Prediction of Tissue Damage Using a User-Independent Machine Learning Algorithm vs. Tmax Threshold Maps
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Patients and Inclusion Criteria
2.3. Imaging Protocol
2.4. Data Processing
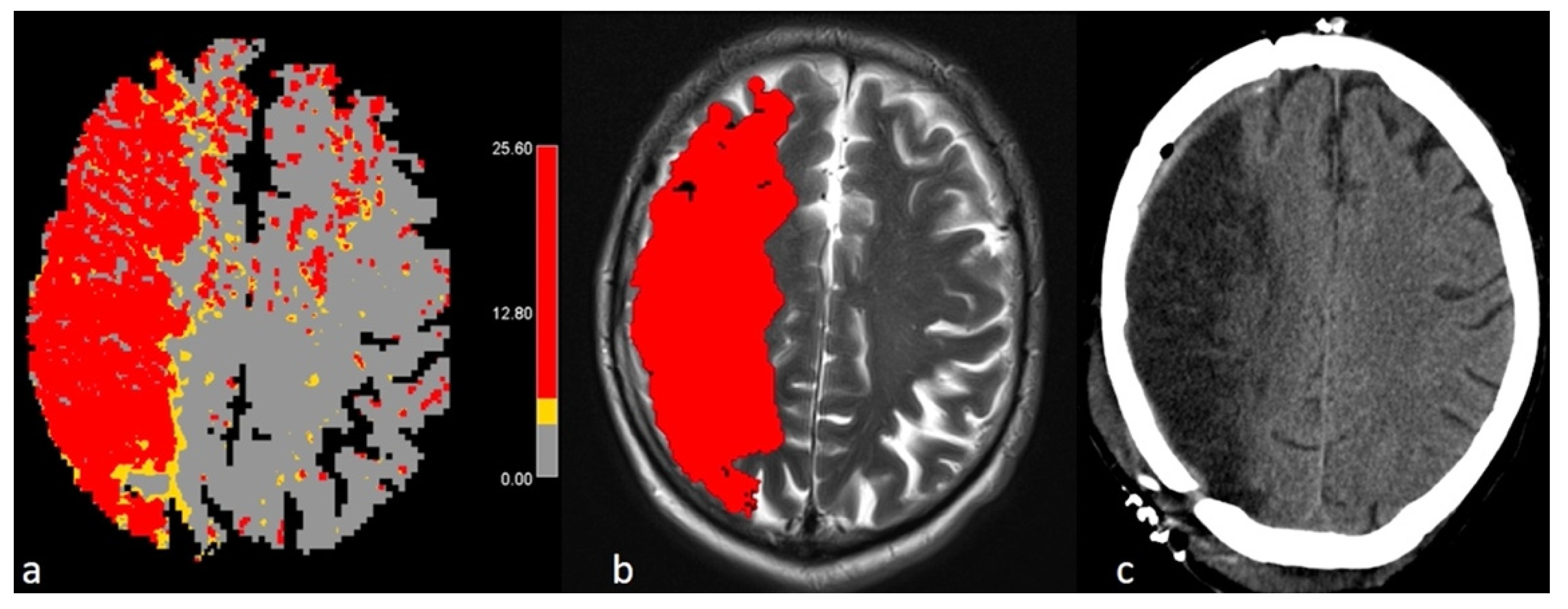
2.5. Manual Segmentation of Hypoperfusion
2.6. Manual Segmentation of Final Infarct Volume
2.7. Postprocessing with FASTER
2.8. Data Comparison
- Infarct core volume: The predicted infarct volume at baseline using FASTER was compared to the final infarct volume on follow-up imaging (MRI or CT) calculated with the slicer.
- Penumbral volume: The estimated penumbral volume by FASTER was compared to the manually delineated volume by Olea using the linear threshold of Tmax (>6 s).
2.9. Statistics
3. Results
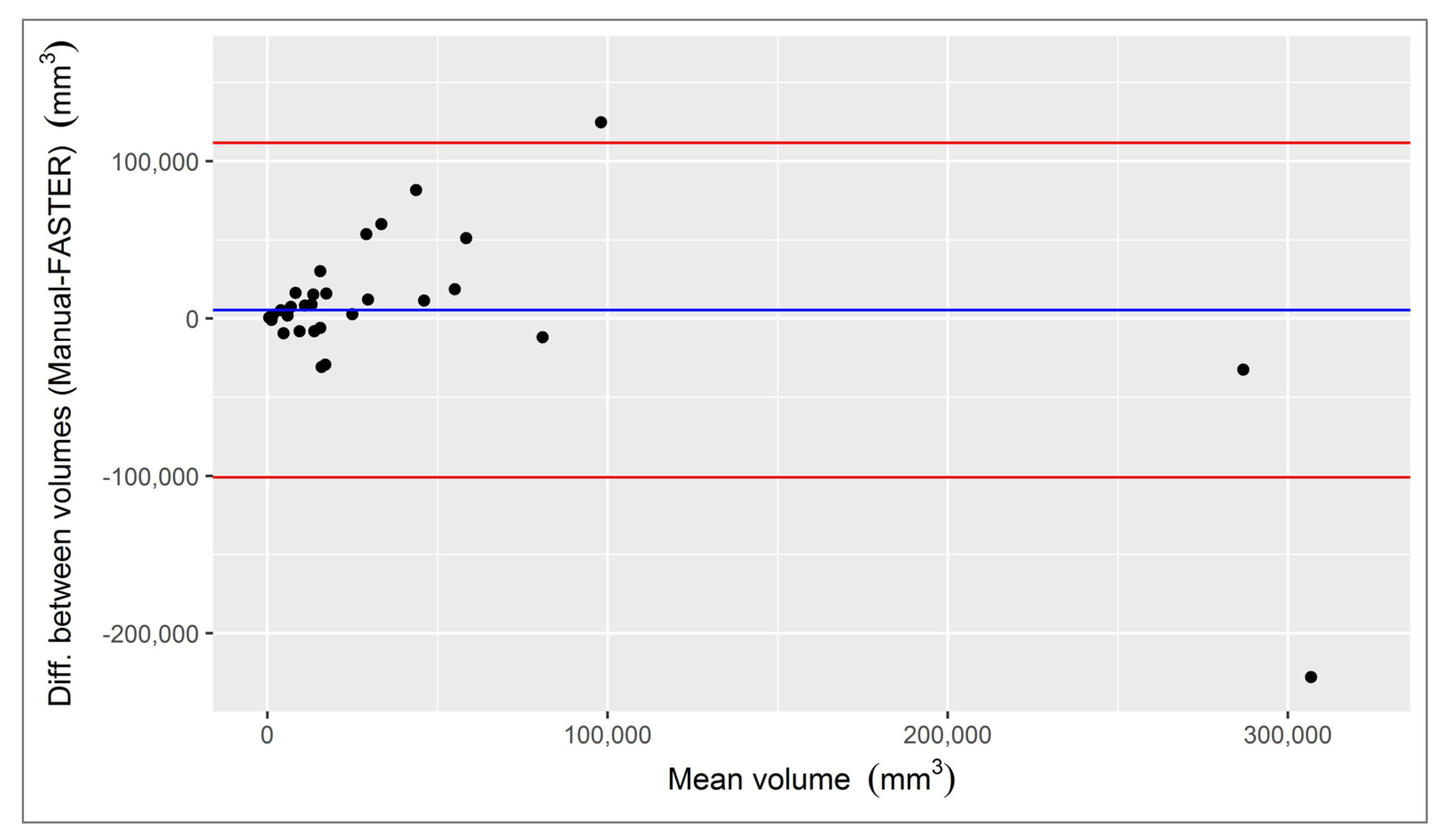
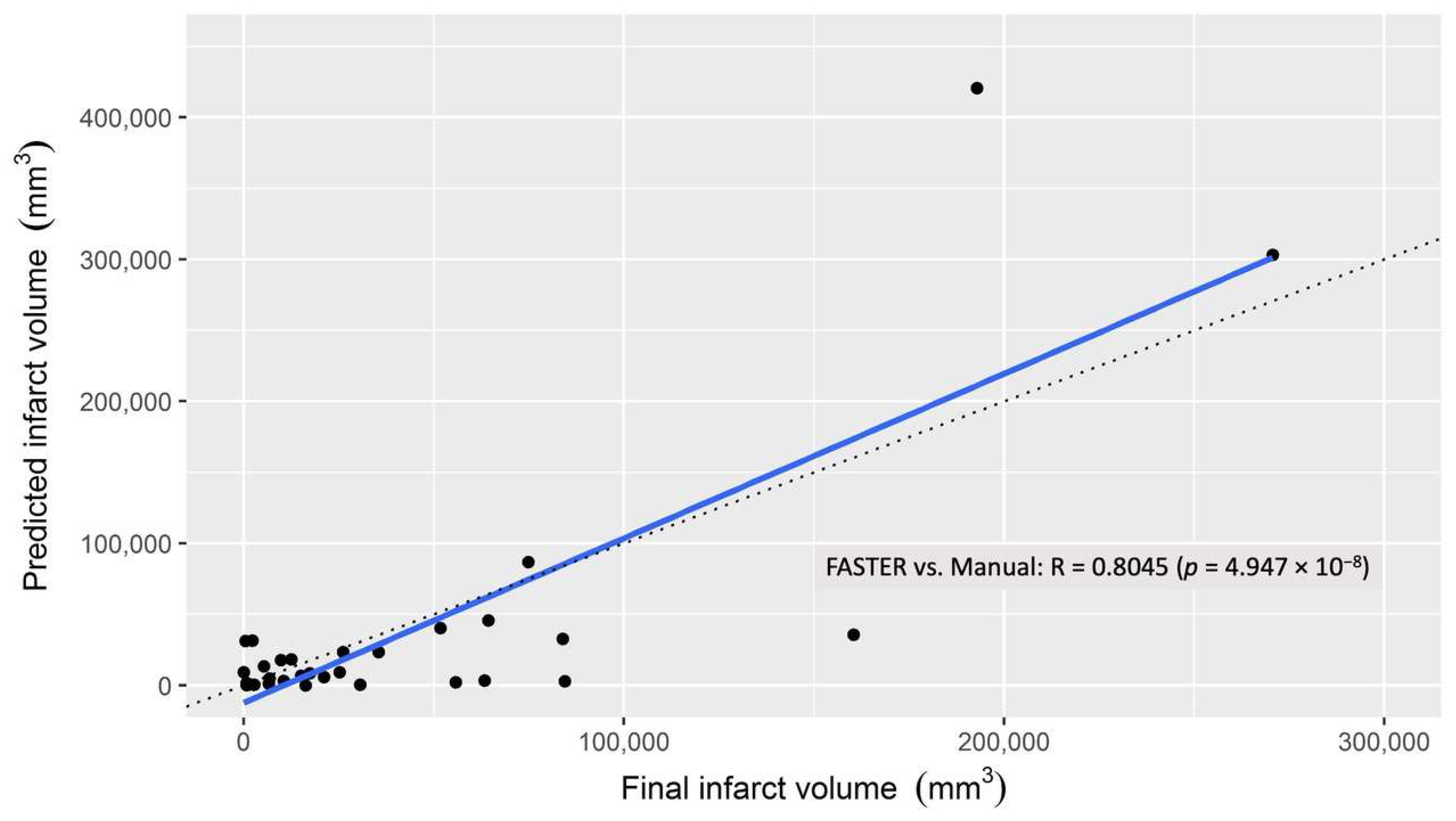
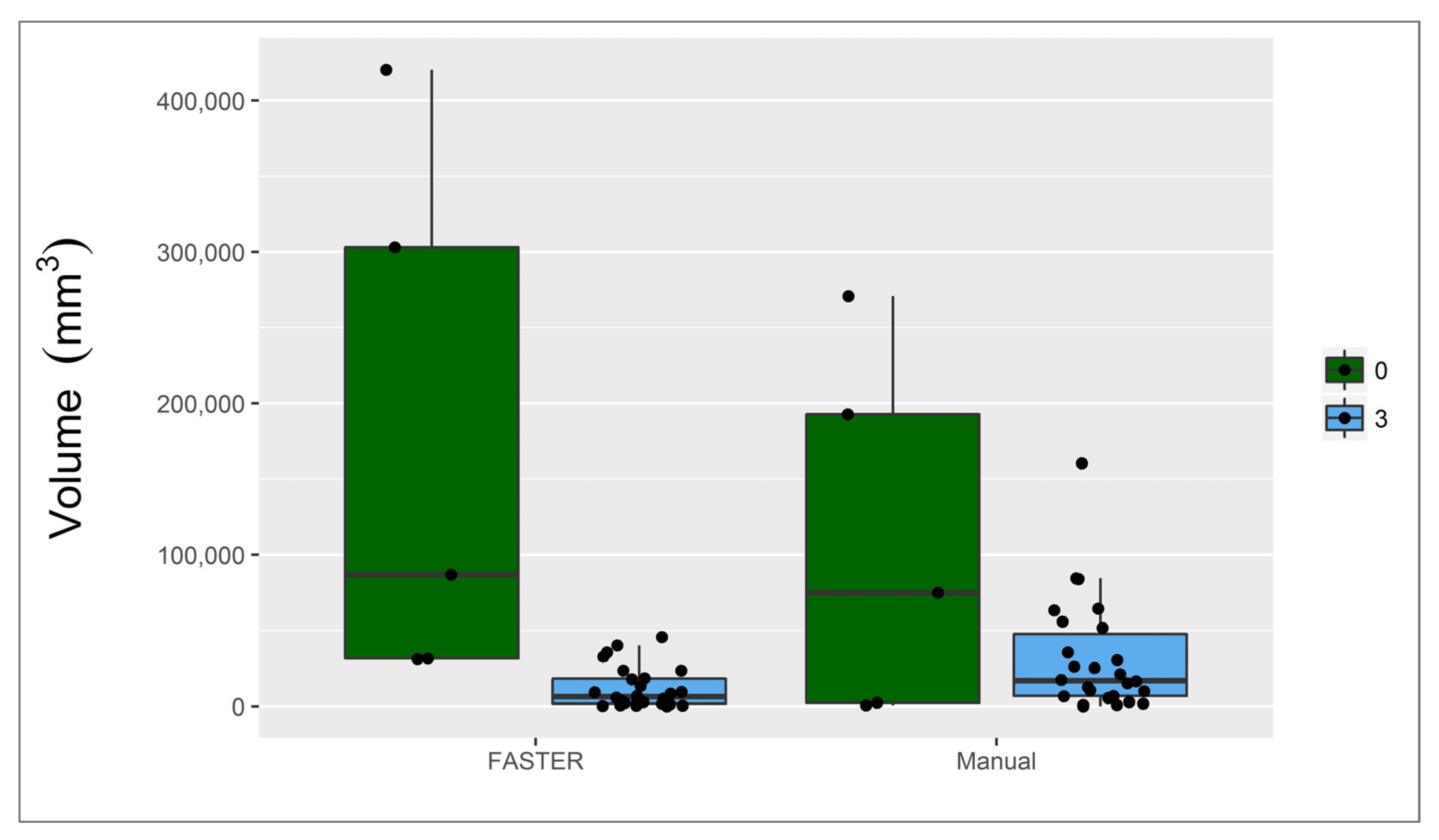
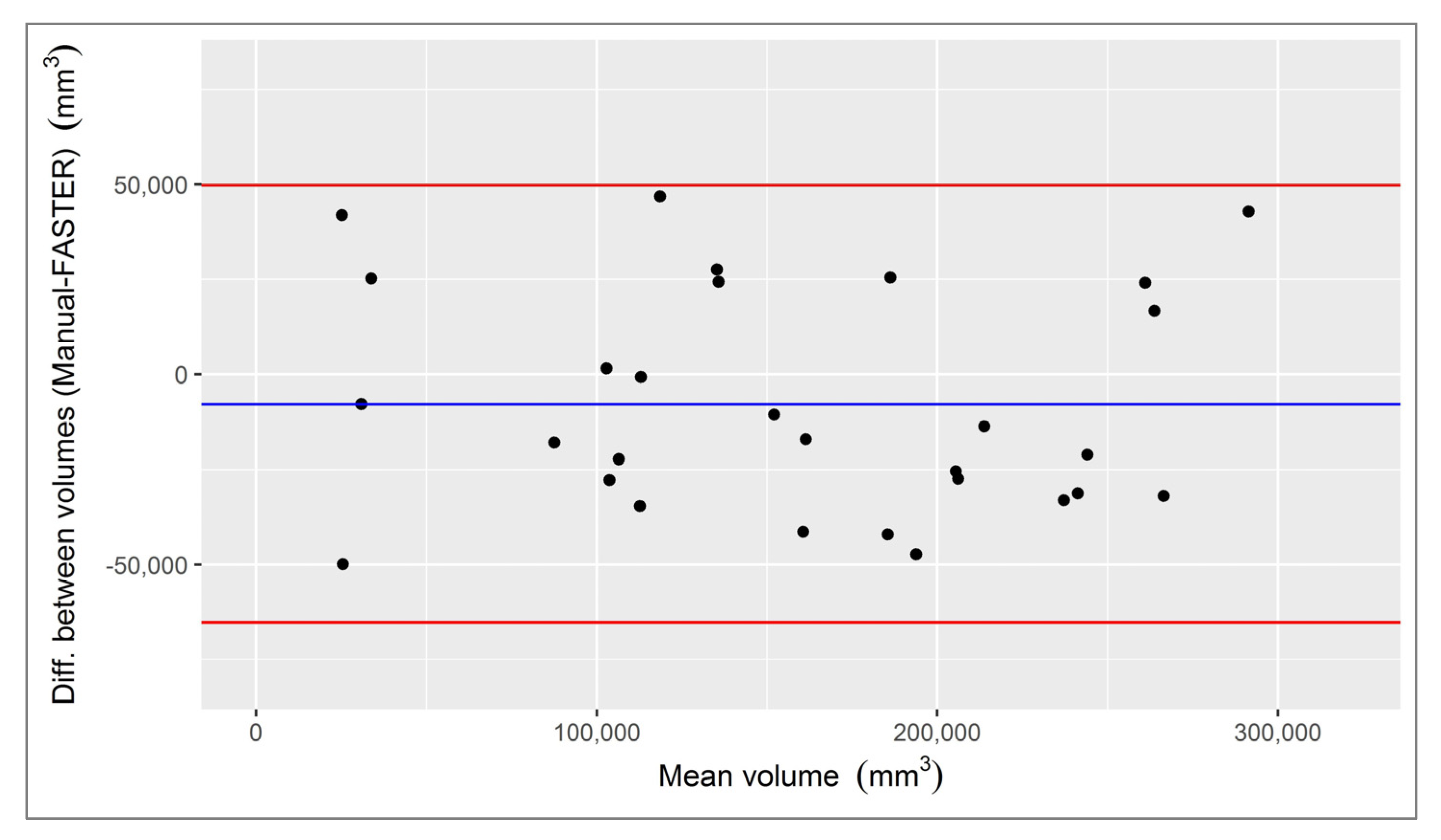
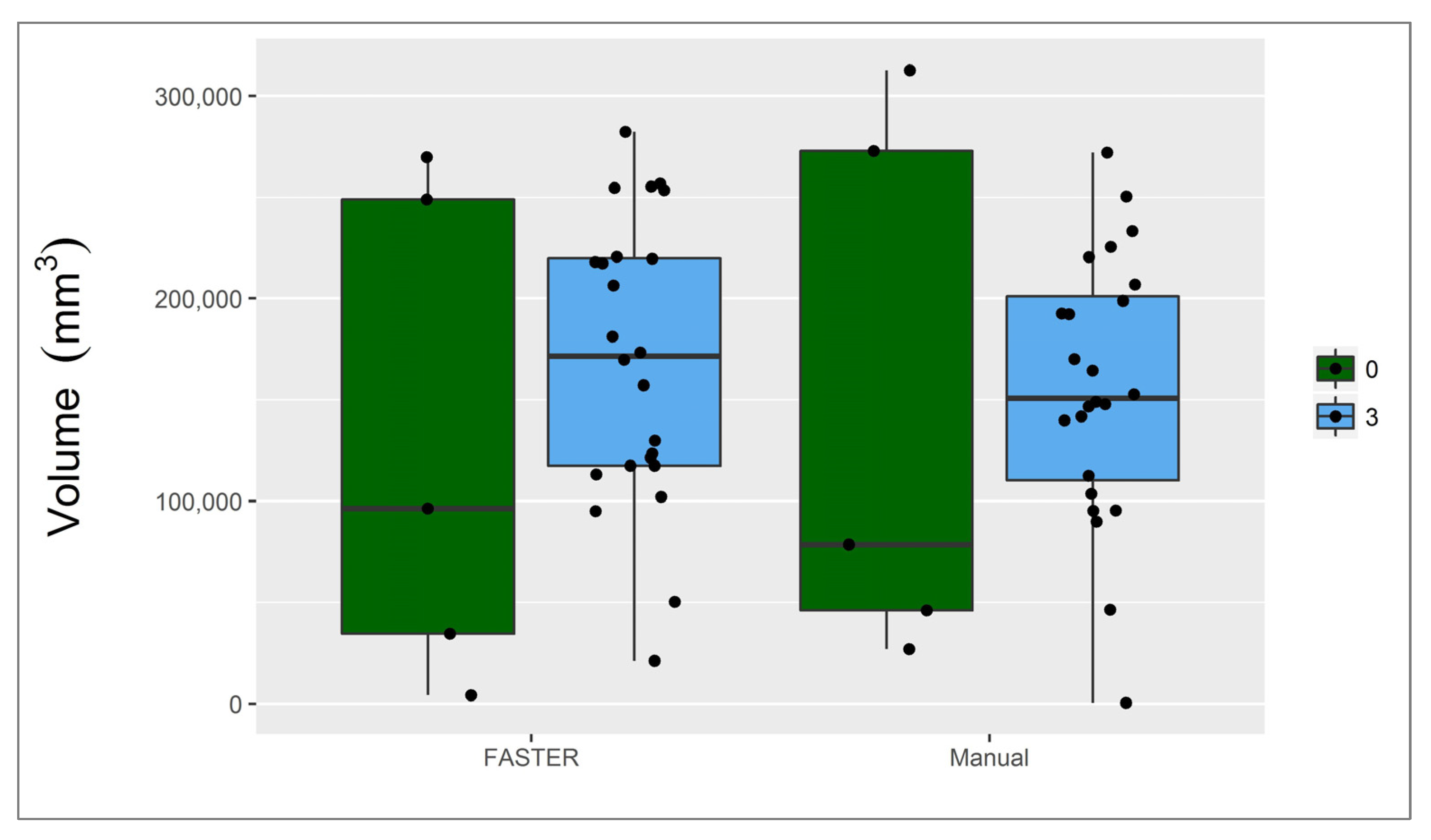
3.1. Infarct Core
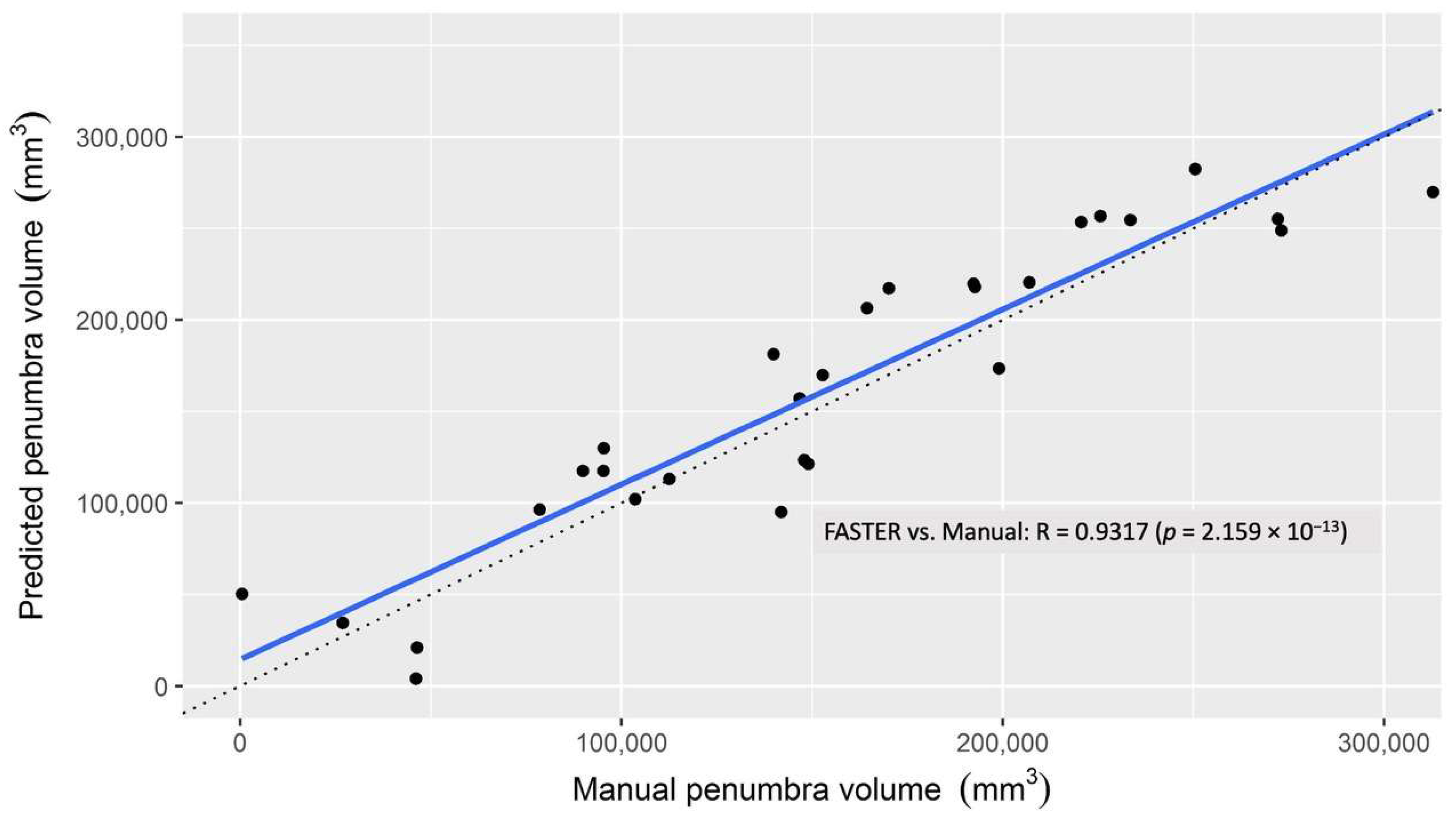
3.2. Penumbra
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, B.; Golsari, A.; Fiehler, J.; Rosenkranz, M.; Gerloff, C.; Thomalla, G. Dynamics of regional distribution of ischemic lesions in middle cerebral artery trunk occlusion relates to collateral circulation. Br. J. Pharmacol. 2010, 31, 36–40. [Google Scholar] [CrossRef]
- Menezes, N.M.; Ay, H.; Zhu, M.W.; Lopez, C.J.; Singhal, A.B.; Karonen, J.O.; Aronen, H.J.; Liu, Y.; Nuutinen, J.; Koroshetz, W.J.; et al. The real estate factor. Stroke 2007, 38, 194–197. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Luo, C.; Lu, W.; Su, B. Analysis of multiple factors involved in acute progressive cerebral infarction and extra- and intracranial arterial lesions. Exp. Ther. Med. 2014, 7, 1495–1505. [Google Scholar] [CrossRef][Green Version]
- Campbell, B.C.V.; Christensen, S.; Tress, B.M.; Churilov, L.; Desmond, P.M.; Parsons, M.W.; Barber, P.A.; Levi, C.; Bladin, C.; Donnan, G.; et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. Br. J. Pharmacol. 2013, 33, 1168–1172. [Google Scholar] [CrossRef]
- Boulouis, G.; Lauer, A.; Siddiqui, A.K.; Charidimou, A.; Regenhardt, R.; Viswanathan, A.; Rost, N.; Leslie-Mazwi, T.M.; Schwamm, L.H. Clinical imaging factors associated with infarct progression in patients with ischemic stroke during transfer for mechanical thrombectomy. JAMA Neurol. 2017, 74, 1361–1367. [Google Scholar] [CrossRef]
- Goda, T.; Oyama, N.; Kitano, T.; Iwamoto, T.; Yamashita, S.; Takai, H.; Matsubara, S.; Uno, M.; Yagita, Y. Factors associated with unsuccessful recanalization in mechanical thrombectomy for acute ischemic stroke. Cerebrovasc. Dis. Extra 2019, 9, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; De Silva, D.A.; MacLeod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Prim. 2019, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef] [PubMed]
- Neumann-Haefelin, T.; Wenserski, F.; Siebler, M. The DWI/PWI mismatch region in acute stroke. Stroke 1999, 30, 1591–1597. [Google Scholar] [CrossRef]
- Purushotham, A.; Campbell, B.; Straka, M.; Mlynash, M.; Olivot, J.-M.; Bammer, R.; Kemp, S.M.; Albers, G.W.; Lansberg, M. Apparent diffusion coefficient threshold for delineation of ischemic core. Int. J. Stroke 2013, 10, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Straka, M.; Albers, G.W.; Bammer, R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J. Magn. Reson. Imaging 2010, 32, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; Purushotham, A.; Christensen, S.; Desmond, P.M.; Nagakane, Y.; Parsons, M.W.; Lansberg, M.; Mlynash, M.; Straka, M.; De Silva, D.A.; et al. The infarct core is well represented by the acute diffusion lesion: Sustained reversal is infrequent. Br. J. Pharmacol. 2011, 32, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Olivot, J.-M.; Mlynash, M.; Thijs, V.N.; Kemp, S.; Lansberg, M.; Wechsler, L.; Bammer, R.; Marks, M.P.; Albers, G.W. Optimal tmax threshold for predicting penumbral tissue in acute stroke. Stroke 2009, 40, 469–475. [Google Scholar] [CrossRef]
- Olivot, J.-M.; Mlynash, M.; Thijs, V.N.; Purushotham, A.; Kemp, S.; Lansberg, M.G.; Wechsler, L.; Gold, G.E.; Bammer, R.; Marks, M.P.; et al. Geography, structure, and evolution of diffusion and perfusion lesions in diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE). Stroke 2009, 40, 3245–3251. [Google Scholar] [CrossRef] [PubMed]
- Soize, S.; Tisserand, M.; Charron, S.; Turc, G.; Ben Hassen, W.; Labeyrie, M.-A.; Legrand, L.; Mas, J.-L.; Pierot, L.; Meder, J.-F.; et al. How sustained is 24-Hour diffusion-weighted imaging lesion reversal? Stroke 2015, 46, 704–710. [Google Scholar] [CrossRef]
- Kidwell, C.S.; Saver, J.L.; Mattiello, J.; Starkman, S.; Vinuela, F.; Duckwiler, G.; Gobin, Y.P.; Jahan, R.; Vespa, P.; Kalafut, M.; et al. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann. Neurol. 2000, 47, 462–469. [Google Scholar] [CrossRef]
- Fiehler, J.; Foth, M.; Kucinski, T.; Knab, R.; von Bezold, M.; Weiller, C.; Zeumer, H.; Röther, J. Severe ADC decreases do not predict irreversible tissue damage in Humans. Stroke 2002, 33, 79–86. [Google Scholar] [CrossRef]
- Labeyrie, M.-A.; Turc, G.; Hess, A.; Hervo, P.; Mas, J.-L.; Meder, J.-F.; Baron, J.-C.; Touzé, E.; Oppenheim, C. Diffusion lesion reversal after thrombolysis. Stroke 2012, 43, 2986–2991. [Google Scholar] [CrossRef]
- Olivot, J.-M.; Mlynash, M.; Thijs, V.N.; Purushotham, A.; Kemp, S.; Lansberg, M.G.; Wechsler, L.; Bammer, R.; Marks, M.P.; Albers, G.W. Relationships between cerebral perfusion and reversibility of acute diffusion lesions in DEFUSE. Stroke 2009, 40, 1692–1697. [Google Scholar] [CrossRef]
- Meier, R.; Lux, P.; McKinley, R.; Jung, S.; Fischer, U.; Gralla, J.; Wiest, R.; Kaesmacher, J. Threshold-based identification of persistent infarction after successful endovascular treatment: Re-evaluating the “core”. medRxiv 2020. [Google Scholar] [CrossRef]
- Wouters, A.; Christensen, S.; Straka, M.; Mlynash, M.; Liggins, J.; Bammer, R.; Thijs, V.; Lemmens, R.; Albers, G.W.; Lansberg, M. A Comparison of relative time to peak and tmax for mismatch-based patient selection. Front. Neurol. 2017, 8, 539. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.; Hansen, M.B.; Tietze, A.; Mouridsen, K. Prediction of tissue outcome and assessment of treatment effect in acute ischemic stroke using deep learning. Stroke 2018, 49, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- McKinley, R.; Häni, L.; Gralla, J.; El-Koussy, M.; Bauer, S.; Arnold, M.; Fischer, U.; Jung, S.; Mattmann, K.; Reyes, M.; et al. Fully automated stroke tissue estimation using random forest classifiers (FASTER). Br. J. Pharmacol. 2016, 37, 2728–2741. [Google Scholar] [CrossRef]
- Brosch, T.; Yoo, Y.; Tang, L.Y.; Li, D.K.; Traboulsee, A.; Tam, R. Deep Convolutional Encoder Networks for Multiple Sclerosis Lesion Segmentation; Lecture Notes in Computer Science, Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics; Crimi, A., Menze, B., Maier, O., Reyes, M., Handels, H., Eds.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Jovin, T.G.; Chamorro, A.; Cobo, E.; De Miquel, M.A.; Molina, C.A.; Rovira, A.; Román, L.S.; Serena, J.; Abilleira, S.; Ribo, M.; et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N. Engl. J. Med. 2015, 372, 2296–2306. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.; Dinnes, J.; D′Amico, R.; Sowden, A.J.; Sakarovitch, C.; Song, F.; Petticrew, M.; Altman, D.G. Evaluating non-randomised intervention studies. Health Technol. Assess. 2003, 7, 1–179. [Google Scholar] [CrossRef]
- Higashida, R.T.; Furlan, A.J.; Roberts, H.; Tomsick, T.; Connors, B.; Barr, J.; Dillon, W.; Warach, S.; Broderick, J.; Tilley, B.; et al. Erratum: Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003, 34, 2774. [Google Scholar] [CrossRef]
- Jung, S.; Gilgen, M.; Slotboom, J.; El-Koussy, M.; Zubler, C.; Kiefer, C.; Luedi, R.; Mono, M.-L.; Heldner, M.R.; Weck, A.; et al. Factors that determine penumbral tissue loss in acute ischaemic stroke. Brain 2013, 136, 3554–3560. [Google Scholar] [CrossRef]
- Jung, S.; Wiest, R.; Gralla, J.; McKinley, R.; Mattle, H.; Liebeskind, D. Relevance of the cerebral collateral circulation in ischaemic stroke: Time is brain, but collaterals set the pace. Swiss Med. Wkly. 2017, 147, w14538. [Google Scholar] [CrossRef]
- McKinley, R.; Hung, F.; Wiest, R.; Liebeskind, D.S.; Scalzo, F. A machine learning approach to perfusion imaging with dynamic susceptibility contrast MR. Front. Neurol. 2018, 9, 717. [Google Scholar] [CrossRef]
- Meier, R.; Lux, P.; Med, B.; Jung, S.; Fischer, U.; Gralla, J.; Reyes, M.; Wiest, R.; McKinley, R.; Kaesmacher, J. Neural network–derived perfusion maps for the assessment of lesions in patients with acute ischemic stroke. Radiol. Artif. Intell. 2019, 1, e190019. [Google Scholar] [CrossRef] [PubMed]
- Cereda, C.W.; Christensen, S.; Campbell, B.C.V.; Mishra, N.; Mlynash, M.; Levi, C.; Straka, M.; Wintermark, M.; Bammer, R.; Albers, G.W.; et al. A benchmarking tool to evaluate computer tomography perfusion infarct core predictions against a DWI standard. Br. J. Pharmacol. 2016, 36, 1780–1789. [Google Scholar] [CrossRef]
- Kudo, K.; Sasaki, M.; Yamada, K.; Momoshima, S.; Utsunomiya, H.; Shirato, H.; Ogasawara, K. Differences in CT perfusion maps generated by different commercial software: Quantitative analysis by using identical source data of acute stroke patients. Radiology 2010, 254, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Fahmi, F.; Marquering, H.; Streekstra, G.; Beenen, L.; Velthuis, B.; van Bavel, E.; Majoie, C. Differences in CT perfusion summary maps for patients with acute ischemic stroke generated by 2 software packages. Am. J. Neuroradiol. 2012, 33, 2074–2080. [Google Scholar] [CrossRef]
- Kudo, K.; Christensen, S.; Sasaki, M.; Østergaard, L.; Shirato, H.; Ogasawara, K.; Wintermark, M.; Warach, S. Accuracy and reliability assessment of CT and MR perfusion analysis software using a digital phantom. Radiology 2013, 267, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Zussman, B.M.; Boghosian, G.; Gorniak, R.J.; Olszewski, M.E.; Read, K.M.; Siddiqui, K.M.; Flanders, A.E. The relative effect of vendor variability in CT perfusion results: A method comparison study. Am. J. Roentgenol. 2011, 197, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Rava, R.A.; Snyder, K.V.; Mokin, M.; Waqas, M.; Zhang, X.; Podgorsak, A.R.; Allman, A.B.; Senko, J.; Bhurwani, M.M.S.; Hoi, Y.; et al. Assessment of computed tomography perfusion software in predicting spatial location and volume of infarct in acute ischemic stroke patients: A comparison of Sphere, Vitrea, and RAPID. J. NeuroInterv. Surg. 2021, 13, 130–135. [Google Scholar] [CrossRef]
- Rekik, I.; Allassonnière, S.; Carpenter, T.K.; Wardlaw, J.M. Medical image analysis methods in MR/CT-imaged acute-subacute ischemic stroke lesion: Segmentation, prediction and insights into dynamic evolution simulation models. A critical appraisal. NeuroImage Clin. 2012, 1, 164–178. [Google Scholar] [CrossRef]
- Maier, O.; Menze, B.; von der Gablentz, J.; Häni, L.; Heinrich, M.P.; Liebrand, M.; Winzeck, S.; Basit, A.; Bentley, P.; Chen, L.; et al. ISLES 2015—A public evaluation benchmark for ischemic stroke lesion segmentation from multispectral MRI. Med. Image Anal. 2017, 35, 250–269. [Google Scholar] [CrossRef]
- Winzeck, S.; Hakim, A.; McKinley, R.; Pinto, J.A.; Alves, V.; Silva, C.; Pisov, M.; Krivov, E.; Belyaev, M.; Monteiro, M.; et al. ISLES 2016 and 2017—Benchmarking ischemic stroke lesion outcome prediction based on multispectral MRI. Front. Neurol. 2018, 9, 679. [Google Scholar] [CrossRef]
- Ho, K.C.; Scalzo, F.; Sarma, K.V.; El-Saden, S.; Arnold, C.W. A temporal deep learning approach for MR perfusion parameter estimation in stroke. In Proceedings of the 2016 23rd International Conference on Pattern Recognition (ICPR), Cancun, Mexico, 4–8 December 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 1315–1320. [Google Scholar]
- Hess, A.; Meier, R.; Kaesmacher, J.; Jung, S.; Scalzo, F.; Liebeskind, D.; Wiest, R.; McKinley, R. Synthetic Perfusion Maps: Imaging Perfusion Deficits in DSC-MRI with Deep Learning; Springer International Publishing: New York, NY, USA, 2019; pp. 447–455. [Google Scholar]
- Ulas, C.; Tetteh, G.; Thrippleton, M.J.; Armitage, P.A.; Makin, S.D.; Wardlaw, J.M.; Davies, M.E.; Menze, B. Direct Estimation of Pharmacokinetic Parameters from DCE-MRI Using Deep CNN with Forward Physical Model Loss; Springer: Berlin/Heidelberg, Geramany, 2018; pp. 39–47. [Google Scholar]
- Pinto, A.; Amorim, J.; Hakim, A.; Alves, V.; Reyes, M.; Silva, C.A. Prediction of stroke lesion at 90-day follow-up by fusing raw DSC-MRI with parametric maps using deep learning. IEEE Access 2021, 9, 26260–26270. [Google Scholar] [CrossRef]
- Hakim, A.; Christensen, S.; Winzeck, S.; Lansberg, M.G.; Parsons, M.W.; Lucas, C.; Robben, D.; Wiest, R.; Reyes, M.; Zaharchuk, G. Predicting infarct core from computed tomography perfusion in acute ischemia with machine learning: Lessons from the ISLES challenge. Stroke 2021, 52, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.; Pereira, S.; Meier, R.; Wiest, R.; Alves, V.; Reyes, M.; Silva, C.A. Combining unsupervised and supervised learning for predicting the final stroke lesion. Med. Image Anal. 2021, 69, 101888. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.; McKinley, R.; Alves, V.; Wiest, R.; Silva, C.A.; Reyes, M. Stroke lesion outcome prediction based on MRI imaging combined with clinical Information. Front. Neurol. 2018, 9, 1060. [Google Scholar] [CrossRef]
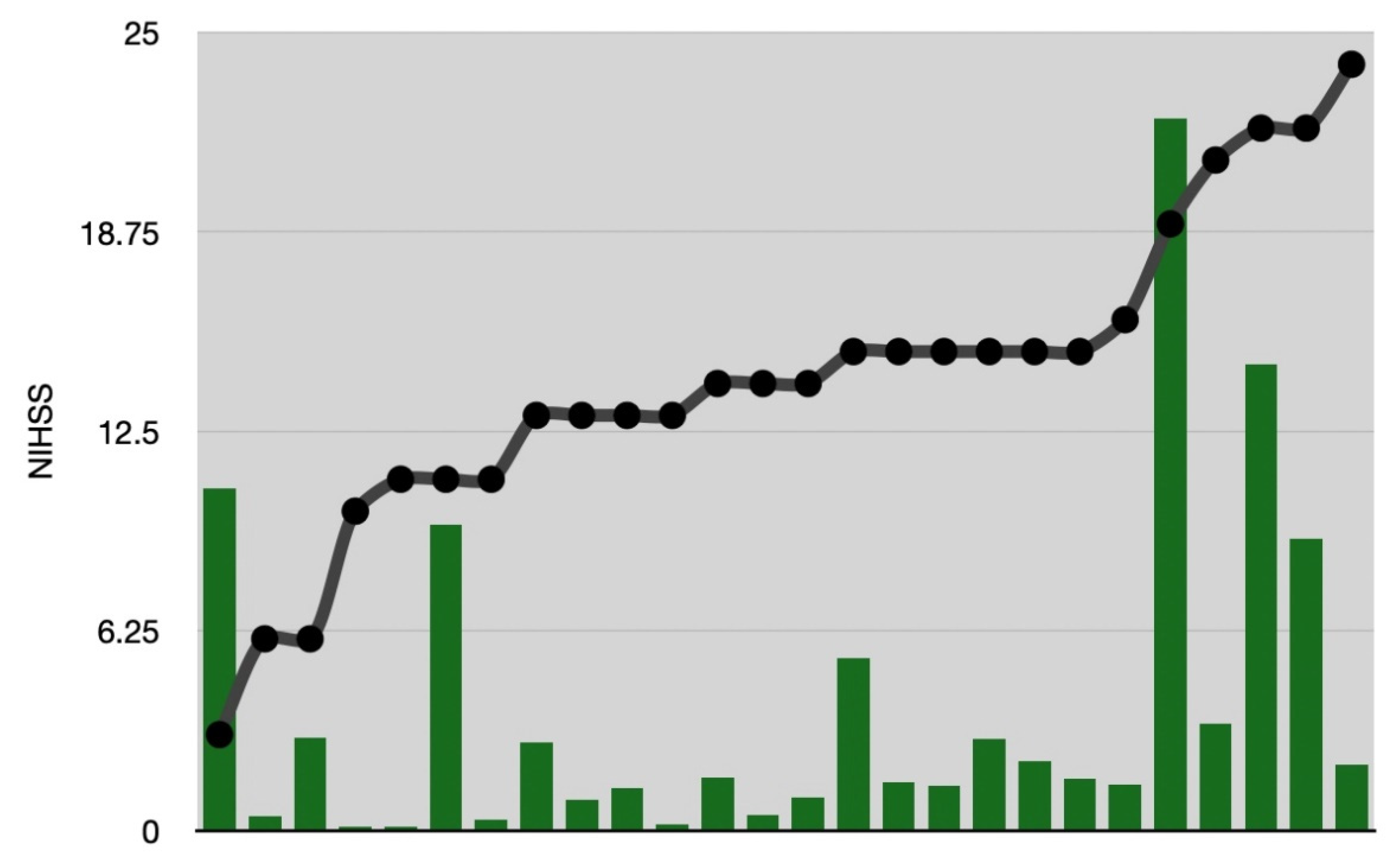
| Number | Age | Gender | NIHSS | Time to Imaging (min) | Time to i.v. Thrombolysis (min) | Time to DSA (min) | Time to Thrombectomy /i.a. Thrombolysis (min) | TICI | Follow-Up | Infarction Core FASTER (cm3) | Final Infarction Ground Truth (cm3) | Penumbra FASTER (cm3) | Penumbra Tmax > 6 s (cm3) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 61 | m | 22 | 120 | -- | 283 | 295 | 0 | CT | 303.1 | 270.7 | 248.9 | 273.1 |
| 2 | 79 | m | 19 | 76 | -- | 150 | 215 | 0 | CT | 420.6 | 192.9 | 270.0 | 312.8 |
| 3 | 64 | f | 11 | 180 | -- | 239 | 313 | 0 | FLAIR | 86.8 | 74.9 | 96.5 | 78.6 |
| 4 | 86 | f | 7 | 172 | 180 | 245 | -- | 0 | CT | 31.6 | 2.3 | 4.2 | 46.1 |
| 5 | 54 | f | 0 | 217 | 230 | 450 | -- | 0 | FLAIR | 31.3 | 0.5 | 34.7 | 26.9 |
| 6 | 56 | m | 22 | 149 | -- | 211 | 268 | 3 | T2 | 2.7 | 84.5 | 48.3 | N/A |
| 7 | 69 | f | 22 | 240 | -- | 360 | 390 | 3 | FLAIR | 32.7 | 84.0 | 220.6 | 207.0 |
| 8 | 32 | m | 14 | 250 | -- | 310 | 370 | 3 | CT | 9.3 | 0.0 | 123.5 | 147.9 |
| 9 | 70 | m | 15 | 105 | 150 | 192 | 252 | 3 | FLAIR | 0.4 | 30.6 | 4.7 | N/A |
| 10 | 73 | m | 10 | 114 | 150 | 188 | 253 | 3 | T2 | 1.6 | 0.7 | 218.1 | 192.7 |
| 11 | 61 | f | 15 | 123 | 152 | 154 | 330 | 3 | T2 | 6.8 | 15.2 | 206.5 | 164.4 |
| 12 | 67 | m | 15 | 168 | 200 | 260 | 290 | 3 | T2 | 17.7 | 9.8 | 219.7 | 192.4 |
| 13 | 86 | m | 11 | 110 | 140 | 183 | 200 | 3 | T2 | 0.1 | 0.8 | 117.6 | 89.9 |
| 14 | 72 | m | 21 | 100 | 135 | 194 | 205 | 3 | FLAIR | 45.6 | 64.4 | 157.2 | 146.8 |
| 15 | 73 | f | 6 | 114 | 160 | 201 | 227 | 3 | T2 | 0.3 | 2.8 | 50.3 | 0.5 |
| 16 | 45 | m | 14 | 85 | -- | 195 | 207 | 3 | T2 | 23.5 | 26.2 | 121.4 | 149.0 |
| 17 | 59 | f | 15 | 102 | -- | 222 | 235 | 3 | T2 | 9.2 | 25.3 | 217.4 | 170.1 |
| 18 | 55 | m | 19 | 83 | -- | 150 | 194 | 3 | T2 | 35.6 | 160.4 | 173.5 | 199.0 |
| 19 | 58 | m | 14 | 748 | -- | 840 | 860 | 3 | T2 | 18.3 | 12.5 | 254.6 | 233.5 |
| 20 | 70 | f | 6 | 260 | -- | 343 | 503 | 3 | T2 | 0.0 | 16.3 | 21.1 | 46.4 |
| 21 | 51 | m | 13 | 104 | -- | 166 | 180 | 3 | T2 | 5.7 | 21.1 | 102.1 | 103.6 |
| 22 | 72 | m | 13 | 107 | -- | 190 | 204 | 3 | FLAIR | 1.2 | 6.6 | 255.3 | 272.1 |
| 23 | 76 | m | 13 | 83 | 140 | 157 | 200 | 3 | T2 | 3.1 | 10.6 | 130.0 | 95.3 |
| 24 | 85 | f | 16 | -- | -- | * | * | 3 | FLAIR | 13.4 | 5.3 | 253.6 | 220.6 |
| 25 | 76 | m | 15 | 217 | 250 | 279 | 281 | 3 | CT | 23.4 | 35.5 | 95.1 | 141.9 |
| 26 | 78 | m | 11 | 103 | -- | 158 | 164 | 3 | FLAIR | 2.2 | 55.8 | 256.8 | 225.6 |
| 27 | 63 | m | 3 | 76 | -- | 171 | 190 | 3 | FLAIR | 3.4 | 63.4 | 113.2 | 112.6 |
| 28 | 84 | f | 15 | 148 | -- | 195 | 222 | 3 | CT | 8.4 | 17.4 | 169.8 | 152.8 |
| 29 | 63 | f | 11 | 127 | -- | 153 | 219 | 3 | FLAIR | 4.9 | 6.8 | 117.5 | 95.2 |
| 30 | 71 | f | 24 | -- | -- | * | * | 3 | FLAIR | 40.2 | 51.8 | 282.4 | 250.5 |
| 31 | 74 | f | 13 | 96 | -- | 127 | 176 | 3 | FLAIR | 0.5 | 1.6 | 181.3 | 139.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakim, A.; Messerli, B.; Meier, R.; Dobrocky, T.; Bellwald, S.; Jung, S.; McKinley, R.; Wiest, R. Prediction of Tissue Damage Using a User-Independent Machine Learning Algorithm vs. Tmax Threshold Maps. Clin. Transl. Neurosci. 2021, 5, 21. https://doi.org/10.3390/ctn5030021
Hakim A, Messerli B, Meier R, Dobrocky T, Bellwald S, Jung S, McKinley R, Wiest R. Prediction of Tissue Damage Using a User-Independent Machine Learning Algorithm vs. Tmax Threshold Maps. Clinical and Translational Neuroscience. 2021; 5(3):21. https://doi.org/10.3390/ctn5030021
Chicago/Turabian StyleHakim, Arsany, Benjamin Messerli, Raphael Meier, Tomas Dobrocky, Sebastian Bellwald, Simon Jung, Richard McKinley, and Roland Wiest. 2021. "Prediction of Tissue Damage Using a User-Independent Machine Learning Algorithm vs. Tmax Threshold Maps" Clinical and Translational Neuroscience 5, no. 3: 21. https://doi.org/10.3390/ctn5030021
APA StyleHakim, A., Messerli, B., Meier, R., Dobrocky, T., Bellwald, S., Jung, S., McKinley, R., & Wiest, R. (2021). Prediction of Tissue Damage Using a User-Independent Machine Learning Algorithm vs. Tmax Threshold Maps. Clinical and Translational Neuroscience, 5(3), 21. https://doi.org/10.3390/ctn5030021






