Comparison of Halmágyi–Curthoys Head Impulse (Thrust) Test with Romberg’s Test in Detection of Vestibular Hypofunctioning in Vertigo Patients
Abstract
1. Introduction
1.1. Semicircular Canals
- Left superior semicircular canal (LSCC)—situated in the left temporal bone, roughly parallel to the ground when standing upright, it primarily senses head rotations in the roll plane (tilting side to side).
- Right superior semicircular canal (RSCC)—its mirrored counterpart on the right side, the RSCC also detects roll plane movements, along with pitch (forward and backward tilting) to some extent.
- Posterior semicircular canal (PSCC)—nestled deeper within the skull, near the brainstem, this canal reigns supreme in perceiving yaw movements (turning left and right).
1.1.1. Curthoys–Halmágyi Test for Differentiating between Peripheral and Central Vestibular Disorders
1.1.2. Reflexes behind the Curthoys–Halmágyi Head Impulse Test and Romberg’s Test
1.1.3. Vestibular Hypofunctioning
1.1.4. Cochlear Symptoms in Vestibular Hypofunctioning
- Tinnitus—subjective phantom sounds experienced due to altered neuronal activity, increased central gain, or shared neurochemical factors.
- Hearing loss can result from direct cochlear harm, metabolic disorders, or retrocochlear lesions, causing difficulties in audio signal transmission and processing.
1.1.5. Causes of Vestibular Hypofunctioning
1.1.6. Halmágyi–Curthoys Head Impulse Test (HIT)
1.2. Vestibular Labyrinth and the VOR
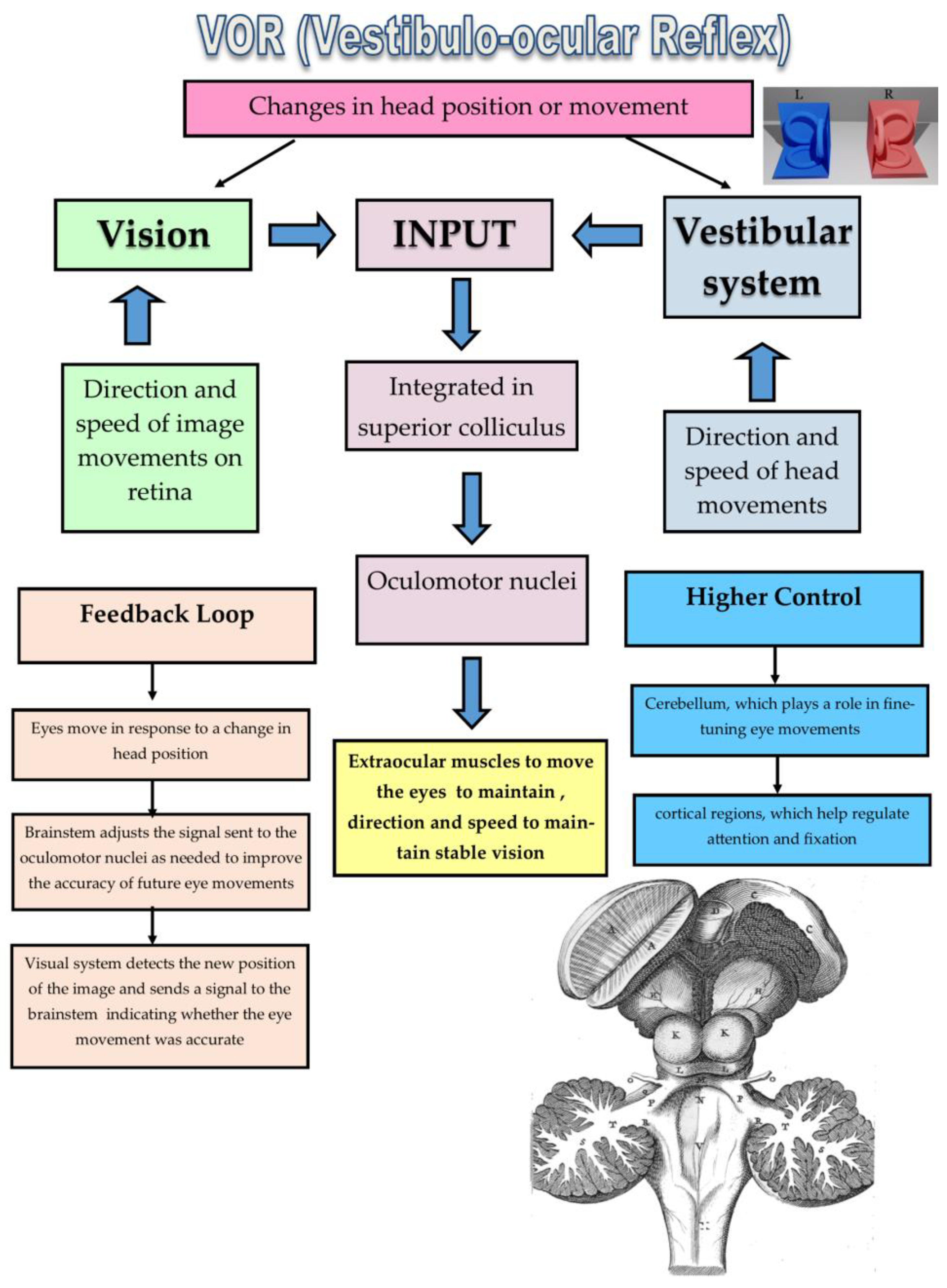
1.2.1. Mechanism of the VOR
1.2.2. Biological Role of the VOR
1.2.3. Accurate Assessment of the VOR
1.3. Performing the HIT
1.4. Interpreting the HIT
1.5. Clinical Significance
1.6. Advantages of the HIT
1.7. Limitations of the HIT
1.8. Beyond Bedside Test
1.9. Romberg’s Test: Exploring the Neurologic Roots of Balance
1.9.1. Performance of Romberg’s Test
Modified Romberg’s Tests Are depicted in the Figure 4C–G
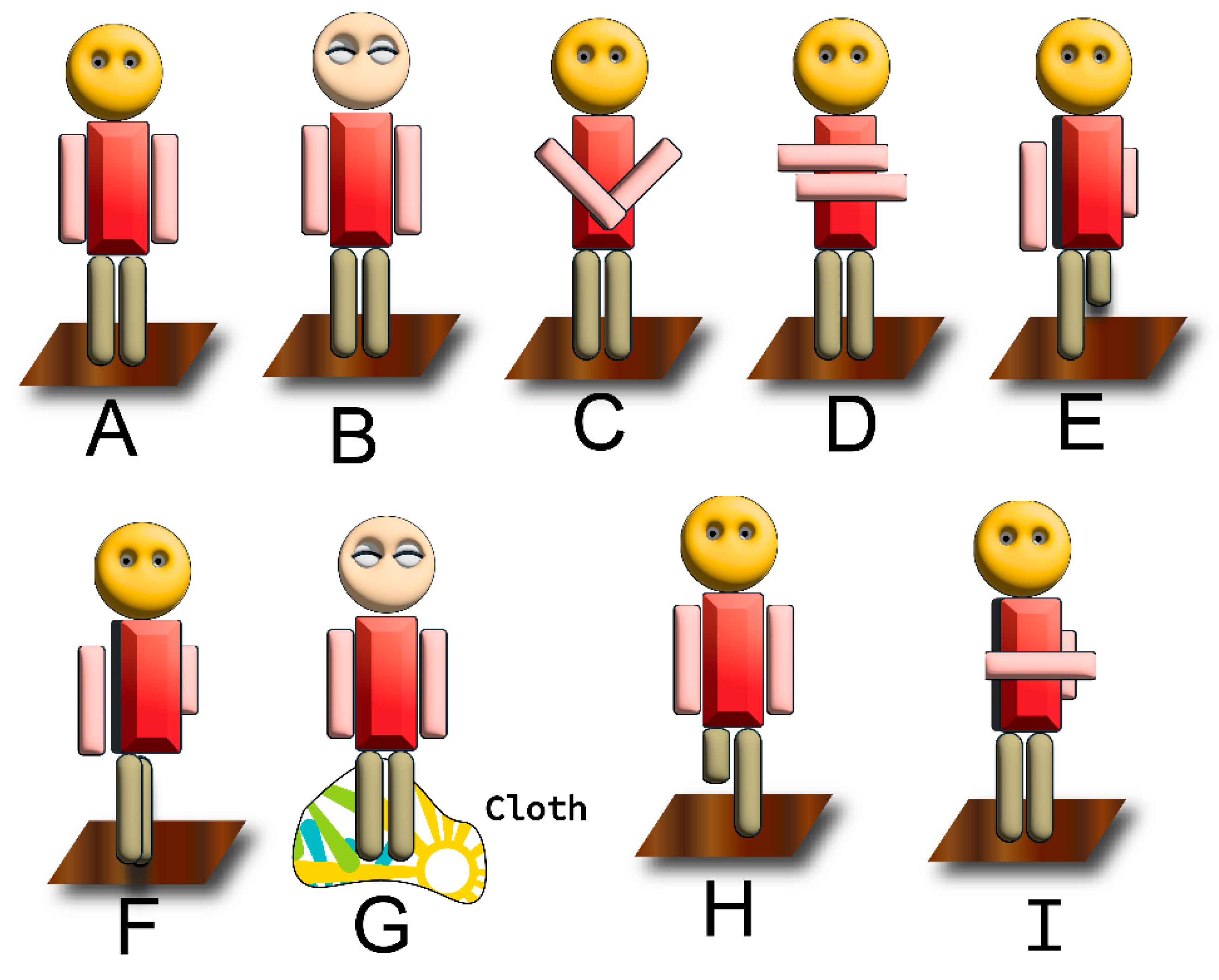
1.9.2. Neurologic Underpinnings
- Vision: provides direct information about our spatial orientation.
- Proprioception: informs the brain about the position and movement of our body parts.
- Vestibular system: senses head movement and spatial orientation through fluid shifts in the inner ear canals.
1.9.3. Diagnostic Scope
- Diabetes mellitus: uncontrolled blood sugar levels can damage peripheral nerves, including those involved in the vestibular system, ultimately impacting balance and coordination.
- Guillain–Barre syndrome: an autoimmune disorder that attacks the myelin sheath surrounding nerve fibers, disrupting signal transmission, and potentially causing vestibular symptoms such as vertigo, nausea, and imbalance.
- Toxins and medications: exposure to certain chemicals, heavy metals, or drugs, such as aminoglycoside antibiotics, can induce peripheral neuropathies and affect the vestibular system, contributing to balance difficulties.
1.9.4. Beyond the Binary Outcomes
1.9.5. Limitations and Refinements
- Degenerative joint diseases: conditions like osteoarthritis can limit joint mobility and increase pain, making it challenging for elderly individuals to maintain their center of gravity during the test.
- Muscle weakness: age-related muscle loss (sarcopenia) and related joint instability can contribute to increased sway during Romberg’s test, even without true vestibular dysfunction.
- Pain: persistent joint pain can distract patients and interfere with their ability to concentrate on maintaining balance during the test, skewing the results and potentially masking genuine vestibular deficits.
2. Materials and Methods
2.1. Patient Selection
2.1.1. Participant Groups
2.1.2. Inclusion Criteria
- Diagnosis of vertigo based on clinical evaluation by an experienced vestibular specialist or neurologist.
- Age between 18 and 70 years old.
- Ability to understand instructions and perform testing procedures.
2.1.3. Exclusion Criteria
- An abnormal MRI brain scan.
- History of head trauma or brain injury within six months prior to enrolment.
- Current use of medications known to affect vestibular function or central nervous system like Labyrinthine sedatives.
- Uncontrolled medical conditions affecting balance or equilibrium like uncontrolled hypoglycemia.
- Pregnancy or lactation.
2.1.4. Grouping
2.2. Diagnostic Methods
- Halmágyi–Curthoys head impulse (thrust) test: Patients underwent systematic head thrust maneuvers to assess the vestibulo–ocular reflex. Any corrective saccades were recorded as indicative of vestibular hypofunctioning [22].
- Romberg’s test: Postural stability during quiet standing was evaluated with patients in various conditions, including eyes open and closed. Deviations from the expected postural stability were noted [23].
2.3. Data Collection
2.4. Data Analysis Using SciPy—Python
2.5. Biostatical Tests of Inference
3. Results
3.1. Demographic, Clinical Characteristics
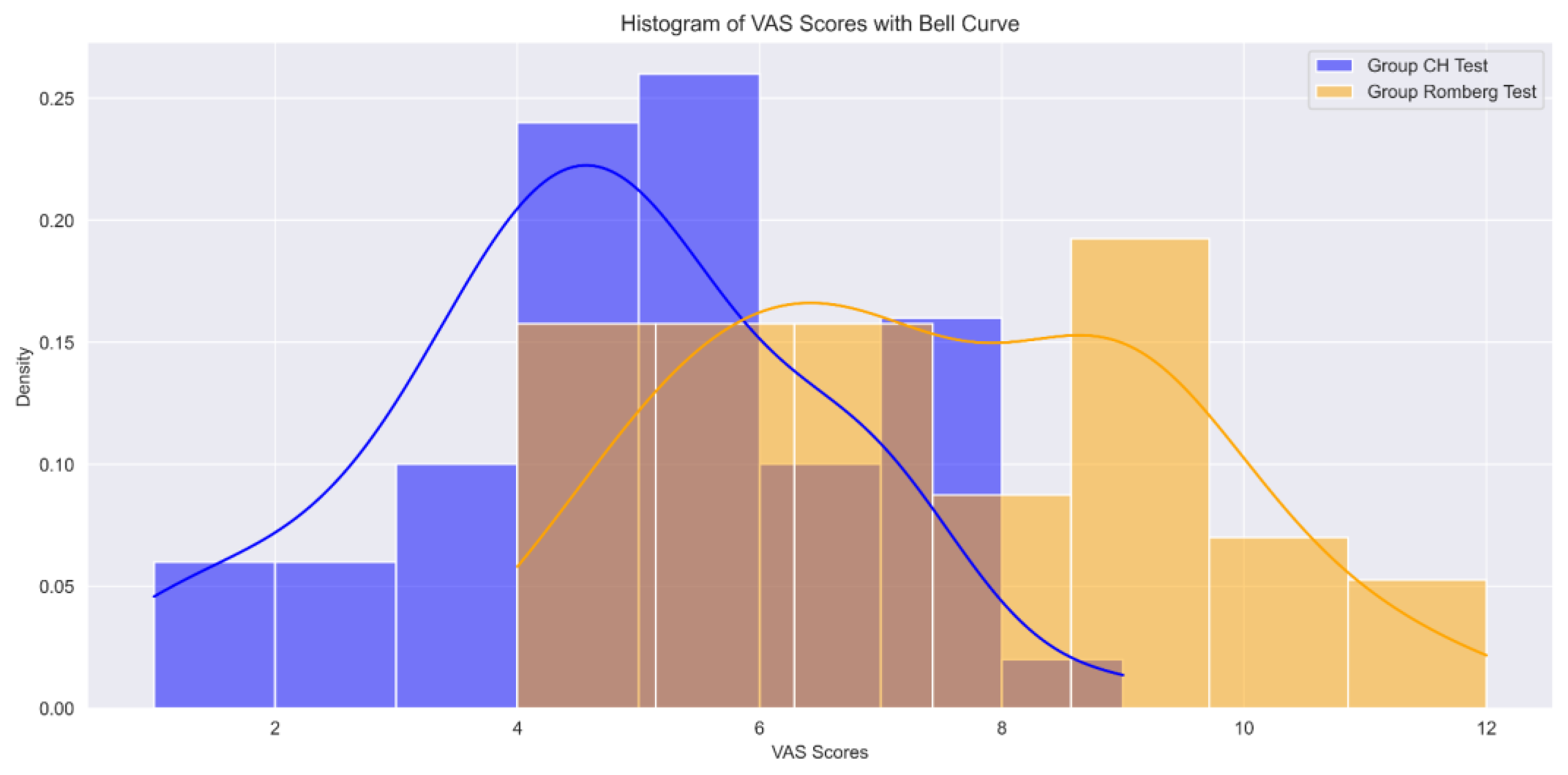
3.2. Analysis of Scores
3.3. Overall Observations
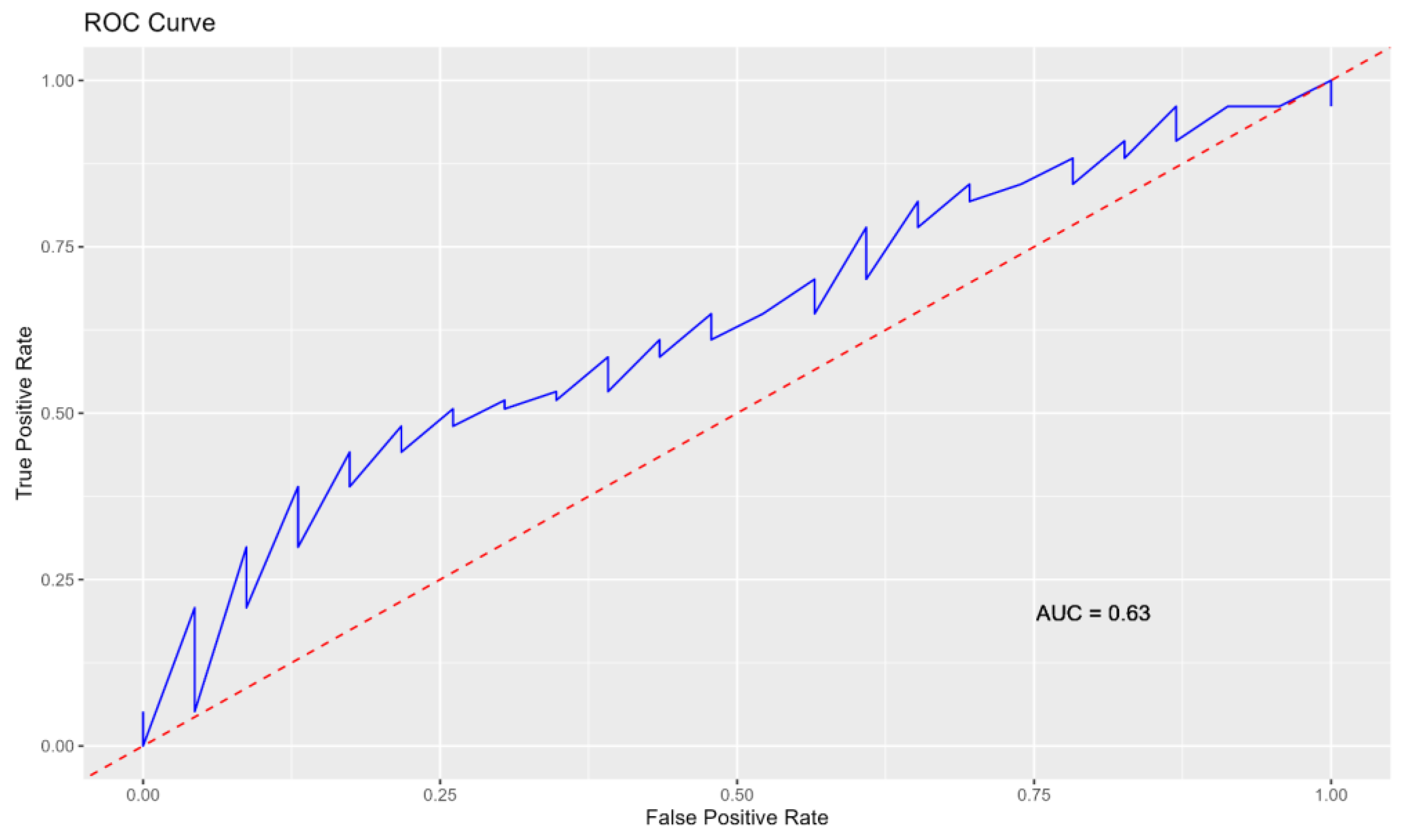
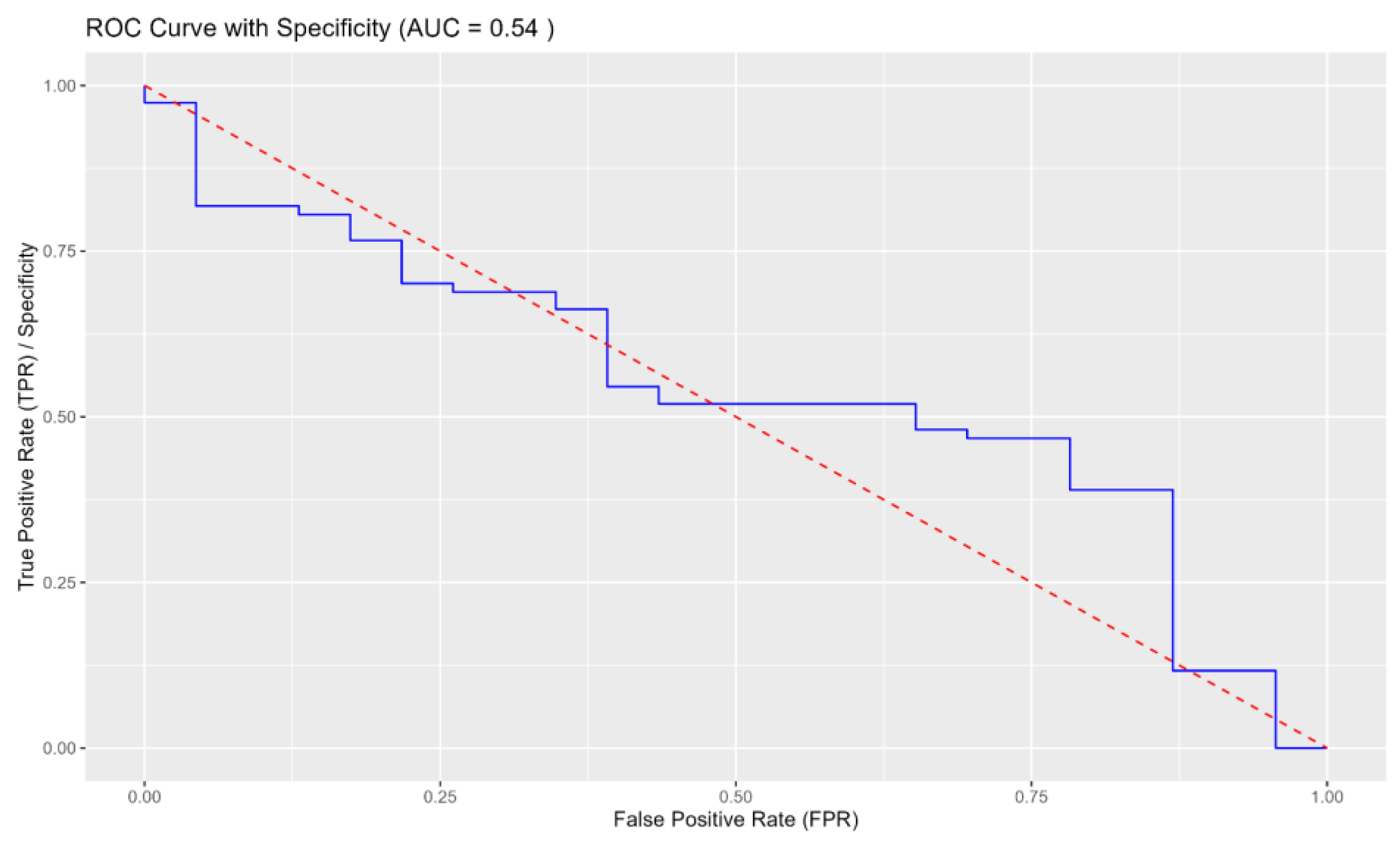
3.4. Comparison between Tests
Overall Implications
4. Discussion
4.1. Applications in Specific Populations
4.1.1. Functional Head Impulse Test (fHIT)
4.1.2. Limitations and Challenges
4.2. Clinical Implications
4.3. Limitations
4.4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kristinsdottir, E.K.; Fransson, P.A.; Magnusson, M. Vestibular rehabilitation with virtual reality for enhanced vestibular response. J. Vestib. Res. 2001, 11, 51–64. [Google Scholar]
- Gerb, J.; Becker-Bense, S.; Zwergal, A.; Huppert, D. Vestibular Syndromes after COVID-19 Vaccination: A Prospective Cohort Study. Eur. J. Neurol. 2022, 29, 3693–3700. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Kim, H.J.; Kim, J.S. Recent advances in head impulse test findings in central vestibulardisorders. Neurology 2018, 90, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.H.; Brodsky, J.; Ishiyama, G.; Sabatti, C.; Baloh, R.W. Vestibular rehabilitation for sensory organization deficits in patients with migraine and dizziness. Otol. Neurotol. 1996, 17, 635–640. [Google Scholar]
- Grill, E.; Strupp, M.; Muller, M.; Jahn, K. Improvement in postural control in patients with peripheral vestibulopathy. J. Neurol. 2014, 261, 118–124. [Google Scholar]
- Macdougall, H.G.; Weber, K.P.; McGarvie, L.A.; Halmagyi, G.M.; Curthoys, I.S. Human vertical vestibuloocular reflex initiation: Normal values and variability. J. Neurophysiol. 2005, 93, 20–29. [Google Scholar]
- Kline-Mangione, K.; Denham, T. Sensitivity of a Clinical Screen for Vestibular Hypofunctioning. Neurol. Rep. 1996, 20, 24. [Google Scholar] [CrossRef]
- Fitzgerald, J.E.; Keough, K.; Curthoys, I.S. Vestibular rehabilitation for dizziness and imbalance: A Canadian case-based survey. Physiother. Can. 2019, 71, 137–144. [Google Scholar]
- Yang, Y.; Chen, K.; Hsiu, H.; Wang, P.; Wang, R. Vestibular rehabilitation exercises in the treatment of dizziness and imbalance: A review. J. Chin. Med. Assoc. 2014, 77, 1–8. [Google Scholar]
- Herdman, S.J. Vestibular Rehabilitation; F.A. Davis Company: Philadelphia, PA, USA, 2014. [Google Scholar]
- von Brevern, M.; Radtke, A.; Lezius, F.; Feldmann, M.; Ziese, T.; Lempert, T.; Neuhauser, H. Epidemiology of benign paroxysmal positional vertigo: A population based study. J. Neurol. Neurosurg. Psychiatry 2015, 86, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Hidayati, H.B.; Imania, H.A.N.; Octaviana, D.S.; Kurniawan, R.B.; Wungu, C.D.K.; Rida Ariarini, N.N.; Srisetyaningrum, C.T.; Oceandy, D. Vestibular Rehabilitation Therapy and Corticosteroids for Vestibular Neuritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicina 2022, 58, 1221. [Google Scholar] [CrossRef]
- Hallpike, C.S.; Cairns, H. The Effect of Labyrinthine Lesions on the Static Labyrinthine Reflexes; Oxford University Press: Oxford, UK, 1956. [Google Scholar]
- Godemann, F.; Schierz, C.; Figge, C.; Deppe, W.; Cnyrim, C.; Reif, W. The course of dizziness and vertigo in patients with panic disorder. J. Psychosom. Res. 2004, 56, 77–81. [Google Scholar]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0 Contributors. SciPy 1.0 Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Radtke, A.; von Brevern, M.; Neuhauser, H.; Hottenrott, T.; Lempert, T. Randomized controlled trial of migraine prophylaxis in dizzy patients. Cephalalgia 2004, 24, 809–816. [Google Scholar]
- Whitney, S.L.; Marchetti, G.F.; Morris, L.O.; Sparto, P.J. Randomized controlled trial of an antidizziness medication in the treatment of dizziness in primary care. Phys. Ther. 2004, 84, 581–589. [Google Scholar]
- Brandt, T.; Daroff, R.B. Physical therapy for benign paroxysmal positioning vertigo. Arch. Otolaryngol. 1980, 106, 484–485. [Google Scholar] [CrossRef] [PubMed]
- Young, A.S.; Nham, B.; Bradshaw, A.P.; Calic, Z.; Pogson, J.M.; Gibson, W.P.; Halmágyi, G.M.; Welgampola, M.S. Clinical, Oculographic and Vestibular Test Characteristics of Ménière’s Disease. J. Neurol. 2022, 269, 1927–1944. [Google Scholar] [CrossRef]
- Kim, S.; Jung, Y.K.; Kim, M.J.; Kim, K.-S.; Kim, H.J. Diagnostic Evolution of Vestibular Neuritis after Long-Term Monitoring. Braz. J. Otorhinolaryngol. 2022, 88 (Suppl. S1), S14–S17. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.; Wang, S.; Li, X.; Zhao, H.; Liu, S.; Lyu, Y.; Fan, Z.; Wang, H.; Zhang, D. Effect of Late-Stage Ménière’s Disease and Vestibular Functional Impairment on Hippocampal Atrophy. Laryngoscope 2024, 134, 410–418. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Z.; Xie, Y.; Jin, S. Effects of Vestibular Rehabilitation Training Combined with Anti-Vertigo Drugs on Vertigo and Balance Function in Patients with Vestibular Neuronitis: A Systematic Review and Meta-Analysis. Front. Neurol. 2023, 14, 1278307. [Google Scholar] [CrossRef]
- Sun, X.; Li, X.; Yang, D. Efficacy and Safety of Mecobalamin Combined with Vestibular Rehabilitation Training for Acute Vestibular Neuritis: A Systematic Review and Meta-Analysis. Ann. Palliat. Med. 2022, 11, 480–489. [Google Scholar] [CrossRef]
- Jacobson, G.P.; Newman, C.W.; Kartush, J.M. Handbook of Balance Function Testing; Singular Publishing Group: San Diego, CA, USA, 1990. [Google Scholar]
- Kraus, M.; Hassannia, F.; Bergin, M.J.; Al Zaabi, K.; Barake, R.; Falls, C.; Rutka, J.A. Post Headshake Nystagmus: Further Correlation with Other Vestibular Test Results. Eur. Arch. Otorhinolaryngol. 2022, 279, 3911–3916. [Google Scholar] [CrossRef]
- Gkoritsa, E.Z. Recovery Nystagmus in Vestibular Neuritis with Minimal Canal Paresis. Clinical Observation and Interpretation. Brain Sci. 2022, 12, 110. [Google Scholar] [CrossRef]
- Alhabib, S.F.; Saliba, I. Reliability of Monothermal Caloric Test as Screening Test of Vestibular System. J. Clin. Med. 2022, 11, 6977. [Google Scholar] [CrossRef]
- Gufoni, M.; Casani, A.P. The Role of Vestibular Cold Caloric Tests in the Presence of Spontaneous Nystagmus. Acta Otorhinolaryngol. Ital. 2023, 43, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Torres-Carretero, L.; Otero-Rodríguez, Á.; Alejos-Herrera, M.V.; Vázquez-Casares, G.; García-Martín, A.; Garrido-Ruiz, P.A. Utility of the intraoperative neurophysiological monitoring as a prognostic value of postoperative facial paresis in vestibular schwannomas. Neurocirugia 2023, 34, 238–246. [Google Scholar] [CrossRef]
- Horak, F.B.; Diener, H.C. Romberg Test Revisited: Clinical Significance and Modifications. Neurotherapeutics 2014, 11, 544–552. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Smith, W.J. Romberg’s sign revisited: A critical evaluation of the historical background and current usage. J. Rehabil. Med. 1987, 19 (Suppl. S1), 23–28. [Google Scholar] [CrossRef]
- Shumway-Cook, A.E.; Woollacott, M.H.; Collins, D.E. Static Posture: Theory and Practical Applications; Mosby Elsevier: Maryland Heights, MO, USA, 2013. [Google Scholar]
- Khedmat, A.A.; Mohammed, A.; Al-Khalafah, M.A.; Al-Anazi, F.A.; Al-Hajeri, M.A.; Al-Qahtani, A.A.; Al-Zahrani, A.M. Comparison of Romberg’s test and Berg Balance Scale in patients with spinocerebellar ataxia type 3. J. Phys. Ther. Sci. 2017, 29, 3161–3164. [Google Scholar] [CrossRef]
- Morita, T.; Yasushi, N.; Takeshi, N.; Yoshiki, I.; Yuki, O.; Yasuhiro, H. Evaluation of Romberg’s test for diagnosis of multisystem atrophy: Comparison with functional reach test and timed up and go test. Eur. J. Neurol. 2016, 23, 122–128. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.Y.; Choi, M.S.; Cho, S.H. Usefulness of Romberg’s test for the detection of vestibular dysfunction in patients with Menière’s disease. Int. J. Otolaryngol. 2017, 11, 1–7. [Google Scholar] [CrossRef]
- Halmágyi, G.; Curthoys, I.S. Rapid head turning elicits nystagmus only if the semicircular canal is irrigated. Ann. Otol. Rhinol. Laryngol. 1988, 97, 1–6. [Google Scholar] [CrossRef]
- Herdman, S.G.; Zhang, X.; Lempert, T.; David, N.-T.; Yuri, A. Quantitative assessment of vestibular ocular reflex function using the video head impulse test. Otol. Neurotol. 2012, 33, 1–11. [Google Scholar] [CrossRef]
- Thomas, B.; Dietrich, D.; Strupp, M.; Bense, J.; Mast, H.-J. Automated quantification of spontaneous and gaze evoked nystagmus by means of digital video recordings. Acta Otolaryngol. 1996, 116, 365–371. [Google Scholar] [CrossRef]
- Thiery, J.-P.; Claudio, L.; Cohen, F.G.; Semont, A.; Chays, M. Visual acuity during active and passive head movement: A novel test to evaluate the interaction between the vestibular and oculomotor systems. Investig. Ophthalmol. Vis. Sci. 2012, 53, 156–162. [Google Scholar] [CrossRef]
- Roll, J.; Stoffregen, T.A. Spatial Orientation: From Perception to Action; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Summers, S.M.; Clarke, L.A.; McCloskey, E.V. Interobserver agreement for the interpretation of results obtained from the head thrust test. Am. J. Audiol. 2008, 17, 11–19. [Google Scholar] [CrossRef]
- Wu, W.-C.; Tsai, M.-Y.; Huang, Y.-T.; Liu, C.-Y.; Chen, C.-C. Objective assessment of the horizontal and vertical halmagyi-curthoys head impulse test using electromyography and electrooculography. J. Clin. Neurol. 2019, 15, 121–126. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, C.-H. Vestibular Schwannoma Presenting as Acute Vertigo Mimicking Vestibular Neuritis. Case Rep. Neurol. 2022, 14, 464–468. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajamani, S.K.; Iyer, R.S.; Venkatraman, A. Comparison of Halmágyi–Curthoys Head Impulse (Thrust) Test with Romberg’s Test in Detection of Vestibular Hypofunctioning in Vertigo Patients. J. Otorhinolaryngol. Hear. Balance Med. 2024, 5, 4. https://doi.org/10.3390/ohbm5010004
Rajamani SK, Iyer RS, Venkatraman A. Comparison of Halmágyi–Curthoys Head Impulse (Thrust) Test with Romberg’s Test in Detection of Vestibular Hypofunctioning in Vertigo Patients. Journal of Otorhinolaryngology, Hearing and Balance Medicine. 2024; 5(1):4. https://doi.org/10.3390/ohbm5010004
Chicago/Turabian StyleRajamani, Santhosh Kumar, Radha Srinivasan Iyer, and Anusha Venkatraman. 2024. "Comparison of Halmágyi–Curthoys Head Impulse (Thrust) Test with Romberg’s Test in Detection of Vestibular Hypofunctioning in Vertigo Patients" Journal of Otorhinolaryngology, Hearing and Balance Medicine 5, no. 1: 4. https://doi.org/10.3390/ohbm5010004
APA StyleRajamani, S. K., Iyer, R. S., & Venkatraman, A. (2024). Comparison of Halmágyi–Curthoys Head Impulse (Thrust) Test with Romberg’s Test in Detection of Vestibular Hypofunctioning in Vertigo Patients. Journal of Otorhinolaryngology, Hearing and Balance Medicine, 5(1), 4. https://doi.org/10.3390/ohbm5010004





