Abstract
In March 2021, cerebral venous sinus thrombosis and thrombocytopenia after vaccination with adenovirus-based vaccine against SARS-CoV-2 were first reported. The underlining condition has been termed vaccine-induced immune thrombocytopenia (VITT). Anti-platelet factor 4 antibodies have been proposed as a central component of the pathomechanism. Treatment recommendations entailed immunomodulation with intravenous immunoglobulins, avoidance of heparins and avoidance of platelet transfusions. Although mortality from VITT-associated cerebral venous sinus thrombosis has decreased over time, it remains high. The aim of this narrative review is to describe different aspects of this disease according to the current state of knowledge.
1. Introduction
Blood clots in the brain’s cerebral venous system cause cerebral venous sinus thrombosis (CVST) (Figure 1). Cerebral venous oedema, infarction and haemorrhage may result after CVST [1]. CVST is a rare disease [2].
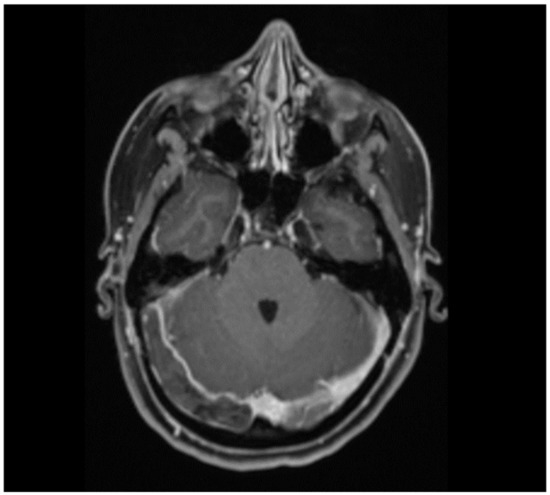
Figure 1.
Thrombosis of the right sinus transverse and sigmoid sinus in a 29-year-old woman who presented with headache, nausea and left-sided hemiparesis. Risk factors for cerebral venous sinus thrombosis were obesity (BMI 30.5 kg/m2) and oral contraception. She was diagnosed with non-VITT CVST.
CVST has been reported in relation with vaccine-induced thrombotic thrombocytopenia (VITT). VITT was first well-described by Greinacher A et al. in March 2021. He published a case series (n = 11) of patients with thrombosis and thrombocytopenia 5–16 days after administration of the adenovirus-based SARS-CoV-2 vaccine ChAdOx1 nCoV-1 (AstraZeneca/Oxford, Oxford, UK) [3]. The authors proposed a pathomechanism similar to autoimmune heparin-induced thrombocytopenia (aHIT), as all VITT patients tested positive for anti-platelet factor (PF) 4/heparin antibodies and for platelet activation assays, although they had no recent exposure to heparin. The condition was named vaccine-induced immune thrombotic thrombocytopenia (VITT). As of March 2021, several reports have been published stating that VITT occurs within a few weeks of administration of the adenovirus-based SARS-CoV-2 vaccines ChAdOx1 nCoV-1 (AstraZeneca/Oxford) and Ad26.COV2.S (Janssen/Johnson & Johnson, New York, NY, USA) [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17].
The aim of this narrative review was to describe different aspects of CVST-VITT according to the current state of knowledge.
2. Methods
Pubmed was searched until March 2022 for original articles, reviews and editorials using the following keywords: ‘vaccine’, ‘cerebral’, ‘thrombosis’, ‘vaccine’ and ‘thrombocytopenia’.
2.1. Pathophysiological Aspects
VITT, in which anti-platelet factor (PF) 4 antibodies are produced after adenovirus-based SARS-CoV-2 vaccination as part of the inflammatory response and immune stimulation, is a condition very similar to HIT, and even more similar to autoimmune HIT (aHIT). Venous thrombosis and/or arterial vessel occlusions and thrombocytopenia are hallmarks of both VITT and aHIT. Heparin-induced thrombocytopenia (HIT) is a systemic hypercoagulable state that occurs when heparin-dependent IgG antibodies bind to PF4/heparin complexes. aHIT is a condition in which anti-PF4 antibodies are produced without recent exposure to heparin [18].
Anti-PF4 antibodies were discovered in VITT, as in aHIT, by an enzyme-linked immunosorbent assay (ELISA) [3,9]. In addition to the similarities in clinical presentation, the thrombus structure in VITT is also similar to aHIT, with platelet-rich ‘white’ venous thrombi as opposed to ‘red’ stasis thrombi seen in classical CVST [4].
It remains to be determined which components of the adenovirus-based SARS-CoV2 vaccines trigger the production of anti-PF4 antibodies [3,19,20]. PF4 is a naturally occurring, positively charged chemokine that binds to negatively charged molecules, such as heparin, bacterial cell walls and polyanionic compounds used in prosthetic surgery [19]. PF4–anionic complexes trigger the production of anti-PF4 antibodies. Greinacher et al. 2021 demonstrated the formation of PF4–vaccine complexes, which stimulated a pro-inflammatory response of B-lymphocytes, a phenomenon that was induced by the addition of DNA [20]. Other mechanisms possibly involved in the pathophysiology of VITT are suspected, such as the downregulation of ACE-receptors, molecular mimicry between the SARS-CoV-2 spike protein and PF4 molecules, RNAemia, and the involvement of vaccine constituents, such as ethylenediaminetetraacetic acid (EDTA) [20].
The occurrence of CVST and other venous thromboses and arterial vessel occlusions after mRNA-based SARS-CoV-2 vaccines have also been described [7,21,22,23,24,25]. However, so far, there is no report on VITT in these patients. The mechanism of venous thrombosis and arterial vessel occlusion after mRNA-based SARS-CoV-2 vaccination is, so far, less understood than after adenovirus-based SARS-CoV-2 vaccination [26]. In the following sections, we focus exclusively on VITT after adenovirus-based SARS-CoV-2 vaccination.
2.2. Epidemiology
The phase 3 trial of ChAdOx1 nCoV-1 (AstraZeneca) randomized 23,848 participants [27], and the trial of Ad26.COV2.S (Janssen) vaccine 39,321 participants [28]. However, these clinical trials, which led to the clinical approval of the adenovirus-based SARS-CoV-2 vaccines, were underpowered to detect rare adverse events such as VITT.
So far, more than 11 billion SARS-CoV-2 vaccinations have been administered worldwide [29]. The largest vaccination campaign in history is still ongoing.
An analysis of patients vaccinated in Europe using the Eudravigilance database from 1 January to 30 July 2021 showed that 38,664,988 people had been vaccinated with ChAdOx1 nCoV-1 (AstraZeneca/Oxford) and 10,972,234 with Ad26.COV2.S (Janssen/Johnson & Johnson) [30]. In this analysis, the rate of CVST with thrombocytopenia during the study period of 6 months was 21.6 (95%CI 20.16-23.11) per 1 million vaccination days for ChAdOx1 nCoV-1 and 11.48 (95%CI 9.57–13.67) per 1 million vaccination days for Ad26.COV2.S vaccination [30]. Another study by the UK Medicines and Healthcare Products Regulatory Agency and the US Centers of Disease Control and Prevention came to very similar conclusions [31].
An analysis of hospitalization or death associated with thrombocytopenia, venous thromboses and arterial vessel occlusions within 28 days after vaccination with ChAdOx1 nCoV-1 (AstraZeneca/Oxford) using a case-control design that analysed the data of 30 million vaccinated people in England between 1 December 2020 and 24 April 2021 found a higher risk of thrombocytopenia and venous thromboses after COVID-19 infection than after vaccination with ChAdOx1 nCoV-1 (AstraZeneca/Oxford) (after 1–7 days OR 4.91, 95%CI 3.5–6.89; after 8–14 days 1.91, 95%CI 1.2–3.06) [21].
Overall, VITT and CVST-VITT are rare after SARS-CoV-2 vaccination; CVST after COVID-19 is more common, and ischaemic strokes or disabling or deadly events show even higher incidence rates [32].
CVST-VITT was observed mainly after the application of the first dose of adenovirus-based SARS-CoV-2 vaccines, and in young-to-middle-aged, predominantly white and female individuals [3,8,9,10,11,12,13,14,21]. Based on the observed age distribution, many national medical agencies in various countries around the world currently recommend avoiding the administration of adenovirus-based SARS-CoV-2 vaccines in young-to-middle-aged people [32,33].
Several conventional CVST risk factors are known, but they seem to be less present in patients with CVST-VITT. Conventional CVST risk factors may be temporary (prothrombogenic drugs, pregnancy and puerperium, infections) or permanent (common prothrombotic disorders such as thrombophilia, antiphospholipid syndrome, myeloproliferative disorders and malignancies) [8,10,11,17,34].
The occurrence of thrombosis with thrombocytopenia caused by idiopathic thrombocytopenic purpura (ITP) has been described after the administration of the measles–mumps–rubella vaccine [35]. Given the rarity (1 case for every 40,000 doses) and the lower total number of doses administered compared with COVID-19 vaccines, the evidence on ITP following measles–mumps–rubella vaccination in the context of post SARS-CoV-2 VITT is of limited relevance.
2.3. Definition and Laboratory Features of VITT
Different societies and research groups have proposed criteria for VITT (Table 1) [8,36,37,38,39,40,41]. Some criteria require only the detection of thrombosis and thrombocytopenia, while others require additional laboratory parameters, such as D-dimer and fibrinogen levels and positive anti-PF4 antibodies, for the diagnosis of VITT. In addition, the time window after vaccination varies considerably depending on the definition (Table 1) [8,36,37,38,39,40,41].

Table 1.
Case-definition criteria according to different specialized societies.
The symptoms at the time of onset of VITT have been described as varying [42,43]. For example, headache has been suggested as an early symptom of CVST-VITT without radiologic evidence of early stage CVST [43,44,45]. Furthermore, anti-PF4 antibodies are potentially negative at the beginning of the clinical course and only become positive later [42]. In some patients, D-dimer and fibrinogen levels were described to be within the normal range throughout the clinical course [46].
In summary, some patients diagnosed with VITT may not fully meet the proposed VITT definition criteria.
Thrombocytopenia and positive anti-PF4 antibodies have been repeatedly described in CVST-VITT. A descriptive analysis of baseline thrombocytopenia in patients with CVST from the pre-COVID-19 era, conducted by the International Cerebral Venous Thrombosis Consortium (ICVST), found baseline thrombocytopenia—vastly non-severe—in 8% of patients, with obvious explanations for the thrombocytopenia in the majority of cases, such as cancer, inflammation, alcohol dependence or intake of drugs. HIT with anti-PF4/heparin antibodies was diagnosed in one single patient. In the pre-COVID-19 era, baseline thrombocytopenia was uncommon in patients with CVST, and HIT and anti-PF4/heparin antibodies were rare. The results suggest a causal association between adenovirus-based SARS-CoV-2 vaccination and CVST with thrombocytopenia [47].
Around 7% of healthy individuals develop antibodies to PF4/polyanion complexes at lower titres than in VITT. This urges caution when screening healthy individuals for VITT, as in a significant proportion of individuals, antibodies against PF4/polyanion complexes have no clinical significance and do not require treatment [48].
Other laboratory features that have been described as typical for VITT include increased D-dimer levels, a shortened pro-thrombin time and an activated partial thromboplastin time (aPTT) (Table 1).
2.4. Location of VITT
Thromboses in VITT have been shown to occur in the cerebral venous system, but also in other, sometimes unusual, locations. According to an analysis of 220 patients with definite or probable VITT, about half of all patients suffered from CVST, one third from pulmonary and deep venous thrombosis, one fifth from splanchnic venous thrombosis, one fifth from arterial vessel occlusion, and 29% had more than two affected vascular beds [8]. Moreover, according to a meta-analysis, about half of the patients with VITT suffered from CVST [19].
A role of PF4 in the interaction of the drainage of the splanchnic and cerebral venous systems with bacteria and viruses from the intestinal and nasal tissues has been proposed as an explanation for the unusual location of thrombosis in VITT. Endothelial activation facilitates the activation of the innate immune system through an interaction between the pathogens and PF4. A higher titre of tissue factor and PF4 in these environments may lead to a higher titre of immunogenic complexes at these sites with stronger local immune responses and consecutive thrombosis [49].
2.5. Clinical Features of VITT
One notable clinical aspect of CVST-VITT is the occurrence of headache. Headache occurs in 40–60% of cases after vaccination with an adenovirus-based SARS-CoV-2 vaccine [27,28,50,51], but it can also be a symptom of CVST [44,45,52]. However, the timing of the onset of headache could be different for CVST-VITT (after 5 days) than for vaccination (within 48 h) [27,28,44,45,50,51,52,53].
Other symptoms depend on the extent and location of the thromboses and arterial vessel occlusions. It is noteworthy that a considerable number of CVST-VITT patients have severe disease with coma and intracerebral haemorrhage [9,10,35].
2.6. Treatment
The first reports on VITT also included treatment recommendations largely based on the similarities with aHIT and the in vitro inhibition of platelet activation by the VITT antibodies after the addition of intravenous immunoglobulin (IVIG) [3,8,9]. Treatment recommendations consist of IVIG and immunosuppression, administration of non-heparin anticoagulants (anti-thrombin and anti-Xa) and strict avoidance of heparin and platelet transfusions.
There is limited evidence on the efficacy and safety of these recommendations [35,54]. Their scientific validity is also changing due to new insights into the pathophysiology of this disease.
The effectiveness of IVIG is limited to case reports only [55]. Platelet transfusions are not recommended, as they can trigger immune reactions. However, no study has yet investigated how platelet transfusions affect outcome.
Other treatment options include steroids, fibrinogen transfusions, plasma exchange, rituximab and eculizimab [55,56].
2.7. Prognosis
CVST-VITT has been shown to be associated with high mortality, higher than in CVST patients from the pre-pandemic era [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17]. However, a retrospective study by the ICVST consortium using EMA (European Medicine Agency) data (n = 270 CVST with thrombocytopenia) showed a decrease in mortality in patients diagnosed and treated after 28 March 2021 compared to those treated before this date (22% vs. 47%, p < 0.001) [12]. This probably reflects the recognition of less severe cases and the implementation and effectiveness of current treatment recommendations over time. Another study, conducted as part of the ICVST consortium and analysing data from a prospective registry (n = 116), found a lower mortality in patients treated after vs. before March 2021 (42% vs. 61%) [11]. Although mortality has decreased in both analyses, it is still considerable. In comparison, mortality in the pre-COVID-19 era was 3.9% [11].
3. Conclusions
CVST-VITT is rare and differs from CVST in the pre-pandemic era. The benefits of SARS-CoV-2 vaccination far outweigh the small risk of VITT. The risk of CVST after COVID-19 is higher than the risk of CVST-VITT.
CVST-VITT has a heterogeneous and severe clinical picture, and although mortality has decreased over time, it remains considerable.
Due to its heterogeneous presentation, the diagnosis of VITT is challenging. The currently proposed case definitions for VITT are heterogeneous, and the diagnosis of VITT should be made on a case-by-case basis.
The type of SARS-CoV-2 vaccine could be adjusted according to patient characteristics, such as age. For example, adenovirus-based SARS-CoV-2 vaccines may be safer in middle-aged and elderly people than in younger people.
Currently, there is limited evidence to support treatment recommendations for VITT, and data on the long-term prognosis, including mortality, disability and risk of recurrence, and long-term secondary prevention are lacking. Further research is needed.
Author Contributions
Conceptualization and methodology, A.S. and M.R.H.; resources, A.S., J.B., J.A.K.H., M.A. and M.R.H.; data curation, A.S.; writing—original draft preparation, A.S. and M.R.H.; writing—review and editing, A.S., J.B., J.A.K.H., M.A. and M.R.H.; visualization, A.S.; supervision, M.R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coutinho, J.M.; Zuurbier, S.M.; Aramideh, M.; Stam, J. The incidence of cerebral venous thrombosis: A cross-sectional study. Stroke 2012, 43, 3375–3377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Wiedmann, M.; Skattør, T.; Stray-Pedersen, A.; Antal, E.A.; Marthinsen, P.B.; Sorvoll, I.H.; Leiknes Ernstsen, S.; Lund, C.G.; Holme, P.A.; Johansen, T.O.; et al. Vaccine Induced Immune Thrombotic Thrombocytopenia Causing a Severe Form of Cerebral Venous Thrombosis With High Fatality Rate: A Case Series. Front. Neurol. 2021, 12, 721146. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.E.; Luz, B.; Niehaus, L.; Bhogal, P.; Bäzner, H.; Henkes, H. Thrombocytopenia and Intracranial Venous Sinus Thrombosis after “COVID-19 Vaccine AstraZeneca” Exposure. J. Clin. Med. 2021, 10, 1599. [Google Scholar] [CrossRef] [PubMed]
- Tiede, A.; Sachs, U.J.; Czwalinna, A.; Werwitzke, S.; Bikker, R.; Krauss, K.J.; Donnerstag, F.; Weissenborn, K.; Höglinger, G.; Maasoumy, B.; et al. Prothrombotic immune thrombocytopenia after COVID-19 vaccination. Blood 2021, 138, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Andraska, E.A.; Kulkarni, R.; Chaudhary, M.; Sachdev, U. Three cases of acute venous thromboembolism in females following vaccination for COVID-19. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 10, 14–17. [Google Scholar] [CrossRef]
- Pavord, S.; Scully, M.; Hunt, B.J.; Lester, W.; Bagot, C.; Craven, B.; Rampotas, A.; Ambler, G.; Makris, M. Clinical Features of Vaccine-Induced Immune Thrombocytopenia and Thrombosis. N. Engl. J. Med. 2021, 385, 1680–1689. [Google Scholar] [CrossRef]
- Scully, M.; Singh, D.; Lown, R.; Poles, A.; Solomon, T.; Levi, M.; Goldblatt, D.; Kotoucek, P.; Thomas, W.; Lester, W.; et al. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2202–2211. [Google Scholar] [CrossRef]
- Perry, R.J.; Tamborska, A.; Singh, B.; Craven, B.; Marigold, R.; Arthur-Farraj, P.; Yeo, J.M.; Zhang, L.; Hassan-Smith, G.; Jones, M.; et al. CVT After Immunisation Against COVID-19 (CAIAC) collaborators. Cerebral venous thrombosis after vaccination against COVID-19 in the UK: A multicentre cohort study. Lancet 2021, 398, 1147–1156. [Google Scholar] [CrossRef]
- Sánchez van Kammen, M.; Aguiar de Sousa, D.; Poli, S.; Cordonnier, C.; Heldner, M.R.; van de Munckhof, A.; Krzwycka, K.; van Haaps, T.; Ciccone, A.; Middeldorp, S.; et al. Characteristics and Outcomes of Patients With Cerebral Venous Sinus Thrombosis in SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. JAMA Neurol. 2021, 78, e213619. [Google Scholar] [CrossRef] [PubMed]
- Van de Munckhof, A.; Krzywicka, K.; Aguiar de Sousa, D.; Sanchez van Kammen, M.; Heldner, M.R.; Jood, K.; Lindgren, E.; Tatslisumak, T.; Putaala, J.; Kremer Hovinga, J.A.; et al. Declining mortality of cerebral venous sinus thrombosis with thrombocytopenia after SARS-CoV-2 vaccination. Eur. J. Neurol. 2021, 29, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Krzywicka, K.; Heldner, M.R.; Sánchez van Kammen, M.; van Haaps, T.; Hiltunen, S.; Silvis, S.M.; Levi, M.; Kremer Hovinga, J.A.; Jood, K.; Lindgren, E.; et al. Post-SARS-CoV-2-vaccination cerebral venous sinus thrombosis: An analysis of cases notified to the European Medicines Agency. Eur. J. Neurol. 2021, 28, 3656–3662. [Google Scholar] [CrossRef]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.H.; Skattor, T.H.; Tjonnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.R.; Mangion, S.A.; Benger, M.; Stanton, B.R.; Czuprynska, J.; Arya, R.; Sztriha, L.K. Cerebral venous sinus thrombosis and thrombocytopenia after COVID-19 vaccination—A report of two UK cases. Brain Behav. Immun. 2021, 95, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, A.; Zanotti, B. Working group on cerebral venous thrombosis after COVID-19 vaccination. The importance of recognizing cerebral venous thrombosis following anti-COVID-19 vaccination. Eur. J. Intern. Med. 2021, 89, 115–117. [Google Scholar] [CrossRef] [PubMed]
- See, I.; Su, J.R.; Lale, A.; Woo, E.J.; Guh, A.Y.; Shimabukuro, T.T.; Streiff, M.B.; Rao, A.K.; Wheeler, A.P.; Beavers, S.F.; et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA 2021, 325, e217517. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A. Heparin-induced thrombocytopenia. N. Engl. J. Med. 2015, 373, 252–261. [Google Scholar] [CrossRef]
- McGonagle, D.; De Marco, G.; Bridgewood, C. Mechanisms of Immunothrombosis in Vaccine-Induced Thrombotic Thrombocytopenia (VITT) Compared to Natural SARS-CoV-2 Infection. J. Autoimmun. 2021, 121, 102662. [Google Scholar] [CrossRef]
- Greinacher, A.; Selleng, K.; Palankar, R.; Wesche, J.; Handtdke, S.; Wolff, M.; Aurich, K.; Lalk, M.; Methling, K.; Völker, U.; et al. Insights in ChAdOx1 nCov-19 Vaccine-induced Immune Thrombotic Thrombocytopenia (VITT). Blood 2021, 138, 2256–2268. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Patone, M.; Mei, X.W.; Saatci, D.; Dixon, S.; Khunti, K.; Zaccardi, F.; Watkinson, P.; Shankar-Hari, M.; Doidge, J.; et al. Risk of thrombocytopenia and thromboembolism after COVID-19 vaccination and SARS-CoV-2 positive testing: Self-controlled case series study. BMJ 2021, 374, n1931. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.; Sapiai, N.A.; Ghani, A.R.I. Cerebral venous sinus thrombosis 2 weeks after the first dose of mRNA SARS-CoV-2 vaccine. Acta Neurochir. 2021, 163, 2359–2362. [Google Scholar] [CrossRef] [PubMed]
- Syed, K.; Chaudhary, H.; Donato, A. Central Venous Sinus Thrombosis with Subarachnoid Hemorrhage Following an mRNA COVID-19 Vaccination: Are These Reports Merely Co-Incidental? Am. J. Case Rep. 2021, 22, e933397. [Google Scholar] [CrossRef]
- Dias, L.; Soares-Dos-Reis, R.; Meira, J.; Ferrao, D.; Soares, P.R.; Pastor, A.; Gama, G.; Fonseca, L.; Fagundes, V.; Carvalho, M. Cerebral Venous Thrombosis after BNT162b2 mRNA SARS-CoV-2 vaccine. J. Stroke Cereb. Dis. 2021, 30, 105906. [Google Scholar] [CrossRef]
- Fan, B.E.; Shen, J.Y.; Lim, X.R.; Tu, T.M.; Chang, C.C.R.; Khin, H.S.W.; Koh, J.S.; Rao, J.P.; Lau, S.L.; Tan, G.B.; et al. Cerebral venous thrombosis post BNT162b2 mRNA SARS-CoV-2 vaccination: A black swan event. Am. J. Hematol. 2021, 96, E357–E361. [Google Scholar] [CrossRef] [PubMed]
- Talotta, R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases”. Clin. Immunol. 2021, 224, 108665. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cardenas, V.; Shukarev, G.; Grinsztejn, B.; Gpeüfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. ENSEMBLE Study Group. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Bloomberg. Vaccine Tracker. Available online: www.bloomberg.com/graphics/covid-vaccine-tracker-global-distribution/#global (accessed on 2 February 2022).
- Abbattista, M.; Martinelli, I.; Peyvandi, F. Comparison of adverse drug reactions among four COVID-19 vaccines in Europe using the EudraVigilance database: Thrombosis at unusual sites. J. Thromb. Haemost. 2021, 19, 2554–2558. [Google Scholar] [CrossRef]
- Bikdeli, B.; Chatterjee, S.; Arora, S.; Monreal, M.; Jimenez, D.; Krumholz, H.M.; Goldhaber, S.Z.; Elkind, M.S.V.; Piazza, G. Cerebral Venous Sinus Thrombosis in the U.S. Population, After Adenovirus-Based SARS-CoV-2 Vaccination, and After COVID-19. J. Am. Coll. Cardiol. 2021, 78, 408–411. [Google Scholar] [CrossRef]
- Euronews. Finland Suspends AstraZeneca Vaccine despite European Regulator Saying It Is Safe and Effective. Available online: https://www.euronews.com/2021/03/18/norway-sweden-denmark-wait-before-restarting-astrazeneca-vaccinations (accessed on 2 February 2022).
- BfArM-Startseite. Available online: https://www.bfarm.de/DE/Home/_node.html (accessed on 2 February 2022).
- Ferro, J.M.; Aguiar de Sousa, A.; Coutinho, J.M.; Martinelli, I. European stroke organization interim expert opinion on cerebral venous thrombosis occuring after SARS-CoV-2 vaccination. Eur. J. Stroke 2021, 6, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Brighton Collaboration. Updated Proposed Brighton Collaboration Process for Developing a Standard Case Definition for Study of New Clinical Syndrome X, as Applied to Thrombosis with Thrombocytopenia Syndrome (TTS). Available online: https://brightoncollaboration.us/wp-content/uploads/2021/05/TTS-Interim-Case-Definition-v10.16.3-May-23-2021.pdf (accessed on 2 February 2022).
- British Society for Haematology. Available online: https://www.hantshealthcarelibrary.nhs.uk/assets/files/guidelines%20updated%20November%202021%201.pdf (accessed on 2 February 2022).
- Oldenburg, J.; Klamroth, R.; Langer, F.; Albisetti, M.; von Auer, C.; Ay, C.; Korte, W.; Scharf, R.E.; Pötsch, B.; Greinacher, A. Diagnosis and Management of Vaccine-Related Thrombosis following AstraZeneca COVID-19 Vaccination: Guidance Statement from the GTH. Hamostaseologie 2021, 41, 184–189. [Google Scholar] [CrossRef] [PubMed]
- International Society on Thrombosis and Haemostasis. Vaccine-Induced Immune Thrombotic Thrombocytopenia(VITT) Diagnostic Flow Chart (Updated 20 April, 2021). Available online: https://cdn.ymaws.com/www.isth.org/resource/resmgr/news/ISTH_VITT_Flow_Chart_Final.pdf (accessed on 2 February 2022).
- International Society on Thrombosis and Haemostasis. The ISTH Releases Interim Guidance on Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT). Available online: https://www.isth.org/news/561406/The-ISTH-Releases-Interim-Guidance-on-Vaccine-Induced-Immune-Thrombotic-Thrombocytopenia-VITT-.htm (accessed on 2 February 2022).
- Cerebral Venous Thrombosis (thrombosiscanada.ca). Available online: https://thrombosiscanada.ca/wp-uploads/uploads/2021/01/43.-Cerebral-Vein-Thrombosis_17Jan2020.pdf (accessed on 2 February 2022).
- Thiele, T.; Weisser, K.; Schönborn, L.; Funk, M.B.; Weber, G.; Greinacher, A.; Keller-Stanislawski, B. Laboratory confirmed vaccine-induced immune thrombotic thrombocytopenia: Retrospective analysis of reported cases after vaccination with ChAdOx-1 nCoV-19 in Germany. Lancet Reg. Health Eur. 2022, 12, 100270. [Google Scholar] [CrossRef] [PubMed]
- Lavin, M.; Elder, P.T.; O’Keeffe, D.; Enright, E.; Ryan, E.; Kelly, A.; Benson, G.; Le, G.N.; Byrne, M.; Ryan, K.; et al. Vaccine-induced immune thrombotic thrombocytopenia (VITT)—A novel clinico-pathological entity with heterogeneous clinical presentations. Br. J. Haematol. 2021, 195, 76–84. [Google Scholar] [CrossRef]
- Salih, F.; Schönborn, L.; Kohler, S.; Franke, C.; Pille, C.; Graw, J.; Alonso, A.; Pelz, J.; Bayas, A.; Kuramatsu, J.; et al. Vaccine-Induced Thrombocytopenia with Severe Headache. N. Engl. J. Med. 2021, 385, 2103–2105. [Google Scholar] [CrossRef]
- García-Azorín, D.; Do, T.P.; Gantenbein, A.R.; Hansen, J.M.; Souza, M.N.P.; Obermann, M.; Pohl, H.; Schankin, C.J.; Schytz, H.W.; Sinclair, A.; et al. Delayed headache after COVID-19 vaccination: A red flag for vaccine induced cerebral venous thrombosis. J. Headache Pain. 2021, 22, 108. [Google Scholar] [CrossRef]
- De Michele, M.; Iacobucci, M.; Chistolini, A.; Nicolini, E.; Pulcinelli, F.; Cerbelli, B.; Merenda, E.; Schiavo, O.G.; Sbardella, E.; Berto, I.; et al. Malignant cerebral infarction after ChAdOx1 nCov-19 vaccination: A catastrophic variant of vaccine-induced immune thrombotic thrombocytopenia. Nat. Commun. 2021, 12, 4663. [Google Scholar] [CrossRef]
- Sánchez van Kammen, M.; Heldner, M.R.; Brodard, J.; Scutelnic, A.; Silvis, S.; Schroeder, V.; Kremer Hovinga, J.A.; Middeldorp, S.; Levi, M.; Hiltunen, S.; et al. Frequency of Thrombocytopenia and Platelet Factor 4/Heparin Antibodies in Patients With Cerebral Venous Sinus Thrombosis Prior to the COVID-19 Pandemic. JAMA 2021, 326, 332–338. [Google Scholar] [CrossRef]
- Thiele, T.; Ulm, L.; Holtfreter, S.; Schönborn, L.; Kuhn, S.O.; Scheer, C.; Warketin, T.E.; Bröker, B.M.; Becker, K.; Aurich, K.; et al. Frequency of positive anti-PF4/polyanion antibody tests after COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162b2. Blood 2021, 138, 299–303. [Google Scholar] [CrossRef]
- Palaiodimou, L.; Stefanou, M.I.; Katsanos, A.H.; Aguiar de Sousa, D.; Coutinho, J.M.; Lagiou, P.; Michopoulos, I.; Naska, A.; Giannopoulos, S.; Vadikolias, K.; et al. Cerebral venous sinus thrombosis and thrombotic events after vector-based COVID-19 vaccines: A systematic review and meta-analysis. Neurology 2021, 97, e2136–e2147. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, B.; et al. COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Göbel, C.H.; Heinze, A.; Karstedt, S.; Morscheck, M.; Tashiro, L.; Cirkel, A.; Hamid, Q.; Halwani, R.; Temsah, M.H.; Ziemann, M.; et al. Headache Attributed to Vaccination Against COVID-19 (Coronavirus SARS-CoV-2) with the ChAdOx1 nCoV-19 (AZD1222) Vaccine: A Multicenter Observational Cohort Study. Pain Ther. 2021, 27, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Ikenberg, B.; Demleitner, A.F.; Thiele, T.; Wiestler, B.; Götze, K.; Mössmer, G.; Lingor, P. Cerebral venous sinus thrombosis after ChAdOx1 nCov-19 vaccination with a misleading first cerebral MRI scan. Stroke Vasc. Neurol. 2021, 6, 668–670. [Google Scholar] [CrossRef]
- Uzun, G.; Althaus, K.; Singh, A.; Möller, P.; Ziemann, U.; Mengel, A.; Rosenberger, P.; Guthoff, M.; Petzold, G.C.; Müller, J.; et al. The use of IV immunoglobulin in the treatment of vaccine-induced immune thrombotic thrombocytopenia. Blood 2021, 138, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Schell, A.M.; Petras, M.; Szczepiorkowski, Z.M.; Ornstein, D.L. Refractory heparin induced thrombocytopenia with thrombosis (HITT) treated with therapeutic plasma exchange and rituximab as adjuvant therapy. Transfus. Apher. Sci. 2013, 49, 185–188. [Google Scholar] [CrossRef]
- Mastellos, D.C.; Skendros, P.; Lambris, J.D. Is complement the culprit behind COVID-19 vaccine-related adverse reactions? J. Clin. Invest. 2021, 131, 151092. [Google Scholar] [CrossRef]
- Mantadakis, E.; Farmaki, E.; Buchanan, G.R. Thrombocytopenic purpura after measles-mumps-rubella vaccination: A systematic review of the literature and guidance for management. J. Pediatr. 2010, 156, 623–628. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).