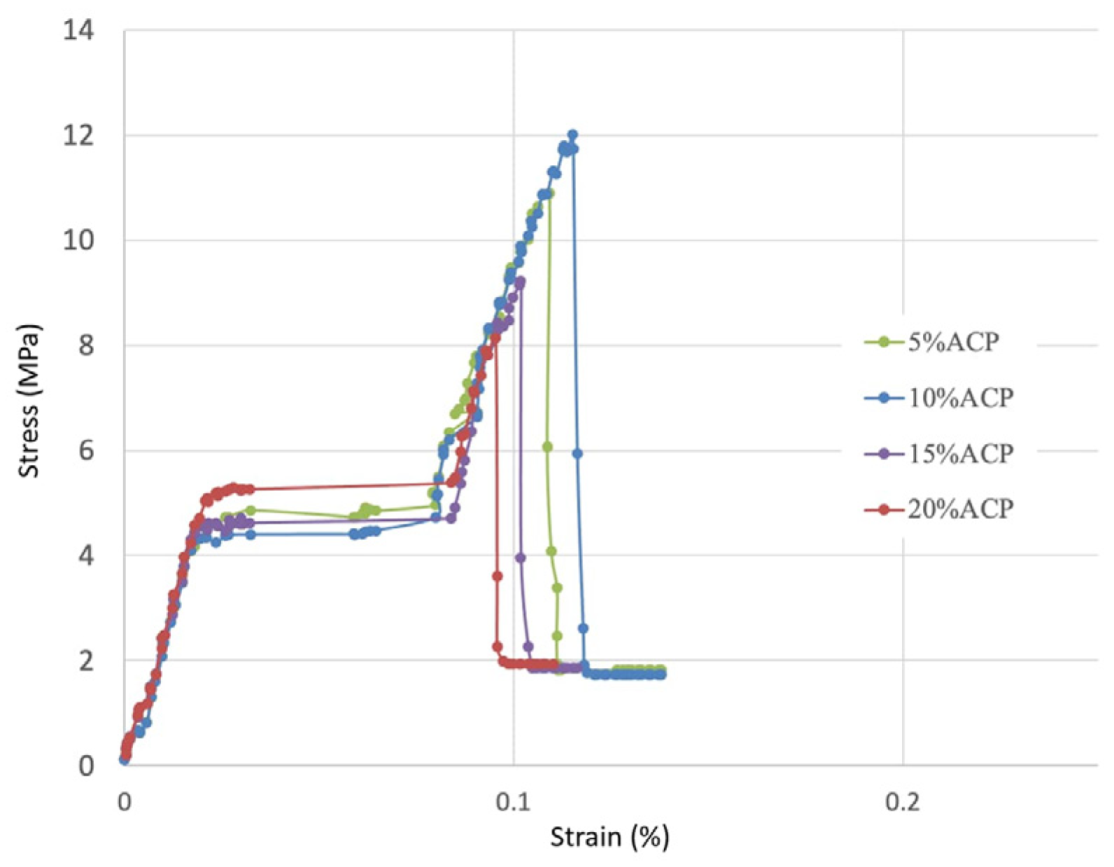
1. Introduction
Accidental and sports-related injuries often result in bone fractures and ligament damage that require immediate stabilization to promote proper healing and reconstruction [
1]. Traditionally, bone screws manufactured from metals—such as titanium alloys, pure titanium, stainless steel, and cobalt–chromium alloys—have been employed to achieve this stabilization [
2,
3]. Despite their excellent mechanical strength, these metallic implants exhibit significant clinical drawbacks. For instance, the wear of the oxidation layer can lead to metal ion release, inciting tissue inflammation and sometimes necessitating secondary removal surgeries, which not only cause patient discomfort but also waste medical resources [
4]. In addition, the high Young’s modulus of metals can result in stress shielding and subsequent bone resorption, a particularly serious problem in pediatric patients where impaired load distribution may affect future bone growth [
5].
To overcome these limitations, the move toward biodegradable alternatives has gained momentum. Magnesium, an essential element with high biocompatibility and degradability, has been investigated as an implant material because its Young’s modulus (40–45 GPa) is closer to that of human bone (5–17 GPa) [
6,
7]. However, when used in biodegradable alloys, magnesium tends to degrade too rapidly in vivo, undermining its long-term stability as an implant [
8].
Polymeric materials such as polylactic acid (PLA) have also been introduced in the manufacture of bone screws. Several companies—including Taiwan’s Union Biomedical and the U.S.-based Arthrex and Biosteon—have developed PLA-based screws that boast good biocompatibility and sufficient mechanical strength for ligament repair [
9]. Nevertheless, PLA presents its own set of clinical challenges. Reports have identified issues with PLA implants, including disintegration, osteolysis, chondrolysis, cyst formation, and allergic reactions [
9]. Moreover, the inherent brittleness of PLA makes it susceptible to rupture after implantation, potentially leading to the inadequate healing of ligament injuries if timely intervention is not pursued [
10,
11].
To address PLA’s brittleness, polycaprolactone (PCL)—a ductile polymer—can be blended with PLA to improve the overall ductility and reduce the risk of cracking. Research by Hassanajili et al. [
12] suggests that PLA/PCL composites not only enhance mechanical performance but also benefit from the incorporation of biomedical ceramics that promote bone cell proliferation and attachment, thereby enhancing integration with host bone.
Calcium phosphates (CaPs) have long been used as bioactive ceramics for bone repair and regeneration. In particular, hydroxyapatite (HAp) closely mimics the inorganic phase of bone due to its similar structure and composition [
13,
14]. However, conventional crystalline HAp often experiences excessive grain growth during high-temperature processing, which diminishes its biological activity. In contrast, amorphous calcium phosphate (ACP)—synthesized via low-temperature wet chemical methods—produces microscale-to-nanoscale particles with higher solubility, leading to improved bioactivity, biodegradability, and osteoblast adhesion [
15].
Advances in micromolding technology further contribute to this field by enabling the fabrication of intricately designed, high-precision biodegradable bone screws that meet rigorous clinical demands [
16]. Integrating nanoscale ACP into PLA/PCL composites via these precise molding techniques represents a cutting-edge strategy for optimizing both mechanical performance and osseointegration [
17]. Recent investigations have also provided valuable insights into PLA’s biodegradation behavior [
18], the microstructure-based magneto-mechanical modeling of composite systems [
19], and composite formulation strategies extrapolated from studies on supplementary cementitious materials [
20]. Furthermore, incorporating ACP into biodegradable polymer matrices has been shown to enhance osteogenic differentiation and bone regeneration capabilities [
21].
Collectively, these studies underscore the critical importance of biocompatibility, mechanical integrity, and controlled degradation in the development of advanced biomaterials for orthopedic applications. Building on this foundation, this study aims to develop biodegradable bone screws composed of a PLA/PCL blend reinforced with ACP. Utilizing low-temperature wet chemical synthesis to produce bioactive ACP and employing injection molding technology, this study endeavors to create a novel, safe, effective, and cost-efficient bone screw implant. The ultimate goal of this study is to enhance the treatment of bone fractures and ligament injuries while reducing patient discomfort and minimizing the need for secondary interventions.
2. Materials and Methods
2.1. Synthesis of Nanoscale Amorphous Calcium Phosphate
Somrania et al. [
22] proposed a low-temperature wet synthesis method for amorphous calcium phosphate (ACP). To begin, a 0.36 M nitrate solution and a 0.16 M phosphate solution were prepared, each containing 40 mL of 28 wt.% ammonium hydroxide (NH
4OH). The nitrate solution was added to the phosphate solution, resulting in the rapid precipitation of ACP. This mixture was then immediately poured into 3 L of deionized water (containing 15 mL of 28 wt.% NH
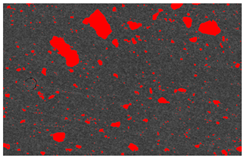
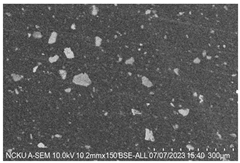
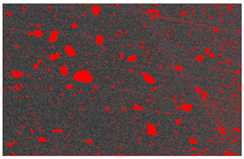
4OH) to inhibit further crystallization. After washing, the solution was divided into bottles and centrifuged to separate the powder from the solution based on density differences. The excess water was decanted, and the ACP was extracted and frozen at −18 °C to prevent crystallization during storage. The frozen ACP was then freeze-dried at −40 °C and 1.3 Pa to sublimate the ice crystals, resulting in the ACP powder. The ACP powder is shown in
Figure 1.
2.2. Preparation of Composite Material
A mixture of 80 wt.% PLA and 20 wt.% PCL exhibited superior mechanical properties [
23,
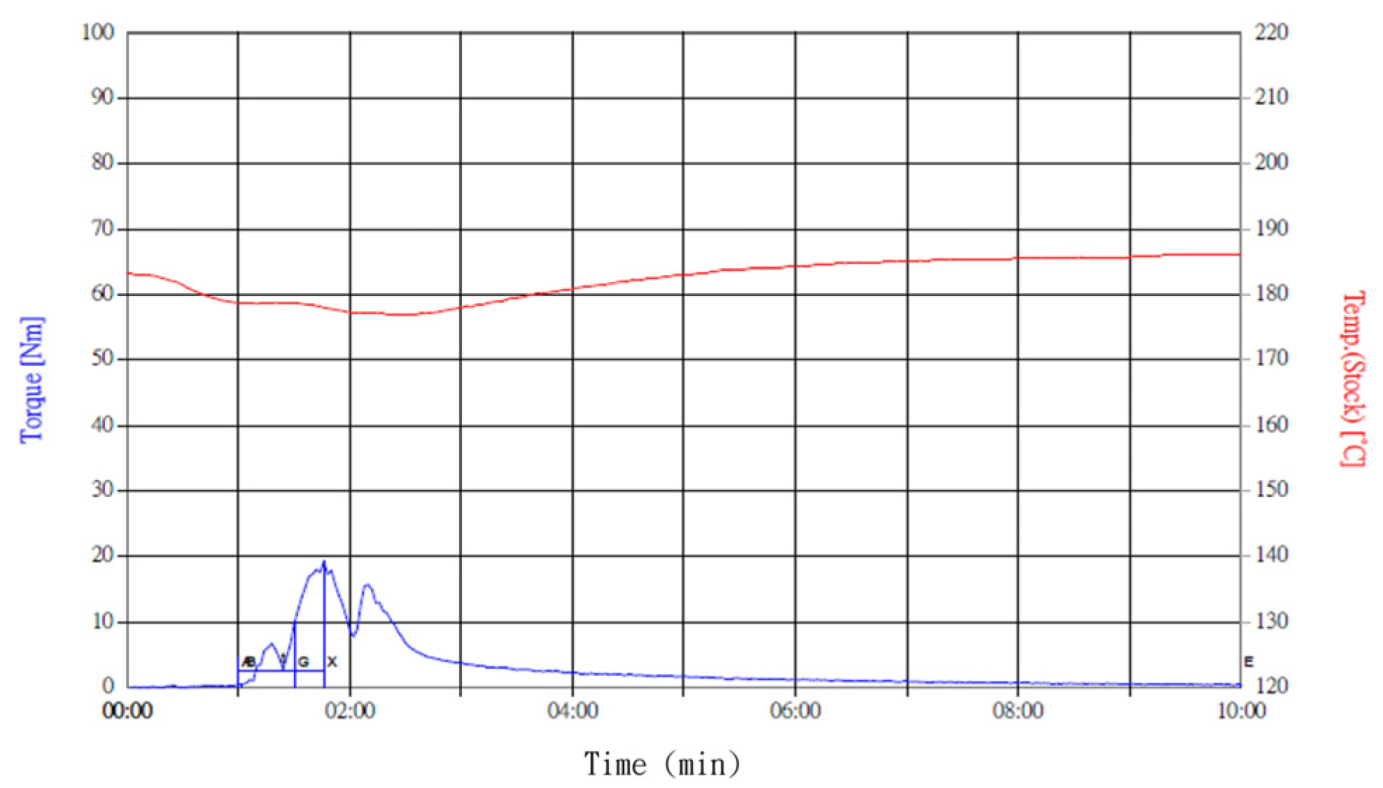
24]; thus, this ratio was used as the polymer composite base in this study. The ACP powder was added in amounts ranging from 5 wt.% to 30 wt.% for experimental testing. PLA (Corbion Purac Group, Luminy@L175)/PCL (Perstorp, Skåne, Sweden, Capa 6800)/ACP composites were mixed using a Brabender mixer (Plasti-Corder Lab-Station, Duisburg, Germany) at 190 °C and 50 rpm for 10 min. Torque and temperature curves were recorded to determine the uniformity of the material mixing, as shown in
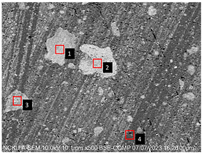
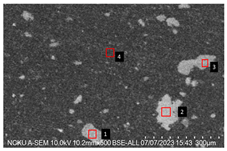
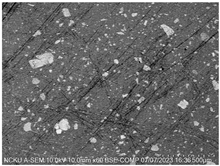
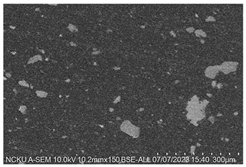
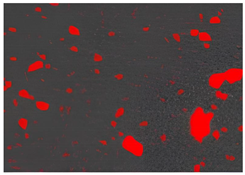
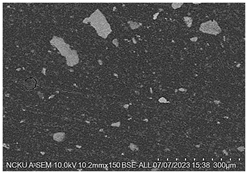
Figure 2. Cross-sectional analysis was conducted on the blended materials and injection-molded bone screws to evaluate the uniformity.
2.3. Material Characterization
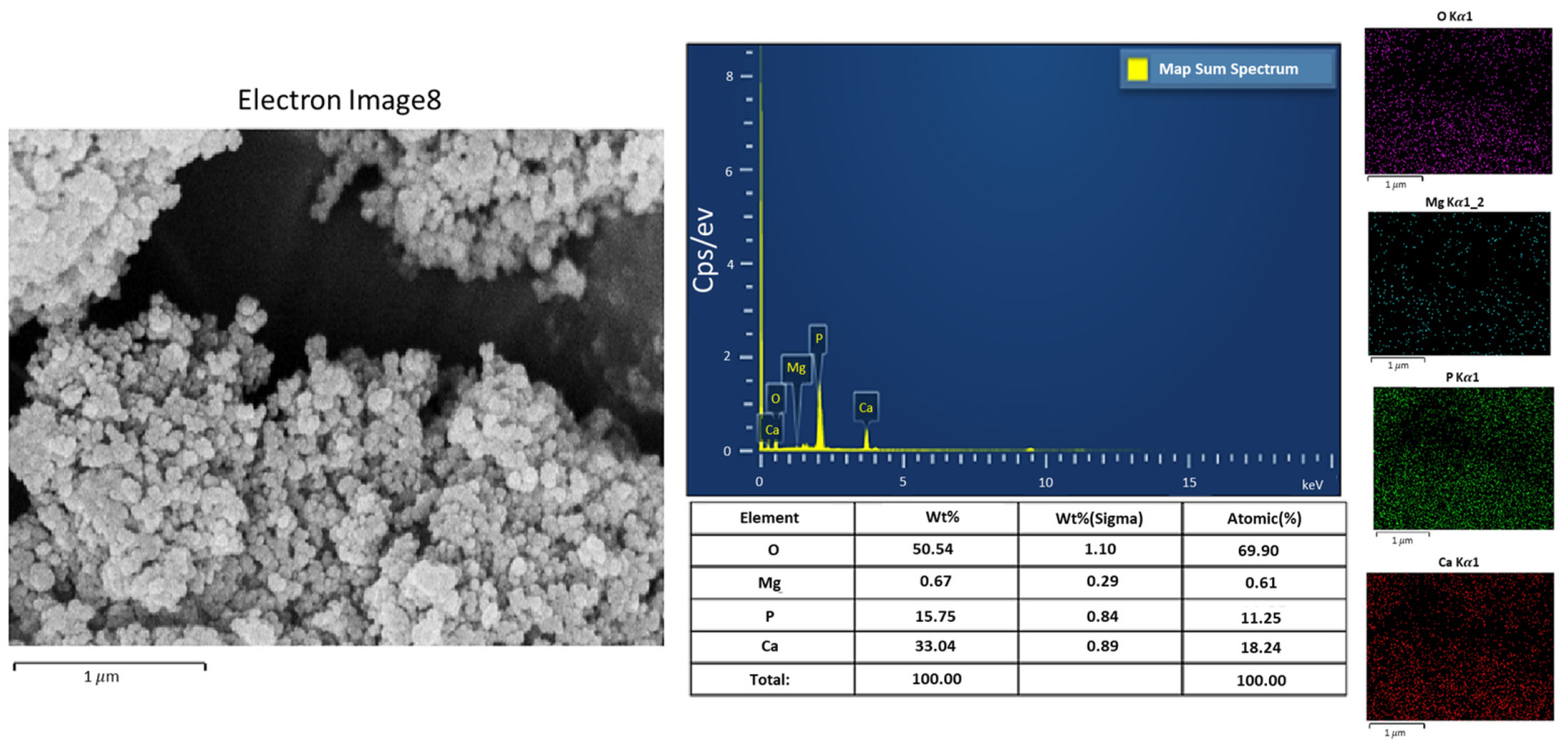
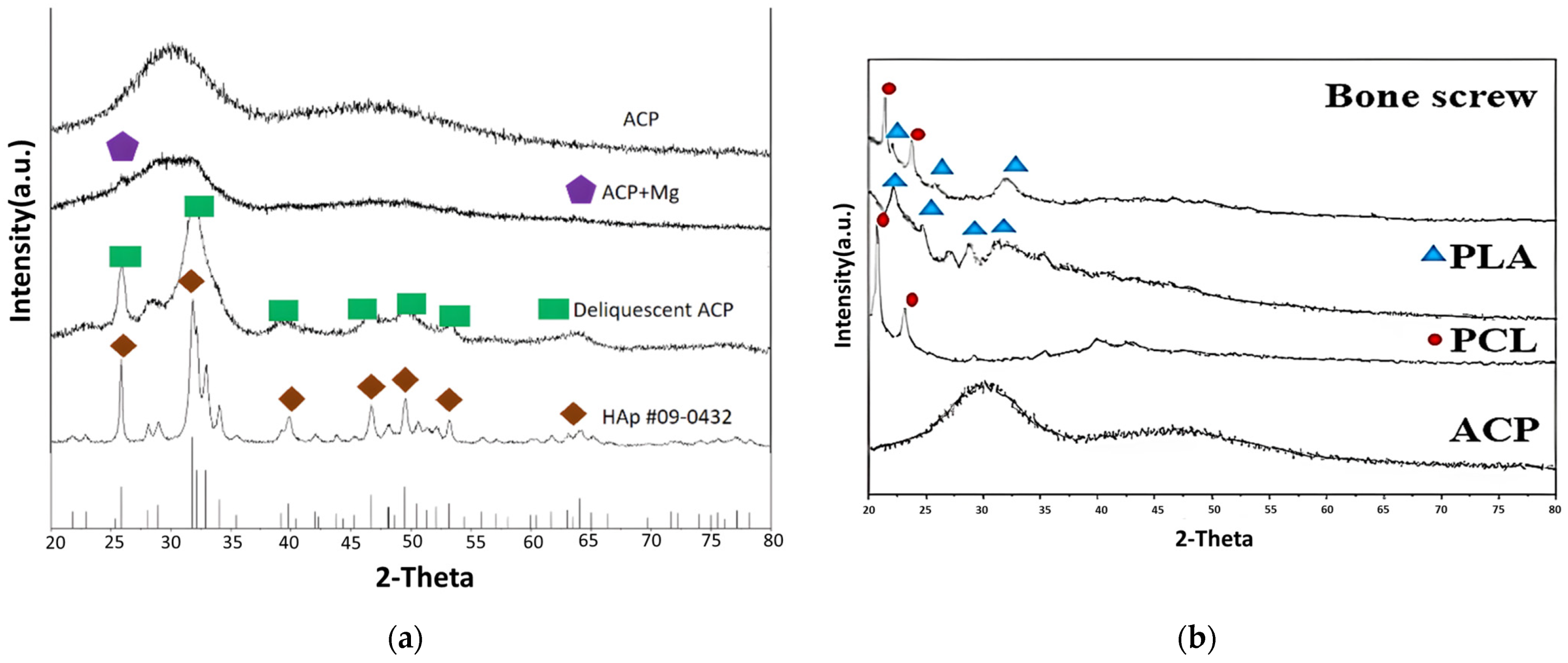
This study used a scanning electron microscope (SEM) to observe the surface characteristics of the ACP powder. To enhance conductivity, the non-conductive synthesized powder was coated with gold using a vacuum sputter coater at 20 mA for 60 s. Energy-dispersive X-ray spectroscopy (EDX) was then employed to analyze its elemental composition. X-ray diffraction (XRD) was used to analyze the crystal structure, chemical composition, and physical properties of the synthesized material. To confirm the material was ACP, XRD was conducted with a 2θ angle range of 10° to 100°, operating at 30 kV and 20 mA.
2.4. Specimen Molding for Tensile Test
For this test, tensile specimens were prepared according to the ASTM-D638 [
25]. The specimens measured 63.5 mm in length, 9.53 mm in width, and 3.2 mm in thickness [
25]. A compression molding machine (Ling Fong 30 Ton Hot Press, Tainan, Taiwan) was used for specimen preparation. The material mixed by the Brabender mixer was cut into appropriate sizes, placed in the mold cavity, melted at a mold temperature of 200 °C, and compressed at a pressure of 25 kg/cm
2 for 10 min. Afterward, the mold temperature was cooled to 60 °C using a water circulation system.
2.5. Injection Molding Experiment
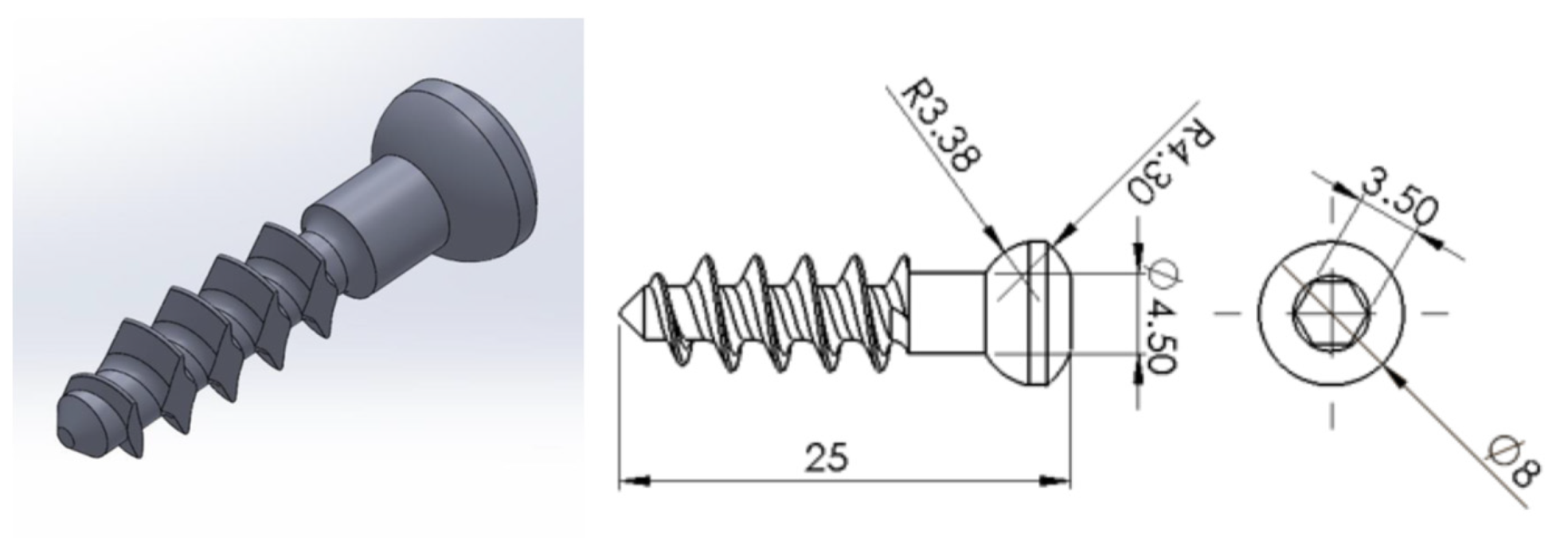
The bone screw design is shown in
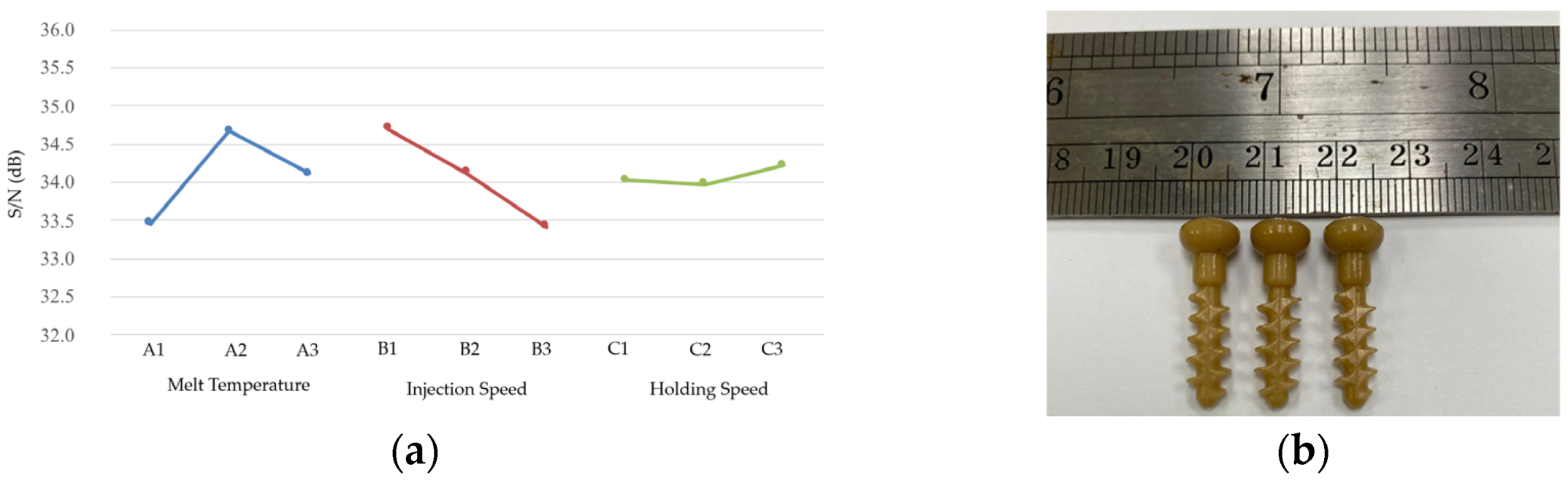
Figure 3. This study utilized a Battenfeld Microsystem 50 micromolding machine for injection molding and employed the Taguchi experimental method to determine the optimal injection molding parameters and their effect on the shrinkage of bone screw injection molding. The control factors selected for the experiment were the melt temperature, injection speed, and holding speed. Before the experiment, Moldex3D analysis was used to determine the fixed parameter values. During actual injection, the processing window was established, the control factor level values were set, and the Taguchi experiment was conducted. The shrinkage rate of the molded bone screw size served as the quality indicator. A smaller shrinkage rate indicated better quality; thus, the experiments were conducted with the objective of achieving the smallest possible shrinkage.
2.5.1. Mold Filling Speed Parameter Setting
From a fluid mechanics perspective, the faster a fluid flows, the greater the resistance it must overcome, resulting in higher pressure requirements. Polymers are non-Newtonian fluids with a shear-thinning effect; as speed increases, shear rate increases and viscosity decreases. Therefore, the injection pressure does not necessarily increase with higher injection speeds. This study utilized different injection speeds to analyze the mold filling flow. As shown in
Figure 4, at an injection speed of 60 mm/s, the required injection pressure was lower. Consequently, 60 mm/s was selected as the starting parameter value for experiments with actual injection molding.
2.5.2. Holding Time Parameter Setting
One criterion for setting the holding time is to adjust it according to the solidification time of the melt at the gate. If pressure holding stops before the melt solidifies, the holding effect is poor and the product shrinks. Conversely, if pressure is maintained after the melt solidifies, it can lead to excessive pressure holding and residual stress. Moldex3D was used to simulate the holding process under conditions of 60 mm/s injection speed, 190 °C melt temperature, and 30 °C mold temperature. As shown in
Figure 5, the gate had not solidified at 2.965 s of packing but had solidified at 3.146 s of holding. Therefore, 4 s was selected as the holding time in the subsequent Taguchi experiment.
2.5.3. Cooling Parameter Settings
The typical mold temperature setting for molding PLA is 20–30 °C [
26] to ensure sufficient cooling. To avoid warping caused by uneven shrinkage after ejection, it is recommended to cool to a temperature difference of less than 10 °C in the part before ejection [
27]. From the mold flow analysis results in
Figure 6, it is evident that, with a cooling time of 35 s, the maximum temperature difference in the mold cavity is 6.1 °C. Therefore, the cooling time for the subsequent molding experiment is set to 35 s.
2.5.4. Processing Window
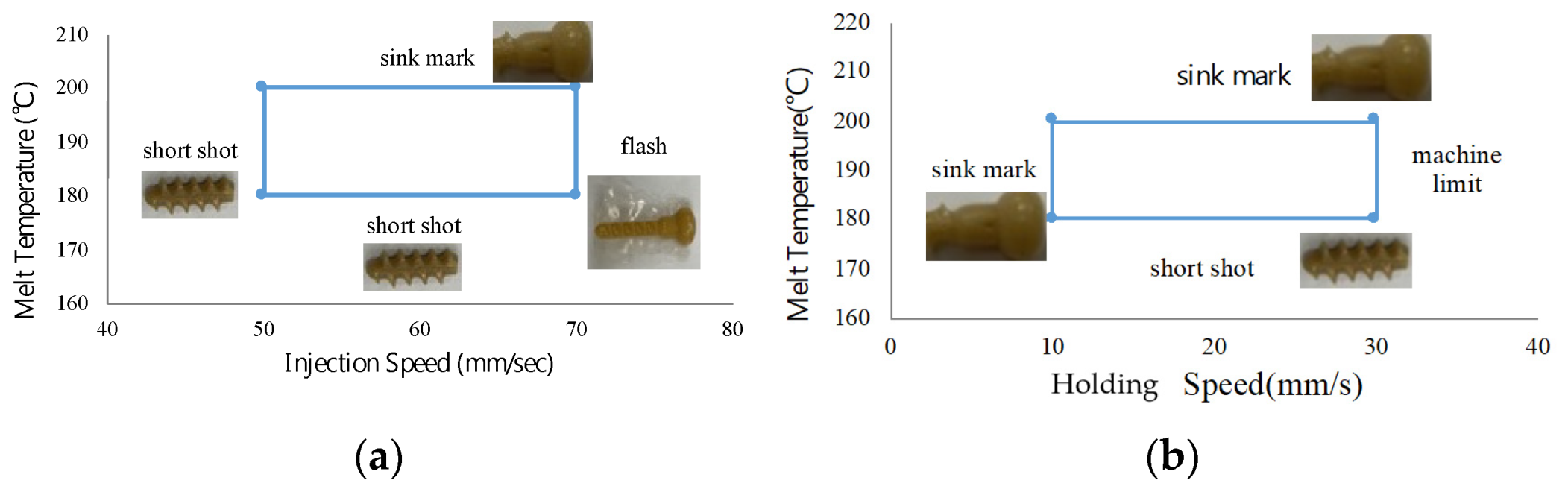
Before planning the Taguchi experiment, this study first identified the processing windows of the control factors through trial molding, as shown in
Figure 7. For the PLA used in this study, a melting temperature of 180–220 °C is recommended [
26]. In the molding test, when the material temperature is below 180 °C, short shots occur due to premature cooling. Conversely, temperatures above 200 °C result in defects such as sink marks and burrs. The injection pressure–injection speed analysis in
Figure 4 indicates that an injection speed of 60 mm/s has a relatively low injection pressure. Therefore, the injection speed processing window experiment started from 60 mm/s. At speeds lower than 50 mm/s, short shots occur, whereas speeds exceeding 70 mm/s result in flash formation on the bone screw. The pressure-holding process of the Microsystem 50 micro injection machine is controlled by the plunger speed rather than pressure, unlike general injection machines. When the holding speed is below 15 mm/s, effective holding cannot be achieved, leading to sink marks on the bone screw surface. However, well-molded bone screws were obtained at all settings from 15 mm/s to the machine’s maximum hold speed of 33 mm/s.
2.5.5. Parameter Settings for Bone Screw Molding Process
The processing window experiment results suggest that the range of the three Taguchi experimental control factors are as follows: a material temperature between 180 and 200 °C, an injection speed between 50 and 60 mm/s, and a holding speed between 10 and 33 mm/s. Each factor has three levels within this processing range, with the highest holding speed set at 30 mm/s.
Table 1 shows the level values of each factor.
The mold flow analysis results in
Figure 5 and
Figure 6 suggest that a holding time of 4 s, a cooling time of 35 s, and a back pressure of 15 bar can avoid bubbles in the finished product. During the Taguchi experiment, the metering amount and the plasticizing screw speed were kept constant, and
Table 2 presents the values of these parameters. Since each of the three control factors has three levels in this experiment, the L9 orthogonal table was used for the Taguchi experiment, as shown in
Table 3.
2.5.6. Taguchi Experiment
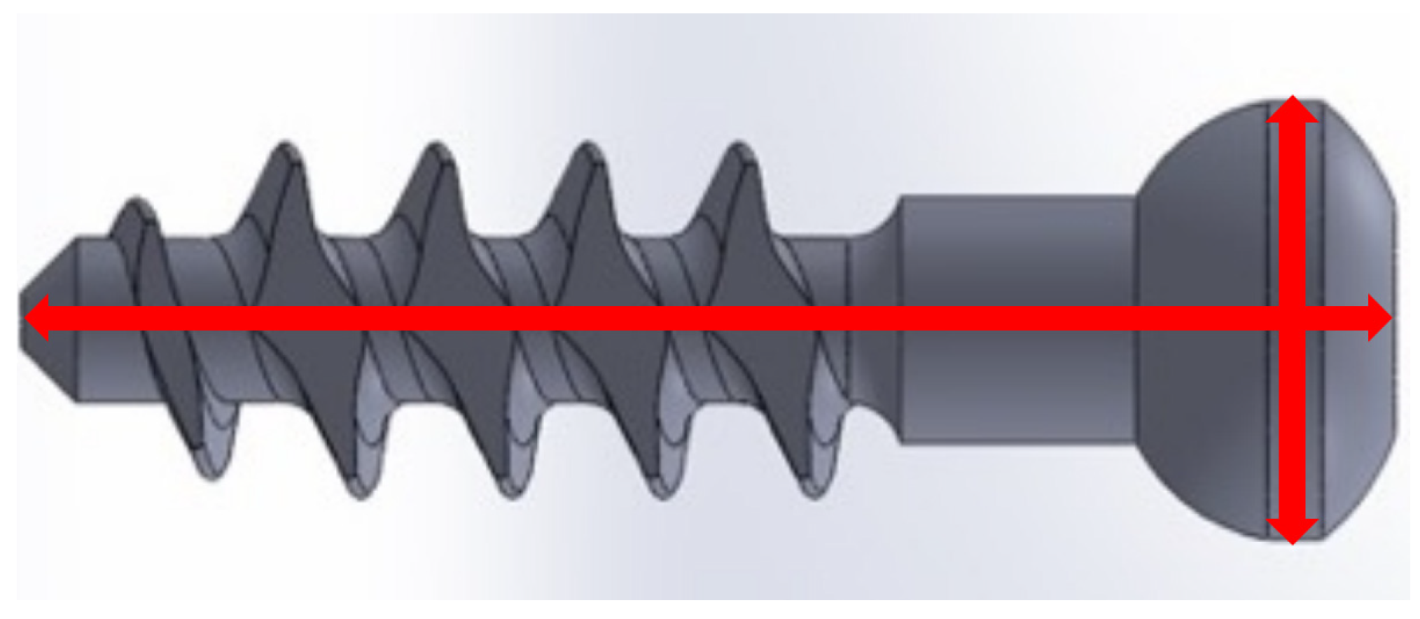
To ensure the optimal bone screw injection molding quality, a 30 min preheating step was performed before the injection molding experiments to stabilize the molding environment. Five bone screws were sampled for each set of molding parameters, with their lengths and outer diameters measured as illustrated in
Figure 8. The mold used for producing the bone screws has a length of 25.009 mm and a head diameter of 8.004 mm. The dimensions of the molded bone screws were compared to those of the mold to calculate the length and radial shrinkage rates using Equation (1). Based on the shrinkage rate, the S/N ratio for each Taguchi group was calculated using Equation (2).
where
yi is the quality characteristic of the
i-th sample, and
n is the number of samples.
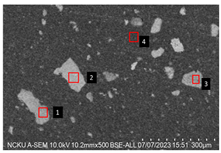
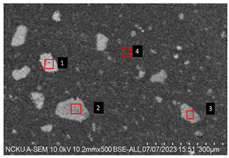
2.5.7. Analysis of Bone Screw Internal Structure
To evaluate the uniformity of ACP powder distribution after injection molding, cross-sectional studies were conducted on the molded bone screws. The bone screws were embedded in BUEHLER cold mounting resin (SamplKwick 20-3560) and prepared for metallographic analysis. Sandpaper with grit sizes ranging from P120 to P4000 was used for grinding, followed by surface polishing. Due to the non-conductive nature of polymer bone screws, gold sputtering was performed on the polished surfaces to facilitate the subsequent SEM analysis.
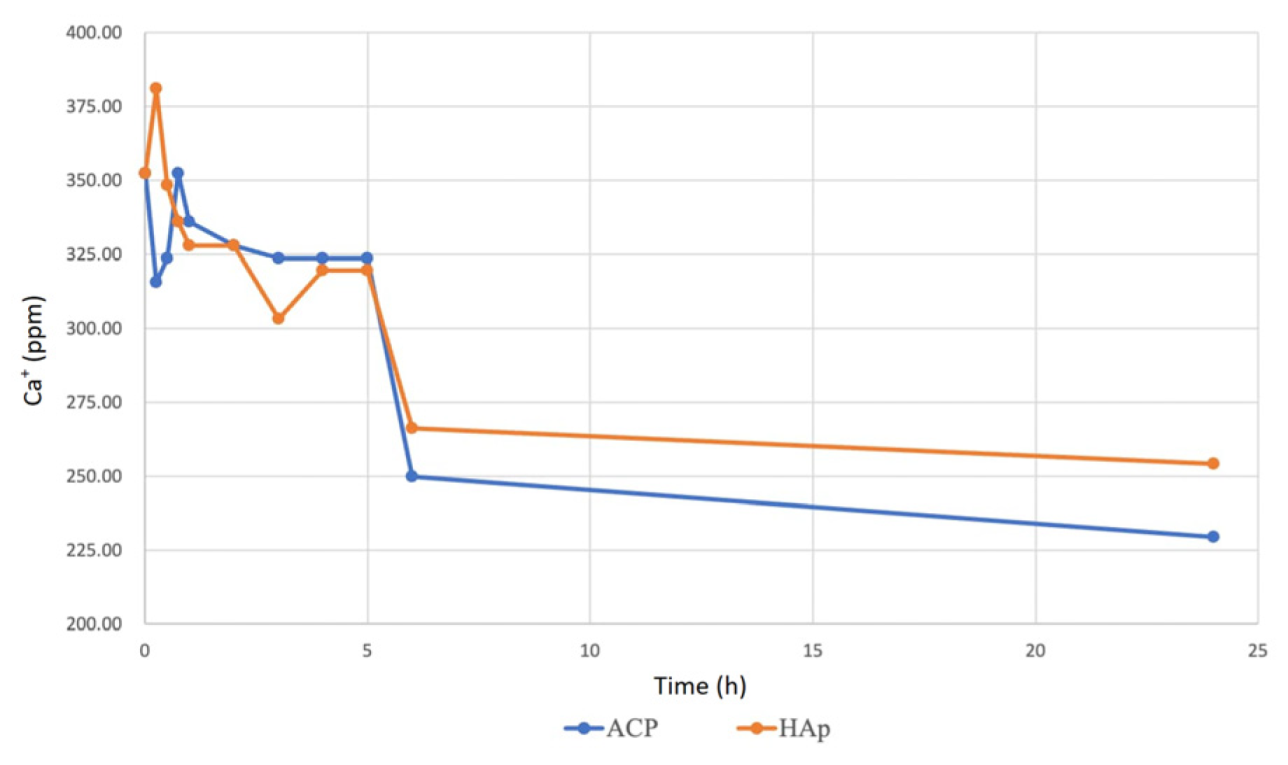
2.6. Water Hardness Test for Calcium Ion Release
This study used water hardness testing to measure calcium ion (Ca⁺) release concentrations from ACP and HAp immersed in simulated body fluid (SBF). ACP and HAp were immersed in an SBF buffer solution containing an indicator that turned wine red. The ethylenediaminetetraacetic acid (EDTA) disodium salt solution was titrated into the sample solution using a burette. When all Ca⁺ ions were chelated, the solution color changed from red to blue, indicating the titration endpoint.
To prepare the standard calcium solution, 0.1 g of anhydrous calcium carbonate was dissolved in 1 M hydrochloric acid, followed by the addition of 100 mL of ultrapure water to achieve a 1 mg/mL (1000 ppm) concentration. The EDTA titration solution was prepared by dissolving 0.14612 g of EDTA in 500 mL of ultrapure water to obtain a 0.001 M solution.
The calcium content of the EDTA solution was determined using Equation (3):
The calcium concentration of ACP and HAp solutions was calculated based on the volume of EDTA titrated, as shown in Equation (4):
2.7. Biocompatibility, Bone Differentiation, and Bone Mineralization Ability Tests
Following the ISO 10993-5 [
28], materials were immersed in bone cell culture media (low-glucose DMEM supplemented with 100 µg/mL ascorbic acid, 10 µg/mL non-essential amino acids (L-Glutamine), 100 µg/mL penicillin/streptomycin, 10% fetal bovine serum (FBS), and 0.01% vitamin C) at 37 °C for 72 h in a shaking water bath to collect the extract solution. NIH/3T3 fibroblast cells (mouse fibroblast cell line) and mouse bone marrow cells (D1 cell line) were used for testing.
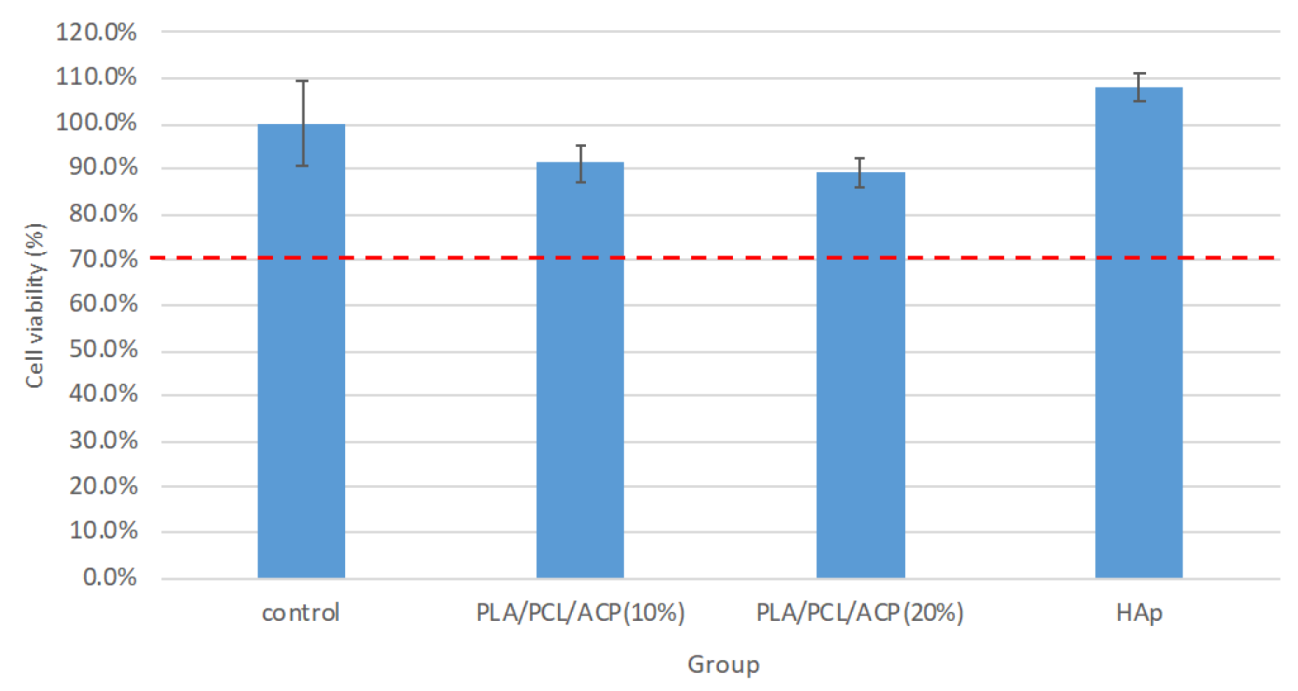
Biocompatibility Test (MTT Assay): Each well of a sterile 96-well plate was seeded with 1 × 104 cells in 100 µL of culture medium. After adding 100 µL of the extract solution, the plate was incubated at 37 °C and 5% CO2 for 24 h. Following incubation, 100 µL of MTT solution was added to each well, and the plate was incubated for an additional 2 h. MTT reacted with mitochondrial enzymes of live cells, producing purple-blue formazan crystals, which were then dissolved in DMSO to form a purple solution. The absorbance of this solution was measured at 570 nm using an ELISA reader, with higher absorbance values indicating better biocompatibility.
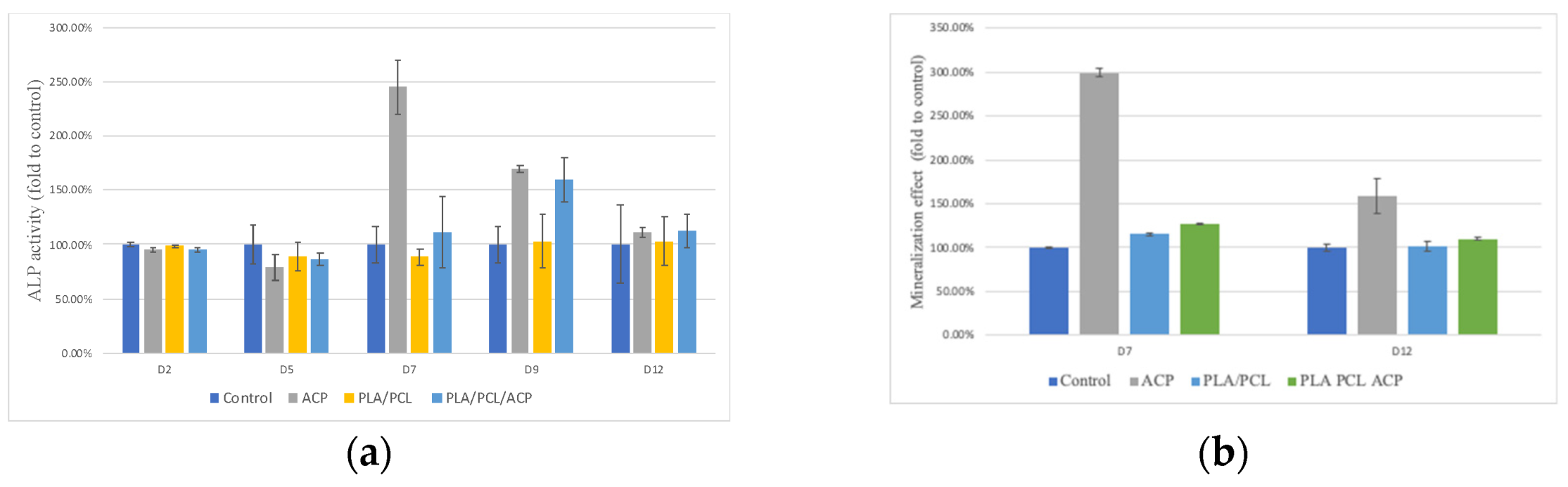
Bone Differentiation Ability Test (ALP Assay): A 48-well plate was prepared with 2 × 104 cells in 200 µL of culture medium. After 24 h, the supernatant was removed and replaced with the extract solution. Following a further 24 h of cell culture, osteoinduction medium (OIM) was introduced to promote cell differentiation and mineralization. After 48 h of culture with OIM, 80 µL of the tissue culture fluid was transferred to a 96-well sterile plate, and 50 µL of phosphate solution was added to react at room temperature in the dark for 1 h. The alkaline phosphatase activity was measured using Biovision’s ALP assay reagent, with the absorbance reading at 405 nm using an ELISA reader. Higher absorbance values indicated higher alkaline phosphatase activity, and the relative percentage of activity was calculated by setting the control group’s measured value as 100%.
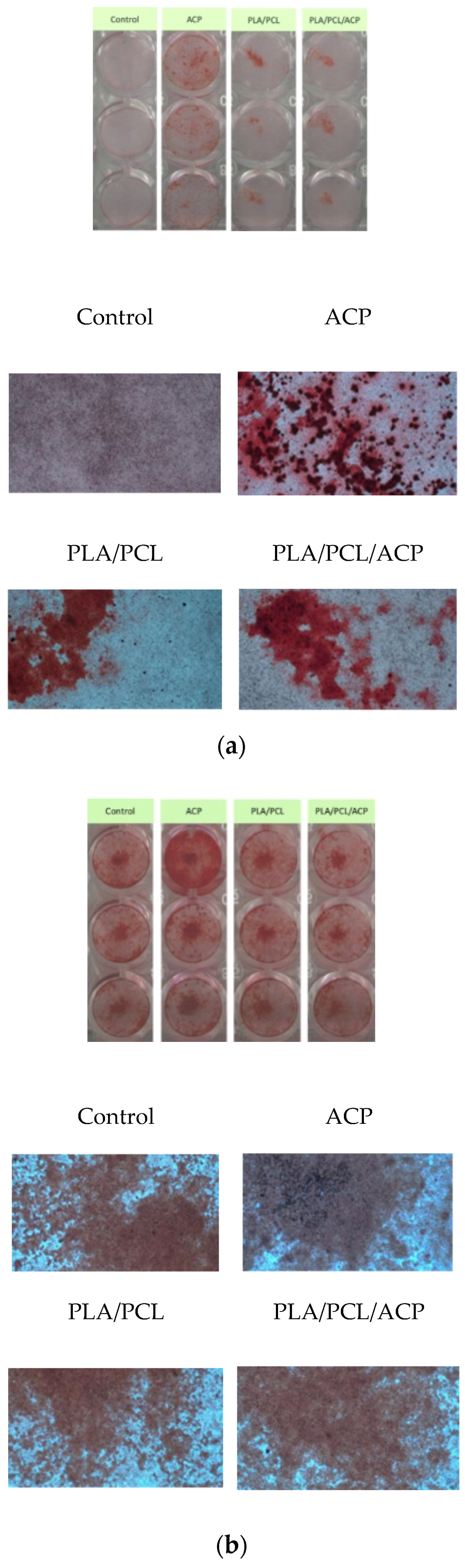
Bone Mineralization Ability Test: Sterile 48-well plates were prepared with 2 × 104 cells per well in 200 µL of culture media. After 24 h of incubation, the supernatant was removed and replaced with the extract solution. The extract solution and cells were incubated for 24 h, followed by the addition of osteoinduction medium (OIM) to promote bone differentiation and mineralization. After washing with phosphate-buffered saline (PBS), the cells were immersed in 10% formalin for 20 min, then washed twice with DI water and stained with Alizarin Red S (ARS) for 10 min. The cells were rinsed with purified water 2-3 times and allowed to dry. Alizarin red stains calcium deposits, forming an orange-red precipitate. Once dried, 200 µL of 10% acetic acid was added to each well, and 100 µL was transferred to a 96-well sterile plate. The absorbance was measured at 405 nm using an ELISA reader (Thermo Fisher Scientific, Waltham, MA, USA). The absorbance value of the tissue culture medium group served as the reference value, and the calcium deposition ability was judged by the absorbance value of the test groups relative to the reference value, with higher absorbance values indicating greater calcium deposition and better bone mineralization induction ability.
2.8. Degradation Test
This test followed the ASTM F1635-11 [
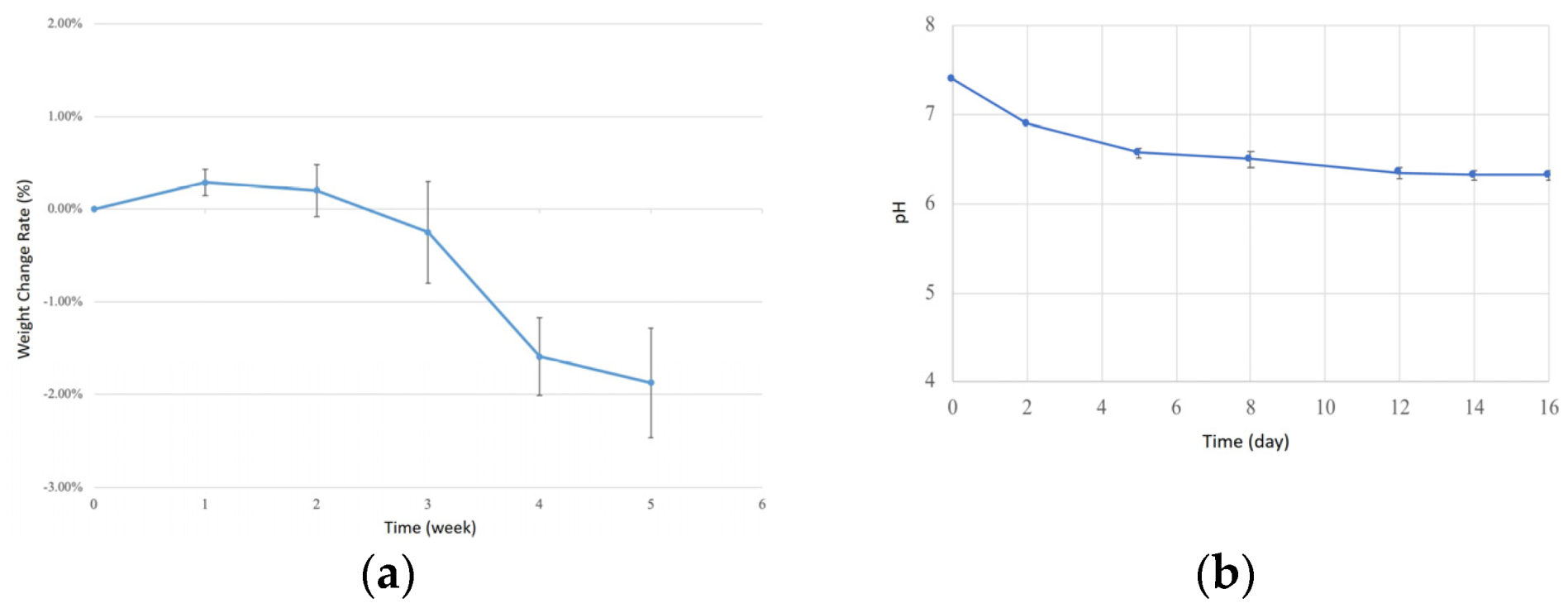
29] to evaluate the degradation of injection-molded bone screws. Bone screws were placed in centrifuge tubes filled with simulated body fluid (SBF) and incubated in a shaking water bath at 37 °C, with agitation set to 10 rpm. Weight and pH changes were recorded weekly for four weeks. Before weighing, the bone screws were dried in an oven at 60 °C for 2 h to remove internal moisture and minimize measurement errors. The collected data were used to plot degradation and pH curves.
To prepare the simulated body fluid (SBF), reagents were sequentially added to 900 mL of ultrapure water using a magnetic stirrer for complete dissolution, following Oyane et al. [
30]. The reagents were added in the following order and amounts (g/L): NaCl (8.035), NaHCO
3 (0.355), KCl (0.225), K
2HPO
4·3H
2O (0.231), MgCl
2·6H
2O (0.311), 1 M HCl (19.5), CaCl
2 (0.292), Na
2SO
4 (0.072), and TRIS (6.118). The solution was then adjusted to a pH of 7.40 at 36.5 °C using 1 M HCl and diluted to a final volume of 1000 mL with ultrapure water.
4. Conclusions
This study demonstrates the potential of biodegradable microinjection-molded PLA/PCL bone screws reinforced with amorphous calcium phosphate (ACP) for biomedical applications. The novel incorporation of nanoscale ACP significantly enhances the mechanical properties, biocompatibility, and bone differentiation/mineralization of the composite—a distinctive approach that combines ceramic reinforcement with polymer matrices in a microscale injection molding process. Moreover, the optimization of molding parameters via the Taguchi experimental design ensures the consistent quality and improved performance of the bone screws.
The evaluation of degradation behavior, mechanical strength, and biocompatibility confirms that the bone screws are promising candidates for orthopedic use. Nevertheless, further insights into their long-term mechanical stability and degradation kinetics under cyclic, load-bearing conditions are needed to fully establish their clinical reliability.
Future research should focus on the following topics:
- -
Composite Optimization and Dynamic Testing: refining the composite formulation to further enhance mechanical strength and assessing its performance under cyclic, load-bearing conditions.
- -
In Vivo Validation: conducting in-depth animal studies and clinical trials to evaluate osseointegration, degradation behavior, and interface stability under physiological loads.
- -
Process Scalability: investigating the scalability of the optimized injection molding process to ensure feasibility for clinical production.
Overall, these findings underscore the critical role of material composition and process optimization in developing advanced biomaterials, while providing clear, actionable directions to bridge the gap between laboratory research and clinical application in this field.