Sex Differences in Ischemic Cerebral Infarction: A Nationwide German Real-Life Analysis from 2014 to 2019
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Statistics
3. Results
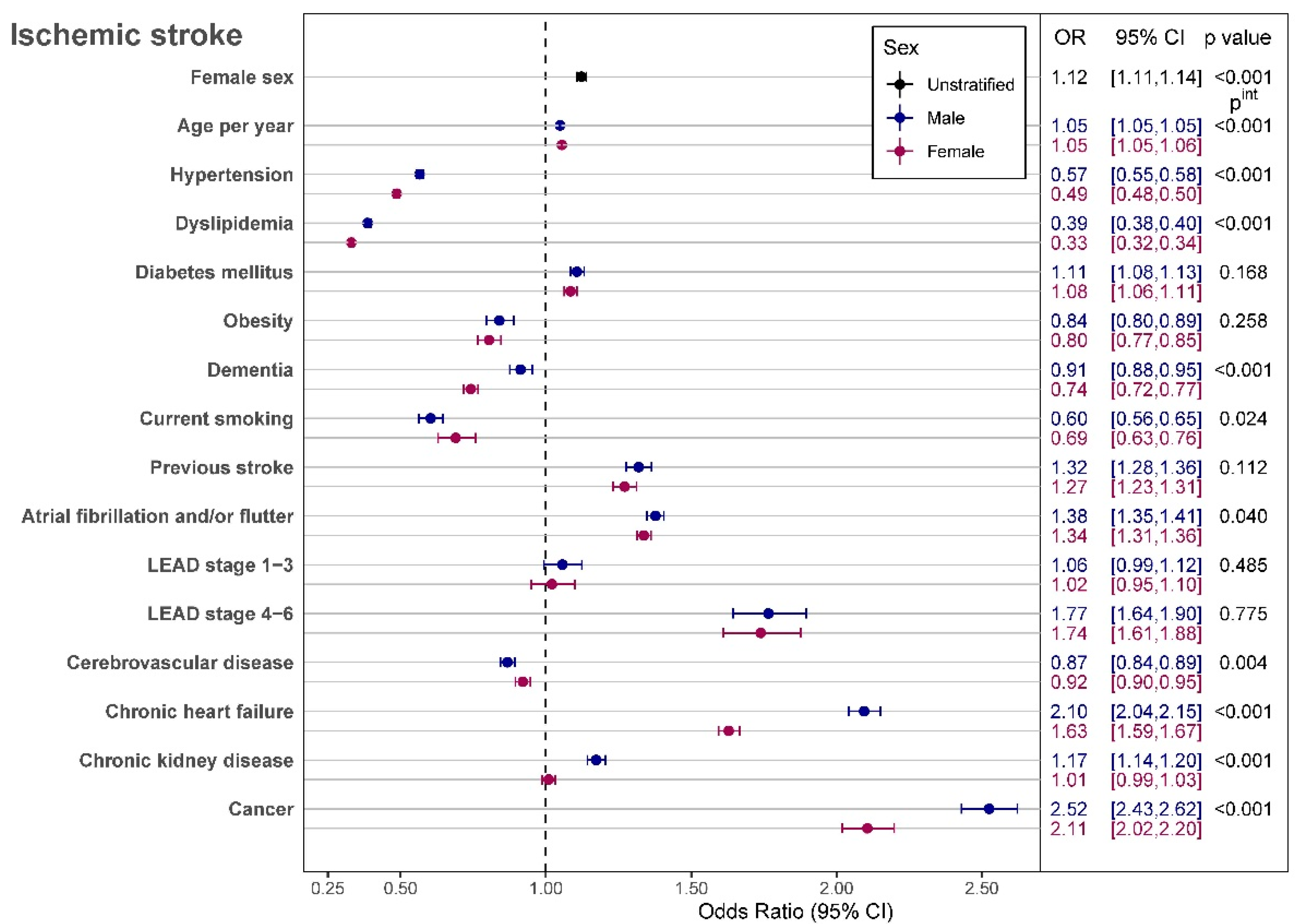
In-Hospital Mortality
4. Discussion
4.1. Impact of In-Hospital Treatment
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKI | Acute kidney injury |
| BMS | Bare-metal stent |
| CABG | Coronary artery bypass grafting |
| CI | Confidence interval |
| DES | Drug-eluting stent |
| DESTATIS | Statistisches Bundesamt (Federal Statistical Office) |
| EA | Endarterectomy |
| GP IIb/IIIa inhibitors | Glycoprotein IIb/IIIa inhibitors |
| ICD | International Statistical Classification of Diseases and Related Health Problems |
| ICH | Intracerebral hemorrhage |
| LEAD | Lower extremity arterial disease |
| LV- CHF | Left ventricular chronic heart failure |
| NYHA | New York Heart Association |
| PCI | Percutaneous coronary intervention |
| OPS | German Procedure Classification |
| OR | Odds ratio |
| RF | Rutherford |
| RV- CHF | Right ventricular chronic heart failure |
| Yr | year |
References
- Persky, R.W.; Turtzo, L.C.; McCullough, L.D. Stroke in Women: Disparities and Outcomes. Curr. Cardiol. Rep. 2010, 12, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Girijala, R.L.; Sohrabji, F.; Bush, R.L. Sex differences in stroke: Review of current knowledge and evidence. Vasc. Med. 2017, 22, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Asdaghi, N.; Romano, J.G.; Wang, K.; Ciliberti-Vargas, M.A.; Koch, S.; Gardener, H.; Dong, C.; Rose, D.Z.; Waddy, S.P.; Robichaux, M.; et al. Sex disparities in Ischemic Stroke Care FL-PR CReSD (Florida-Puerto Rico Collaboration to reduce Stroke disparities). Stroke 2016, 47, 2618–2626. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.M.; Dai, D.; Gubitz, G.J.; Kapral, M.K.; Christian, C.; Phillips, S.J. Gender Differences in Stroke Examined in a 10-Year Cohort of Patients Admitted to a Canadian Teaching Hospital. Stroke 2008, 39, 1090–1095. [Google Scholar] [CrossRef]
- Towfighi, A.; Markovic, D.; Ovbiagele, B. Sex Differences in Revascularization Interventions after Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2013, 22, e347–e353. [Google Scholar] [CrossRef] [PubMed]
- Corbière, S.; Tettenborn, B. Stroke in women: Is it different? Clin. Transl. Neurosci. 2021, 5, 1–8. [Google Scholar] [CrossRef]
- Berglund, A.; Schenck-Gustafsson, K.; von Euler, M. Sex differences in the presentation of stroke. Maturitas 2017, 99, 47–50. [Google Scholar] [CrossRef]
- Spaander, F.H.; Zinkstok, S.M.; Baharoglu, I.M.; Gensicke, H.; Polymeris, A.; Traenka, C.; Hametner, C.; Ringleb, P.; Curtze, S.; Martinez-Majander, N.; et al. Thrombolysis in Ischemic Stroke Patients Collaborators (TrISP). Sex Differences and Functional Outcome After Intravenous Thrombolysis. Stroke 2017, 48, 699–703, Erratum in Stroke 2017, 48, e97. [Google Scholar] [CrossRef]
- Cadilhac, D.A.; Kim, J.; Lannin, N.A.; Kapral, M.K.; Schwamm, L.H.; Dennis, M.S.; Norrving, B.; Meretoja, A. National stroke registries for monitoring and improving the quality of hospital care: A systematic review. Int. J. Stroke 2015, 11, 28–40. [Google Scholar] [CrossRef]
- Kuehnemund, L.; Koeppe, J.; Feld, J.; Wiederhold, A.; Illner, J.; Makowski, L.; Gerß, J.; Reinecke, H.; Freisinger, E. Gender differences in acute myocardial infarction—A nationwide German real-life analysis from 2014 to 2017. Clin. Cardiol. 2021, 44, 890–898. [Google Scholar] [CrossRef]
- Peters, S.A.; Carcel, C.; Millett, E.R.; Woodward, M. Sex differences in the association between major risk factors and the risk of stroke in the UK Biobank cohort study. Neurology 2020, 95, e2715–e2726. [Google Scholar] [CrossRef] [PubMed]
- Emdin, C.A.; Wong, C.; Hsiao, A.J.; Altman, D.G.; Peters, S.; Woodward, M.; Odutayo, A.A. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: Systematic review and meta-analysis of cohort studies. BMJ 2016, 352, h7013. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.; Seyfang, L.; Ferrari, J.; Gattringer, T.; Greisenegger, S.; Willeit, K.; Toell, T.; Krebs, S.; Brainin, M.; Kiechl, S.; et al. Do Women With Atrial Fibrillation Experience More Severe Strokes? Results From the Austrian Stroke Unit Registry. Stroke 2017, 48, 778–780. [Google Scholar] [CrossRef]
- Christensen, H.; Bushnell, C. Stroke in Women. Contin. Lifelong Learn. Neurol. 2020, 26, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, F.; Fassbender, P.; Zander, W.; Ulbrich, L.; Kuhr, K.; Adler, C.; Halbach, M.; Reuter, H. The Hypertension Paradox: Survival Benefit After ST-Elevation Myocardial Infarction in Patients with History of Hypertension. A Prospective Cohort- and Risk-Analysis. Front. Cardiovasc. Med. 2022, 9, 785657. [Google Scholar] [CrossRef]
- Ali, M.; van Os, H.J.; van der Weerd, N.; Schoones, J.W.; Heymans, M.W.; Kruyt, N.D.; Visser, M.C.; Wermer, M.J. Sex Differences in Presentation of Stroke: A Systematic Review and Meta-Analysis. Stroke 2022, 53, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Lisabeth, L.D.; Reeves, M.J.; Baek, J.; Skolarus, L.E.; Brown, D.; Zahuranec, D.B.; Smith, M.A.; Morgenstern, L.B. Factors Influencing Sex Differences in Poststroke Functional Outcome. Stroke 2015, 46, 860–863. [Google Scholar] [CrossRef]
- Gall, S.L.; Donnan, G.; Dewey, H.M.; Macdonell, R.; Sturm, J.; Gilligan, A.; Srikanth, V.; Thrift, A.G. Sex differences in presentation, severity, and management of stroke in a population-based study. Neurology 2010, 74, 975–981. [Google Scholar] [CrossRef]
- Gall, S.; Phan, H.; Madsen, T.E.; Reeves, M.; Rist, P.; Jimenez, M.; Lichtman, J.; Dong, L.; Lisabeth, L.D. Focused Update of Sex Differences in Patient Reported Outcome Measures After Stroke. Stroke 2018, 49, 531–535. [Google Scholar] [CrossRef]
- Strong, B.; Lisabeth, L.D.; Reeves, M. Sex differences in IV thrombolysis treatment for acute ischemic stroke. Neurology 2020, 95, e11–e22. [Google Scholar] [CrossRef]
- Oluwole, S.A.; Wang, K.; Dong, C.; Ciliberti-Vargas, M.A.; Gutierrez, C.M.; Yi, L.; Romano, J.G.; Perez, E.; Tyson, B.A.; Ayodele, M.; et al. Disparities and Trends in Door-to-Needle Time: The FL-PR CReSD Study (Florida-Puerto Rico Collaboration to Reduce Stroke Disparities). Stroke 2017, 48, 2192–2197. [Google Scholar] [CrossRef] [PubMed]
- Colsch, R.; Lindseth, G. Unique Stroke Symptoms in Women: A Review. J. Neurosci. Nurs. 2018, 50, 336–342. [Google Scholar] [CrossRef] [PubMed]
| Male Sex | Female Sex | Total | p Value | |
|---|---|---|---|---|
| Number of cases—n (%) | 816,347 (51.7) | 761,537 (48.3) | 1,577,884 (100.0) | n.a. |
| Median age—Yr (Q1,Q3) | 73 (63,80) | 79 (70,85) | 76 (66,83) | <0.001 |
| Co-Diagnosis | ||||
| LEAD Stages—n (%): LEAD RF 1–3 LEAD RF 4–6 | 20,585 (2.5) 8502 (1.0) | 11,035 (1.5) 5730 (0.8) | 31,620 (2.0) 14,232 (0.9) | <0.001 |
| Arterial hypertension—n (%) | 610,053 (74.7) | 586,667 (77.0) | 1,196,720 (75.8) | <0.001 |
| Atrial fibrillation and/or flutter—n (%) | 208,734 (25.6) | 253,865 (33.3) | 462,599 (29.3) | <0.001 |
| Acute myocardial infarction—n (%) | 10,392 (1.3) | 9493 (1.3) | 19,885 (1.3) | 0.137 |
| Cancer—n (%) | 25,631 (3.1) | 19,353 (2.5) | 44,984 (2.9) | <0.001 |
| Cerebrovascular disease—n (%) | 123,392 (15.1) | 89,418 (11.7) | 212,810 (13.5) | <0.001 |
| Coronary heart disease—n (%) | 153,713 (18.8) | 88,768 (11.7) | 242,481 (15.4) | <0.001 |
| Chronic heart failure—n (%) | 79,261 (9.7) | 93,264 (12.3) | 172,525 (10.9) | <0.001 |
| RV-CHF—n (%) | 15,958 (2.0) | 21,624 (2.8) | 37,582 (2.4) | <0.001 |
| LV-CHF—n (%) NYHA I NYHA II NYHA III NYHA IV | 7486 (0.9) 19,090 (2.3) 19,331 (2.4) 14,012 (1.7) | 7367 (1.0) 22,237 (2.9) 22,219 (2.9) 16,241 (2.1) | 14,853 (0.9) 41,327 (2.2) 41,550 (2.6) 30,253 (1.9) | <0.001 |
| Chronic kidney disease—n (%) | 105,035 (12.9) | 118,463 (15.6) | 223,498 (14.2) | <0.001 |
| Diabetes mellitus—n (%) | 241,587 (29.6) | 200,635 (26.4) | 442,222 (28.0) | <0.001 |
| Dyslipidemia—n (%) | 343,071 (42.0) | 290,487 (38.1) | 633,558 (40.2) | |
| Obesity—n (%) | 38,809 (4.8) | 37,922 (5.0) | 76,731 (4.9) | <0.001 |
| Current smoking—n (%) | 49,429 (6.1) | 21,128 (2.8) | 70,557 (4.5) | <0.001 |
| Prev. Ischemic stroke—n (%) | 61,288 (7.5) | 49,590 (6.5) | 110,878 (7.0) | <0.001 |
| Prev. Intracranial bleeding—n (%) | 4421 (0.5) | 3589 (0.5) | 8010 (0.5) | <0.001 |
| Ischemic heart disease—n (%) | 157,713 (19.3) | 93,694 (12.3) | 251,407 (15.9) | <0.001 |
| Dementia—n (%) | 33,086 (4.1) | 50,080 (6.6) | 83,166 (5.3) | <0.001 |
| Prev. CABG—n (%) | 25,930 (3.2) | 8663 (1.1) | 34,593 (2.2) | <0.001 |
| Prev. Valve replacement—n (%) | 6701 (0.8) | 4400 (0.6) | 11,101 (0.7) | <0.001 |
| Male Sex | Female Sex | Total | p Value | |
|---|---|---|---|---|
| Carotid EA—n (%) | 18,496 (2.3) | 8601 (1.1) | 27,097 (1.7) | <0.001 |
| Carotid stent—n (%) | 11,353 (1.4) | 5126 (0.7) | 16,479 (1.0) | <0.001 |
| Carotid Interposition graft n (%) | 162 (0.0) | 59 (0.0) | 221 (0.0) | <0.001 |
| Decompressive hemicraniectomy—n (%) | 3121 (0.4) | 2563 (0.3) | 5684 (0.4) | <0.001 |
| Evacuation of extracranial hemorrhage—n (%) | 1504 (0.2) | 915 (0.1) | 2419 (0.2) | <0.001 |
| Evacuation of ICH—n (%) | 17 (0.0) | 15 (0.0) | 32 (0.0) | 0.875 |
| Thrombectomy extra/intra cranial | 30,905 (3.8) | 34,919 (4.6) | 65,824 (4.2) | <0.001 |
| PCI—n (%) | 3378 (0.4) | 1707 (0.2) | 5085 (0.3) | <0.001 |
| DES—n (%) | 2419 (0.3) | 1147 (0.2) | 3566 (0.2) | <0.001 |
| BMS, only—n (%) | 211 (0.0) | 108 (0.0) | 319 (0.0) | |
| CABG—n (%) | 88 (0.0) | 25 (0.0) | 113 (0.0) | <0.001 |
| Renal replacement therapy—n (%) | 4937 (0.6) | 3126 (0.4) | 8063 (0.5) | <0.001 |
| Systemic thrombolysis—n (%) | 106,291 (13.0) | 97,864 (12.9) | 204,155 (12.9) | <0.002 |
| Selective thrombolysis—n (%) | 2737 (0.3) | 2688 (0.4) | 5425 (0.3) | 0.058 |
| Intra-arterial thrombolysis—n (%) | 193 (0.0) | 139 (0.0) | 332 (0.0) | 0.020 |
| GpIIb/IIIa inhibitor—n (%) | 1352 (0.2) | 823 (0.1) | 2175 (0.1) | <0.001 |
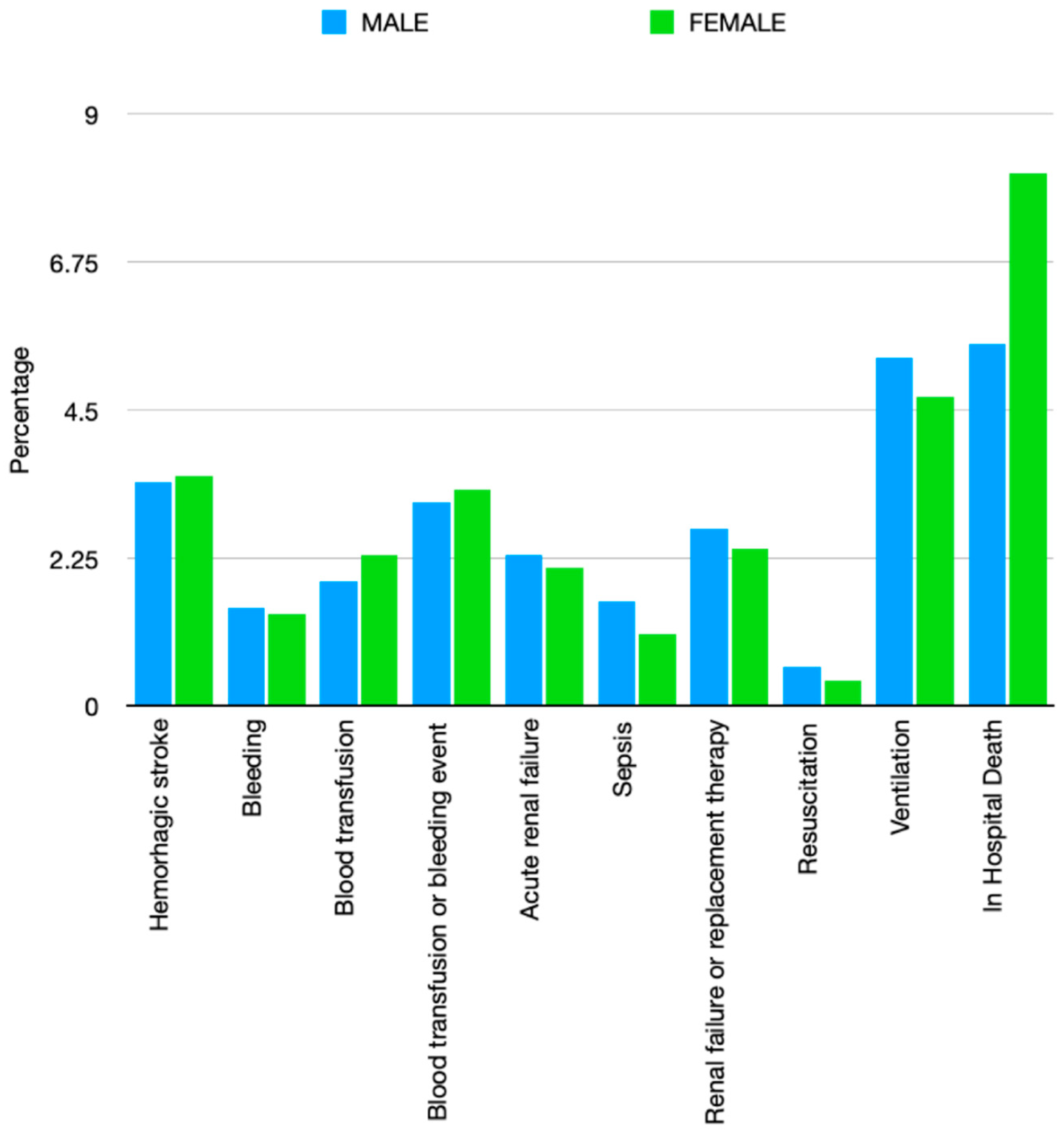
| Hemorrhagic transformation—n (%) | 27,715 (3.4) | 26,977 (3.5) | 54,692 (3.5) | <0.001 |
| Bleeding—n (%) | 12,558 (1.5) | 10,899 (1.4) | 23,457 (1.5) | <0.001 |
| Blood transfusion—n (%) | 15,389 (1.9) | 17,321 (2.3) | 32,710 (2.1) | <0.001 |
| Blood transfusion or bleeding event—n (%) | 25,094 (3.1) | 25,406 (3.3) | 50,500 (3.2) | <0.001 |
| AKI—n (%) | 18,580 (2.3) | 15,661 (2.1) | 34,241 (2.2) | <0.001 |
| Sepsis—n (%) | 12,927 (1.6) | 8251 (1.1) | 21,178 (1.3) | <0.001 |
| AKI or need for dialysis—n (%) | 21,849 (2.7) | 17,931 (2.4) | 39,780 (2.5) | <0.001 |
| Cardiac arrest—n (%) | 4742 (0.6) | 3354 (0.4) | 8096 (0.5) | <0.001 |
| Mechanical ventilation—n (%) | 43,385 (5.3) | 35,996 (4.7) | 79,381 (5.0) | <0.001 |
| Median duration of ventilation—h (IQR) | 115 (308) | 72 (242) | 93 (280) | <0.001 |
| In-Hospital Death—n (%) | 44,518 (5.5) | 61,489 (8.1) | 106,007 (6.7) | <0.001 |
| Mean length of hospital stay—days (SD) | 12.0 (14.3) | 12.1 (12.7) | 12.0 (13.5) | <0.001 |
| Mean charges per case—EUR (SD) | 7145.00 (10,816.93) | 6733.49 (8858.98) | 6946.17 (9921.42) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lappe, C.; Reinecke, H.; Feld, J.; Köppe, J. Sex Differences in Ischemic Cerebral Infarction: A Nationwide German Real-Life Analysis from 2014 to 2019. Clin. Transl. Neurosci. 2022, 6, 23. https://doi.org/10.3390/ctn6030023
Lappe C, Reinecke H, Feld J, Köppe J. Sex Differences in Ischemic Cerebral Infarction: A Nationwide German Real-Life Analysis from 2014 to 2019. Clinical and Translational Neuroscience. 2022; 6(3):23. https://doi.org/10.3390/ctn6030023
Chicago/Turabian StyleLappe, Claudia, Holger Reinecke, Jannik Feld, and Jeanette Köppe. 2022. "Sex Differences in Ischemic Cerebral Infarction: A Nationwide German Real-Life Analysis from 2014 to 2019" Clinical and Translational Neuroscience 6, no. 3: 23. https://doi.org/10.3390/ctn6030023
APA StyleLappe, C., Reinecke, H., Feld, J., & Köppe, J. (2022). Sex Differences in Ischemic Cerebral Infarction: A Nationwide German Real-Life Analysis from 2014 to 2019. Clinical and Translational Neuroscience, 6(3), 23. https://doi.org/10.3390/ctn6030023






