalalgia 2010, 30, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Taylor, F.R.; Larkins, M.V. Headache and Chiari I malformation: Clinical presentation, diagnosis, and controversies in management. Curr. Pain Headache Rep. 2002, 6, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Williams, B. Cough Headache due to Craniospinal Pressure Dissociation. Arch. Neurol. 1980, 37, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Arafah, B.M.; Prunty, D.; Ybarra, J.; Hlavin, M.L.; Selman, W.R. The Dominant Role of Increased Intrasellar Pressure in the Pathogenesis of Hypopituitarism, Hyperprolactinemia, and Headaches in Patients with Pituitary Adenomas. J. Clin. Endocrinol. Metab. 2000, 85, 1789–1793. [Google Scholar] [CrossRef]
- Jennum, P.; Børgesen, S.E. Intracranial Pressure and Obstructive Sleep Apnea. Chest 1989, 95, 279–283. [Google Scholar] [CrossRef]
- Devor, M.; Amir, R.; Rappaport, Z.H. Pathophysiology of Trigeminal Neuralgia: The Ignition Hypothesis. Clin. J. Pain 2002, 18, 4–13. [Google Scholar] [CrossRef]
- Hucklenbroich, P. “Disease Entity” as the Key Theoretical Concept of Medicine. J. Med. Philos. 2014, 39, 609–633. [Google Scholar] [CrossRef]
- Stovner, L.J.; Nichols, E.; Steiner, T.J.; Abd-Allah, F.; Abdelalim, A.; Al-Raddadi, R.M.; Ansha, M.G.; Barac, A.; Bensenor, I.M.; Doan, L.P.; et al. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 954–976. [Google Scholar] [CrossRef]
- Pohl, H. Red flags in headache care. Headache 2022, 62, 534–535. [Google Scholar] [CrossRef]
- Pohl, H.; Do, T.P.; García-Azorín, D.; Hansen, J.M.; Kristoffersen, E.S.; Nelson, S.E.; Obermann, M.; Sandor, P.S.; Schankin, C.J.; Schytz, H.W.; et al. Green Flags and headache: A concept study using the Delphi method. Headache 2021, 61, 300–309. [Google Scholar] [CrossRef]
- García-Azorín, D.; Abelaira-Freire, J.; González-García, N.; Rodriguez-Adrada, E.; Schytz, H.W.; Barloese, M.; Guerrero, A.L.; Porta-Etessam, J.; Martín-Sánchez, F.J. Sensitivity of the SNNOOP10 list in the high-risk secondary headache detection. Cephalalgia 2022, 42, 1521–1531. [Google Scholar] [CrossRef]
- Perry, J.J.; Sivilotti, M.L.A.; Sutherland, J.; Hohl, C.M.; Émond, M.; Calder, L.A.; Vaillancourt, C.; Thiruganasambandamoorthy, V.; Lesiuk, H.; Wells, G.A.; et al. Validation of the Ottawa Subarachnoid Hemorrhage Rule in patients with acute headache. Can. Med. Assoc. J. 2017, 189, E1379–E1385. [Google Scholar] [CrossRef]
- Sempere, A.P.; Porta-Etessam, J.; Medrano, V.; Garcia-Morales, I.; Concepción, L.; Ramos, A.; Florencio, I.; Bermejo, F.; Botella, C. Neuroimaging in the Evaluation of Patients with Non-Acute Headache. Cephalalgia 2005, 25, 30–35. [Google Scholar] [CrossRef]
- Wan, W.H.; Ang, B.T.; Wang, E. The Cushing Response: A case for a review of its role as a physiological reflex. J. Clin. Neurosci. 2008, 15, 223–228. [Google Scholar] [CrossRef]
- Pascual, J.; Iglesias, F.; Oterino, A.; Vazquez-Barquero, A.; Berciano, J. Cough, exertional, and sexual headaches: An analysis of 72 benign and symptomatic cases. Neurology 1996, 46, 1520–1524. [Google Scholar] [CrossRef]
- Cordenier, A.; De Hertogh, W.; De Keyser, J.; Versijpt, J. Headache associated with cough: A review. J. Headache Pain 2013, 14, 42. [Google Scholar] [CrossRef]
- García-Azorín, D.; Do, T.P.; Gantenbein, A.R.; Hansen, J.M.; Souza, M.N.P.; Obermann, M.; Pohl, H.; Schankin, C.J.; Schytz, H.W.; Sinclair, A.; et al. Delayed headache after COVID-19 vaccination: A red flag for vaccine induced cerebral venous thrombosis. J. Headache Pain 2021, 22, 108. [Google Scholar] [CrossRef]
- Peral-Cagigal, B.; Perez-Villar, A.; Redondo-Gonzalez, L.M.; Garcia-Sierra, C.; Morante-Silva, M.; Madrigal-Rubiales, B.; Verrier-Hernandez, A. Temporal headache and jaw claudication may be the key for the diagnosis of giant cell arteritis. Med. Oral Patol. Oral Cir. Bucal. 2018, 23, e290–e294. [Google Scholar] [CrossRef]
- Saper, J.R.; Da Silva, A.N. Medication Overuse Headache: History, Features, Prevention and Management Strategies. CNS Drugs 2013, 27, 867–877. [Google Scholar] [CrossRef]
- van de Beek, D.; de Gans, J.; Spanjaard, L.; Weisfelt, M.; Reitsma, J.B.; Vermeulen, M. Clinical Features and Prognostic Factors in Adults with Bacterial Meningitis. N. Engl. J. Med. 2004, 351, 1849–1859. [Google Scholar] [CrossRef]
- Linn, F.H.; Wijdicks, E.F.; van der Graaf, Y.; Weerdesteyn-van Vliet, F.A.; Bartelds, A.I.; van Gijn, J. Prospective study of sentinel headache in aneurysmal subarachnoid haemorrhage. Lancet 1994, 344, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Cerón, J.; Díaz-Forero, F.; Buitrago, A.; Chinchilla, S. Secondary cluster headache and numb chin syndrome as initial manifestation of high-grade B-lymphoma: A case report. J. Med. Case Rep. 2021, 15, 468. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.S.; Donaldson, L.; Margolin, E. Papilledema: A review of etiology, pathophysiology, diagnosis, and management. Surv. Ophthalmol. 2022, 67, 1135–1159. [Google Scholar] [CrossRef] [PubMed]
- Schievink, W.I. Spontaneous Spinal Cerebrospinal Fluid Leaks and Intracranial Hypotension. JAMA 2006, 295, 2286–2296. [Google Scholar] [CrossRef]
- Hofmann, E.; Behr, R.; Neumann-Haefelin, T.; Schwager, K. Pulsatile Tinnitus: Imaging and differential diagnosis. Dtsch. Arztebl. Int. 2013, 110, 451–458. [Google Scholar] [CrossRef]
- Figatner, J.G. Diagnosis of Night Sweats. JAMA J. Am. Med. Assoc. 1993, 270, 2502. [Google Scholar] [CrossRef]
- Pohl, H.; Tarnutzer, A.A. Acute Angle-Closure Glaucoma. N. Engl. J. Med. 2018, 378, e14. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Tsai, S.-H.; Wang, J.-C.; Kao, H.-W.; Hsu, C.-W.; Liu, W.-H.; Chen, S.-J. Swollen and bloodshot eye following headache. Am. J. Emerg. Med. 2019, 37, 378.e7–378.e9. [Google Scholar] [CrossRef]
- Sakaida, H.; Kobayashi, M.; Ito, A.; Takeuchi, K. Cavernous sinus thrombosis: Linking a swollen red eye and headache. Lancet 2014, 384, 928. [Google Scholar] [CrossRef]
- Bravo Petersen, S.M.; Vardaxis, V.G. The flexion–rotation test performed actively and passively: A comparison of range of motion in patients with cervicogenic headache. J. Man. Manip. Ther. 2015, 23, 61–67. [Google Scholar] [CrossRef]
- Tabak, F.; Murtezaoglu, A.; Tabak, O.; Ozaras, R.; Mete, B.; Kutlubay, Z.; Mert, A.; Ozturk, R. Clinical Features and Etiology of Adult Patients with Fever and Rash. Ann. Dermatol. 2012, 24, 420–425. [Google Scholar] [CrossRef]
- Tsai, J.; Nagel, M.A.; Gilden, D. Skin rash in meningitis and meningoencephalitis. Neurology 2013, 80, 1808–1811. [Google Scholar] [CrossRef]
- Grooters, G.S.; Sluzewski, M.; Tijssen, C.C. How Often Is Thunderclap Headache Caused by the Reversible Cerebral Vasoconstriction Syndrome? Headache 2014, 54, 732–735. [Google Scholar] [CrossRef]
- Cheng, Y.-C.; Kuo, K.-H.; Lai, T.-H. A common cause of sudden and thunderclap headaches: Reversible cerebral vasoconstriction syndrome. J. Headache Pain 2014, 15, 13. [Google Scholar] [CrossRef]
- Schwedt, T.J. Thunderclap Headache. Contin. Lifelong Learn. Neurol. 2015, 21, 1058–1071. [Google Scholar] [CrossRef]
- Suri, H.; Dougherty, C. Presentation and Management of Headache in Pituitary Apoplexy. Curr. Pain Headache Rep. 2019, 23, 61. [Google Scholar] [CrossRef]
- Vinod, K.V.; Reddy, P.; Pillai, V.M. Scalloped tongue: A rare finding in nocturnal bruxism. Natl. Med. J. India 2017, 30, 296. [Google Scholar] [CrossRef]
- Weiss, T.M.; Atanasov, S.; Calhoun, K.H. The Association of Tongue Scalloping With Obstructive Sleep Apnea and Related Sleep Pathology. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol. Head Neck Surg. 2005, 133, 966–971. [Google Scholar] [CrossRef]
- Giuseffi, V.; Wall, M.; Siegel, P.Z.; Rojas, P.B. Symptoms and disease associations in idiopathic intracranial hypertension (pseudotumor cerebri): A case-control study. Neurology 1991, 41, 239–244. [Google Scholar] [CrossRef]
- Do, T.P.; Remmers, A.; Schytz, H.W.; Schankin, C.; Nelson, S.E.; Obermann, M.; Hansen, J.M.; Sinclair, A.J.; Gantenbein, A.R.; Schoonman, G.G. Red and orange flags for secondary headaches in clinical practice: SNNOOP10 list. Neurology 2019, 92, 134–144. [Google Scholar] [CrossRef]
- Robbins, M.S.; Farmakidis, C.; Dayal, A.K.; Lipton, R.B. Acute headache diagnosis in pregnant women: A hospital-based study. Neurology 2015, 85, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Argyriou, A.A.; Chroni, E.; Polychronopoulos, P.; Argyriou, K.; Papapetropoulos, S.; Corcondilas, M.; Lepoura, N.; Heras, P. Headache characteristics and brain metastases prediction in cancer patients. Eur. J. Cancer Care 2006, 15, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wall, M. Epidemiology and Risk Factors for Idiopathic Intracranial Hypertension. Int. Ophthalmol. Clin. 2014, 54, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fallata, A.; Salter, A.; Tyry, T.; Cutter, G.R.; Marrie, R.A. Trigeminal Neuralgia Commonly Precedes the Diagnosis of Multiple Sclerosis. Int. J. MS Care 2017, 19, 240–246. [Google Scholar] [CrossRef]
- Weyand, C.M.; Goronzy, J.J. Clinical practice. Giant-Cell Arteritis and Polymyalgia Rheumatica. N. Engl. J. Med. 2014, 371, 50–57. [Google Scholar] [CrossRef]
- Crawford, P.M.; West, C.R.; Chadwick, D.W.; Shaw, M.D. Arteriovenous malformations of the brain: Natural history in unoperated patients. J. Neurol. Neurosurg. Psychiatry 1986, 49, 1–10. [Google Scholar] [CrossRef]
- Gross, B.A.; Du, R. The natural history of Moyamoya in a North American adult cohort. J. Clin. Neurosci. 2013, 20, 44–48. [Google Scholar] [CrossRef]
- How, J.; Blattner, M.; Fowler, S.; Wang-Gillam, A.; Schindler, S.E. Chemotherapy-associated Posterior Reversible Encephalopathy Syndrome: A Case Report and Review of the Literature. Neurologist 2016, 21, 112–117. [Google Scholar] [CrossRef]
- Short, K.; Emsley, H.C.A. Illicit Drugs and Reversible Cerebral Vasoconstriction Syndrome. Neurohospitalist 2021, 11, 40–44. [Google Scholar] [CrossRef]
- Friedman, D.I. Medication-Induced Intracranial Hypertension in Dermatology. Am. J. Clin. Dermatol. 2005, 6, 29–37. [Google Scholar] [CrossRef]
- Matsumura, Y. Risk Analysis of Eculizumab-Related Meningococcal Disease in Japan Using the Japanese Adverse Drug Event Report Database. Drug Healthc. Patient Saf. 2020, 12, 207–215. [Google Scholar] [CrossRef]
- Amoozegar, F.; Ronksley, P.E.; Sauve, R.; Menon, B.K. Hormonal Contraceptives and Cerebral Venous Thrombosis Risk: A Systematic Review and Meta-Analysis. Front. Neurol. 2015, 6, 7. [Google Scholar] [CrossRef]
- Holle, D.; Obermann, M. The role of neuroimaging in the diagnosis of headache disorders. Ther. Adv. Neurol. Disord. 2013, 6, 369–374. [Google Scholar] [CrossRef]
- Friedman, D.I. Headache and the eye. Curr. Pain Headache Rep. 2008, 12, 296–304. [Google Scholar] [CrossRef]
- Felisati, G.; Lozza, P.; Maccari, A.; Scotti, A.; Leone, M.; Bussone, G. The role of the ear, nose and throat specialist in diagnosing headaches. Neurol. Sci. 2005, 26 (Suppl. S2), s83–s86. [Google Scholar] [CrossRef]
- Kranz, P.G.; Luetmer, P.H.; Diehn, F.E.; Amrhein, T.J.; Tanpitukpongse, T.P.; Gray, L. Myelographic Techniques for the Detection of Spinal CSF Leaks in Spontaneous Intracranial Hypotension. AJR Am. J. Roentgenol. 2016, 206, 8–19. [Google Scholar] [CrossRef]
- Antonakis, J.; Bendahan, S.; Jacquart, P.; Lalive, R. On making causal claims: A review and recommendations. Leadersh. Q. 2010, 21, 1086–1120. [Google Scholar] [CrossRef]
- Michali-Stolarska, M.; Bladowska, J.; Stolarski, M.; Sąsiadek, M.J. Diagnostic Imaging and Clinical Features of Intracranial Hypotension–Review of Literature. Pol. J. Radiol. 2017, 82, 842–849. [Google Scholar] [CrossRef]
- Maurya, V.; Sreedhar, C.M.; Khera, A.; Bhatia, M.; Sharma, V. Trigeminal neuralgia: When does neurovascular contact turn into a conflict? Med. J. Armed Forces India 2019, 75, 134–139. [Google Scholar] [CrossRef]
- Stuginski-Barbosa, J.; Murayama, R.A.; Conti, P.C.; Speciali, J.G. Refractory facial pain attributed to auriculotemporal neuralgia. J. Headache Pain 2012, 13, 415–417. [Google Scholar] [CrossRef][Green Version]
- Campaner, R.; Cerri, M. Manipulative evidence and medical interventions: Some qualifications. Hist. Philos. Life Sci. 2020, 42, 15. [Google Scholar] [CrossRef] [PubMed]
- Foltz, E.L.; Ward, A.A., Jr. Communicating Hydrocephalus from Subarachnoid Bleeding. J. Neurosurg. 1956, 13, 546–566. [Google Scholar] [CrossRef] [PubMed]
- Yiangou, A.; Mitchell, J.; Markey, K.A.; Scotton, W.; Nightingale, P.; Botfield, H.; Ottridge, R.; Mollan, S.P.; Sinclair, A.J. Therapeutic lumbar puncture for headache in idiopathic intracranial hypertension: Minimal gain, is it worth the pain? Cephalalgia 2019, 39, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Rozen, T.D. Daily persistent headache after a viral illness during a worldwide pandemic may not be a new occurrence: Lessons from the 1890 Russian/Asiatic flu. Cephalalgia 2020, 40, 1406–1409. [Google Scholar] [CrossRef]
| Red Flag | Associated Condition |
|---|---|
| Abnormal neurologic examination | Headaches with different aetiologies including mass lesion, hydrocephalus, and dural fistula [115] |
| Arterial hypertension | Pheochromocytoma, hypertensive crisis, pre-eclampsia and eclampsia, acute pressure response to an exogenous agent, and acute increase in intracranial pressure (Cushing response) [116] |
| Cough headache | Chiari malformation type 1 [117] and posterior fossa lesion [118] |
| Delayed headache after COVID-19 vaccination | Sinus thrombosis [119] |
| Exertional headache | Subarachnoid haemorrhage, sinusitis, and brain metastases [117] |
| Fever | Systemic infection, meningitis, and encephalitis |
| Headache associated with sexual activity | Subarachnoid haemorrhage [117] |
| Jaw claudication | Temporal arteritis, temporomandibular joint dysfunction, and myofascial pain [120] |
| Morning headache | Brain tumour [101], medication overuse headache [121], and sleep apnoea [89] |
| Neck stiffness | Meningitis [122] and intracranial haemorrhage [123] |
| Numb chin | Metastatic tumour (infrequently associated with pain) [124] |
| Papilledema | Raised intracranial pressure [125] |
| Positional headache | Intracranial hypertension and intracranial hypotension [126] |
| Pulsatile tinnitus | Intracranial hypertension, arterio-venous malformation, and arterio-venous fistula [127] |
| Recent unwanted weight loss, night sweat | Systemic disorders including infection, malignancy (e.g., lymphoma), autoimmune (e.g., polymyalgia rheumatica), and endocrinologic disorders (e.g., carcinoid syndrome) [128] |
| Reddening of one eye | Glaucoma [129], carotid-cavernous fistula [130], and cavernous sinus thrombosis [131] |
| Reduced range of motion in the flexion-rotation test | Cervicogenic headache [132] |
| Skin rash | Systemic infection, meningitis, meningoencephalitis due to, e.g., measles, Mediterranean spotted fever, Syphilis, Neisseria meningitides, varicella zoster virus, and West Nile virus [133,134] |
| Tenderness upon palpation of the temporal and masseter muscles | Temporal-mandibular dysfunction and temporal arteritis [120] |
| Thunderclap headache | Subarachnoid haemorrhage [10], reversible cerebral vascular constriction syndrome (RCVS) [135,136], cervical artery dissection, cerebral venous sinus thrombosis, spontaneous intracranial hypotension [137], and pituitary apoplexy [138] |
| Tongue Scalloping | Bruxism [139] and sleep apnoea [140] |
| Transient visual obscuration | Intracranial hypertension [141] |
| Mechanism | Associated Conditions | Direct Mechanistic Evidence | Indirect Mechanistic Evidence |
|---|---|---|---|
| Craniospinal pressure dissociation | Chiari malformation type 1 | Measurement of the pressure gradient [105] | Headache attacks from coughing [47] |
| Decreased intracranial pressure | Spontaneous CSF leakage and postdural headache | Invasive evidence of a pressure gradient | MRI signs of reduced intracranial pressure [160] and positional headaches [126] |
| Focal demyelination of the trigeminal nerve | Neuralgia | - | MR evidence of nerve vessel conflict with thinning, grooving, or distortion of the nerve [161], presence of a trigger point or a trigger zone, or pain restricted to a skin area innervated by a specific sensory nerve [5,162] |
| Inflammation | Systemic or localised inflammation | - | Elevated inflammatory parameters, skin rash |
| Medication overuse | Medication overuse headache | - | Medication overuse and presence of morning headaches [121] |
| Raised intracranial pressure | IIH and brain tumour | Measurement of the pressure | Papilledema in fundoscopy, MRI signs of raised intracranial pressure, vomiting, impaired consciousness, bradycardia, hypertension (Cushing response) [116], and positional headaches [101] |
| Sleep-related hypoxia | Sleep apnoea | Reduced oxygen saturation during sleep | Morning headaches [89] |
| Stimulation of arterial nociceptors | SAH and RCVS | - | Thunderclap headache [10,135,136] and imaging evidence of a bleeding or vasospasm |
| Traumatic stimulation of nociceptors | Trauma to the head, whiplash, and craniotomy | Witness of the impact | Traces of the trauma, e.g., scars |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pohl, H. Approaching Headaches—A Guide to Differential-Diagnostic Considerations and Causal Claims. Clin. Transl. Neurosci. 2023, 7, 17. https://doi.org/10.3390/ctn7030017
Pohl H. Approaching Headaches—A Guide to Differential-Diagnostic Considerations and Causal Claims. Clinical and Translational Neuroscience. 2023; 7(3):17. https://doi.org/10.3390/ctn7030017
Chicago/Turabian StylePohl, Heiko. 2023. "Approaching Headaches—A Guide to Differential-Diagnostic Considerations and Causal Claims" Clinical and Translational Neuroscience 7, no. 3: 17. https://doi.org/10.3390/ctn7030017
APA StylePohl, H. (2023). Approaching Headaches—A Guide to Differential-Diagnostic Considerations and Causal Claims. Clinical and Translational Neuroscience, 7(3), 17. https://doi.org/10.3390/ctn7030017






