The Preparation and Characterization of Poly(lactic Acid)/Poly(ε-caprolactone) Polymer Blends: The Effect of Bisphenol A Diglycidyl Ether Addition as a Compatibilizer
Abstract
1. Introduction
2. Materials and Methods
2.1. Compounding and Processing of Materials
2.2. Characterization
3. Results
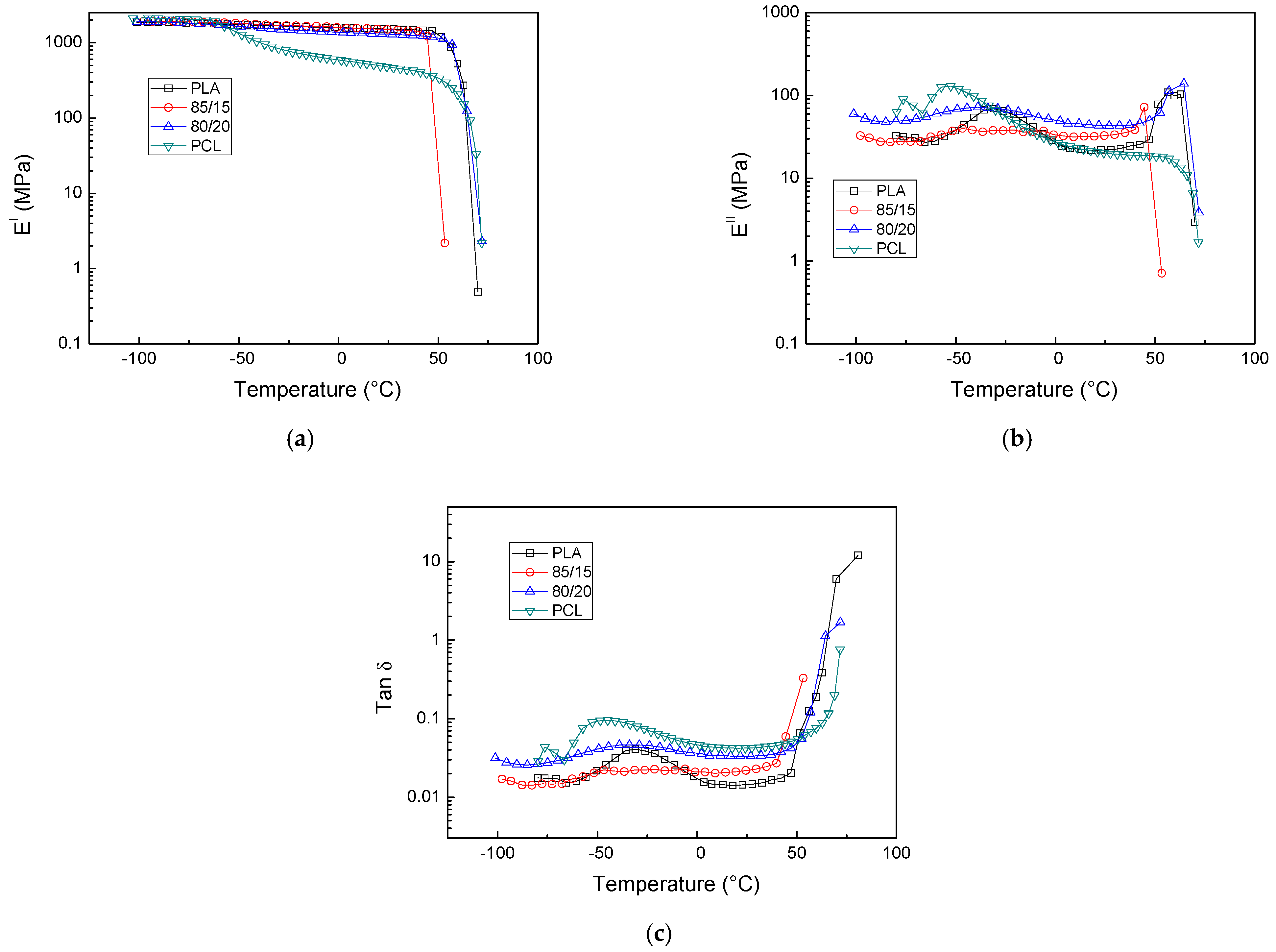
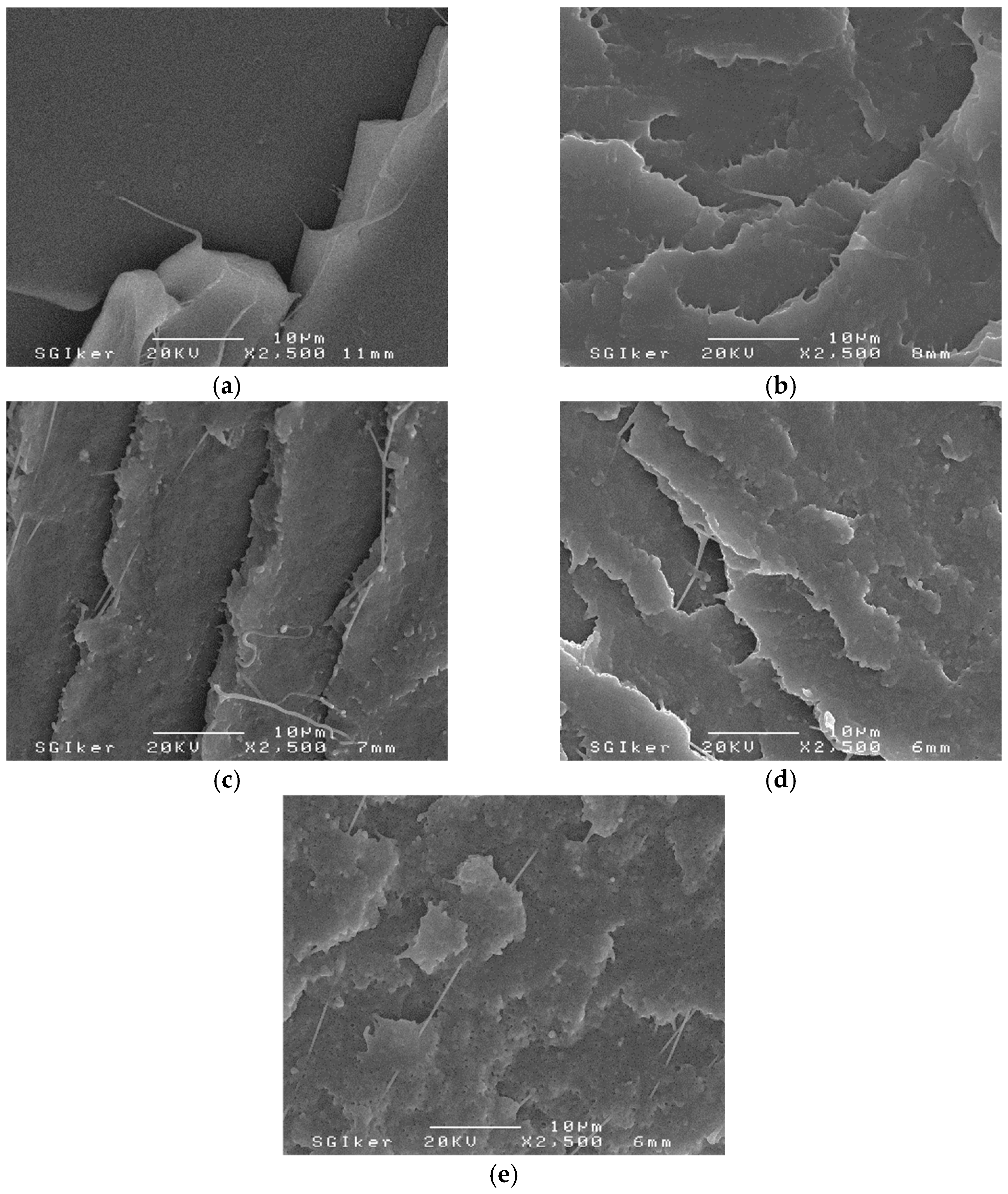
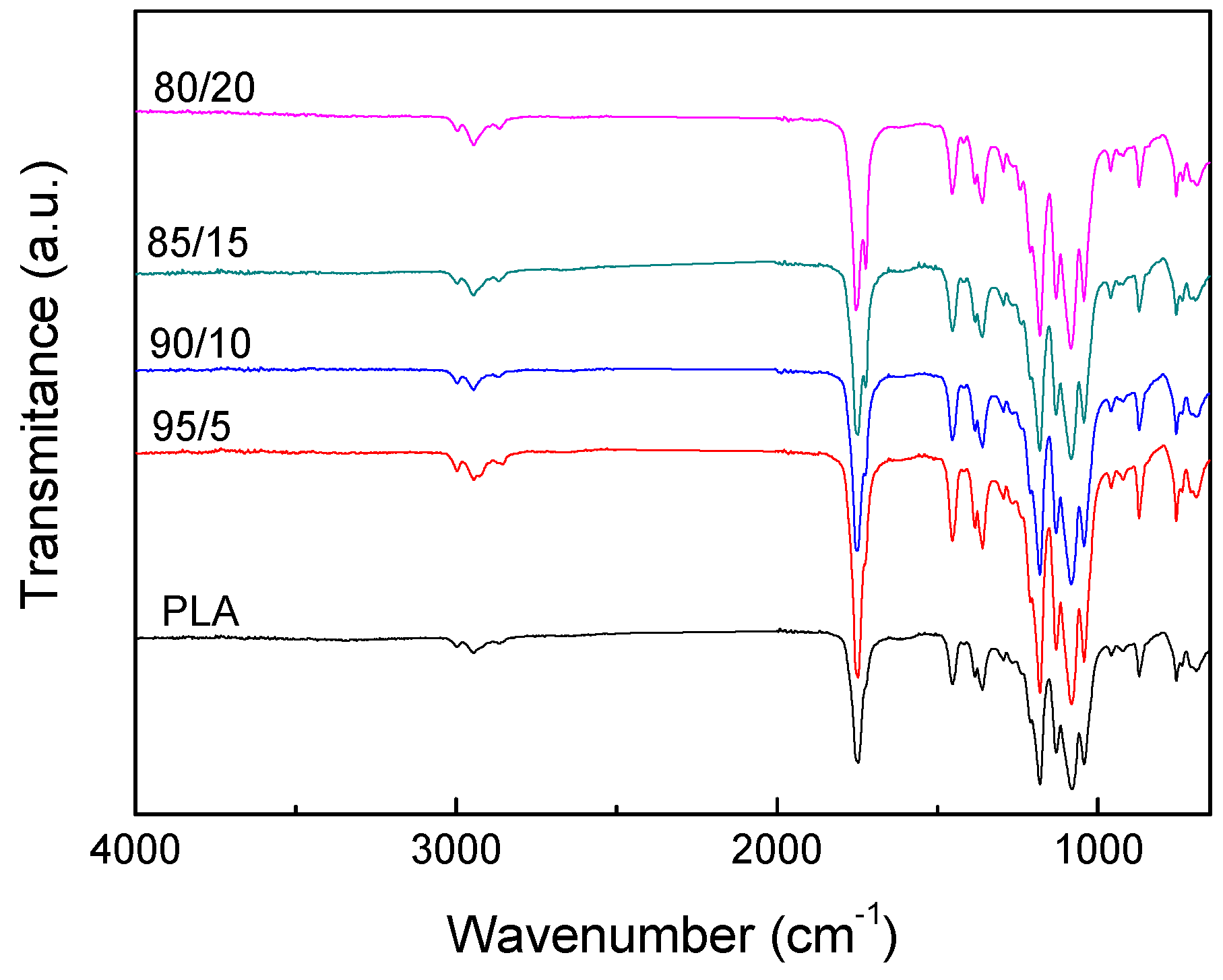
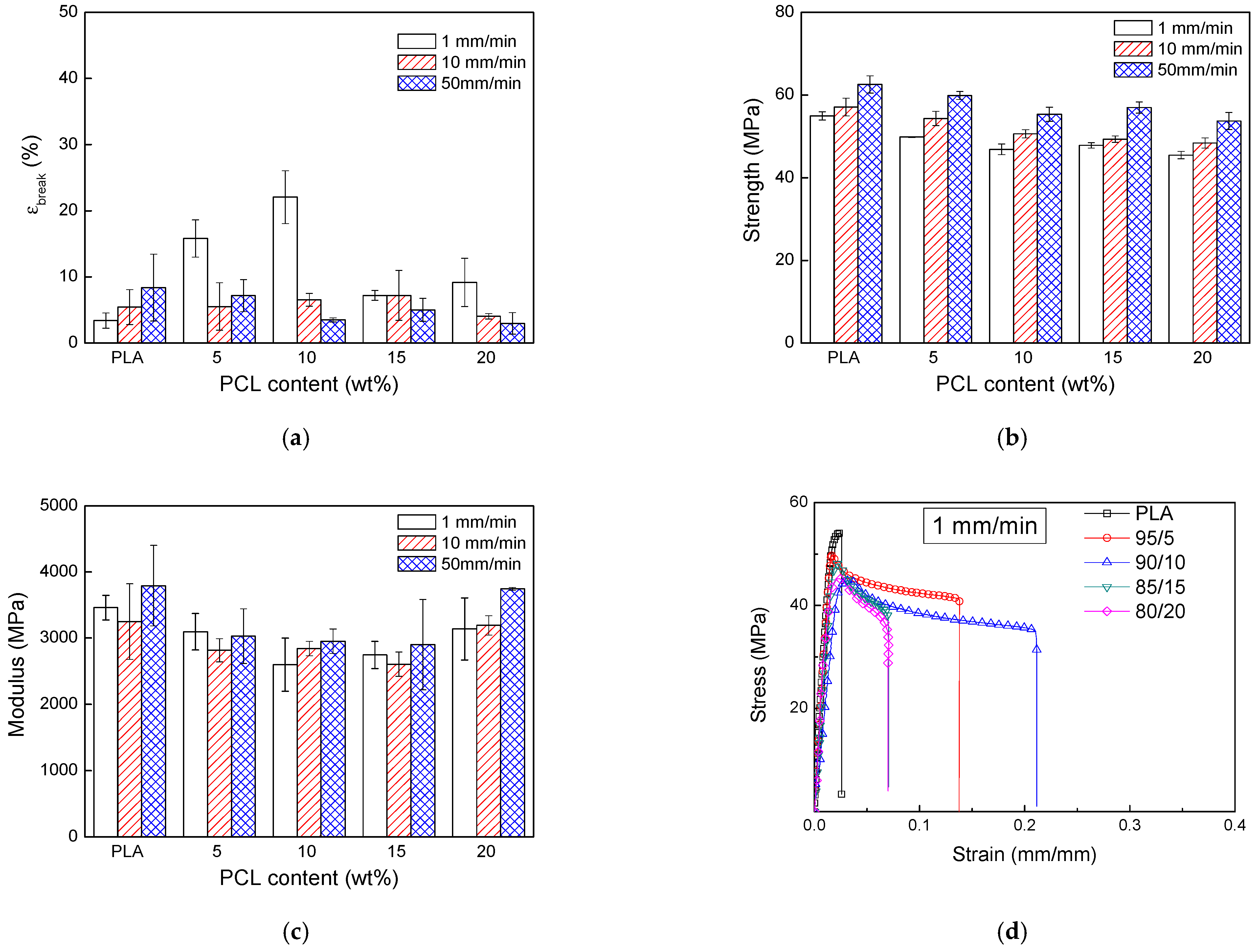
3.1. PLA/PCL Blends
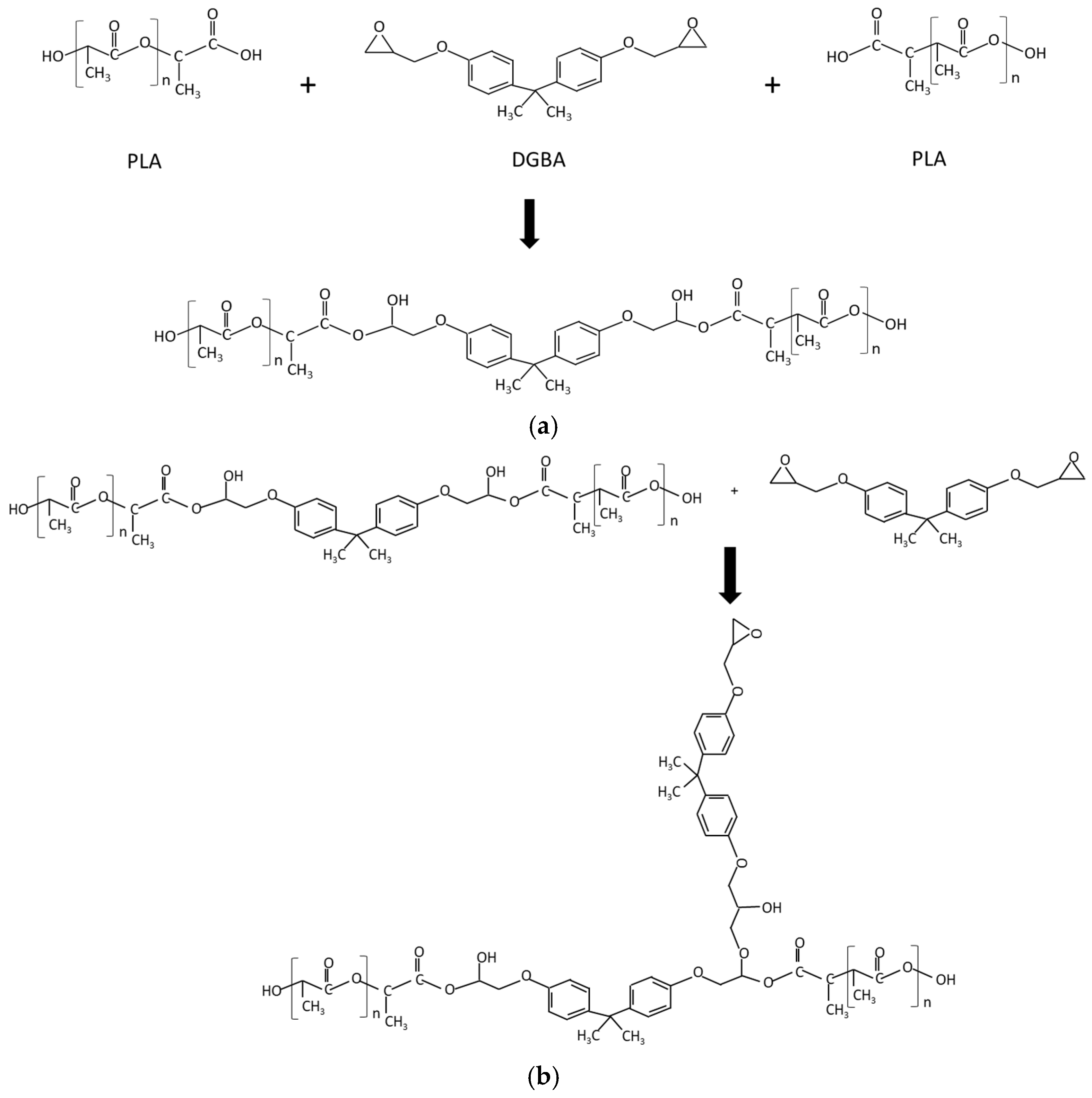
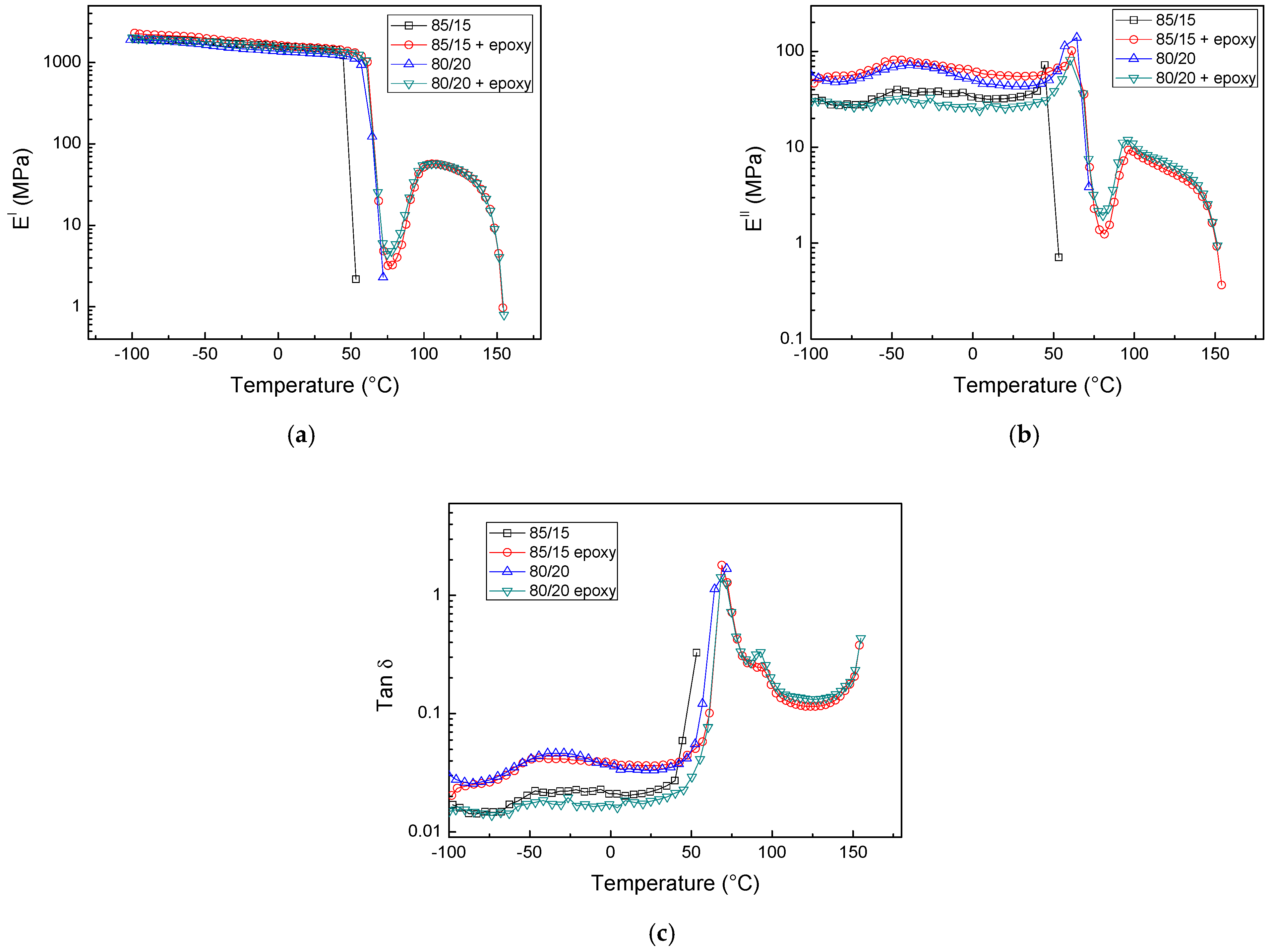
3.2. PLA/PCL Blends Modified with DGEBA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fortelný, I.; Ostafińska, A.; Michálková, D.; Jůza, J.; Mikešová, J.; Šlouf, M. Phase structure evolution during mixing and processing of poly(lactic acid)/polycaprolactone (PLA/PCL) blends. Polym. Bull. 2015, 72, 2931–2947. [Google Scholar] [CrossRef]
- Malinowski, R. Mechanical properties of PLA/PCL blends crosslinked by electron beam and TAIC additive. Chem. Phys. Lett. 2016, 662, 91–96. [Google Scholar] [CrossRef]
- Gardella, L.; Calabrese, M.; Monticelli, O. PLA maleation: An easy and effective method to modify the properties of PLA/PCL immiscible blends. Colloid Polym. Sci. 2014, 292, 2391–2398. [Google Scholar] [CrossRef]
- Navarro-Baena, I.; Sessini, V.; Dominici, F.; Torre, L.; Kenny, J.M.; Peponi, L. Design of biodegradable blends based on PLA and PCL: From morphological, thermal and mechanical studies to shape memory behavior. Polym. Degrad. Stab. 2016, 132, 97–108. [Google Scholar] [CrossRef]
- Takayama, T.; Todo, M. Improvement of impact fracture properties of PLA/PCL polymer blend due to LTI addition. J. Mater. Sci. 2006, 41, 4989–4992. [Google Scholar] [CrossRef]
- Dell’Erba, R.; Groeninckx, G.; Maglio, G.; Malinconico, M.; Migliozzi, A. Immiscible polymer blends of semicrystalline biocompatible components: Thermal properties and phase morphology analysis of PLLA/PCL blends. Polymer 2001, 42, 7831–7840. [Google Scholar] [CrossRef]
- Bouakaz, B.S.; Habi, A.; Grohens, Y.; Pillin, I. Organomontmorillonite/graphene-PLA/PCL nanofilled blends: New strategy to enhance the functional properties of PLA/PCL blend. Appl. Clay Sci. 2017, 139, 81–91. [Google Scholar] [CrossRef]
- Ostafinska, A.; Fortelny, I.; Nevoralova, M.; Hodan, J.; Kredatusova, J.; Slouf, M. Synergistic effects in mechanical properties of PLA/PCL blends with optimized composition, processing, and morphology. RSC Adv. 2015, 5, 98971–98982. [Google Scholar] [CrossRef]
- Maglio, G.; Malinconico, M.; Migliozzi, A.; Groeninckx, G. Immiscible Poly(L-lactide)/Poly(ε-caprolactone) Blends: Influence of the Addition of a Poly(L-lactide)-Poly(oxyethylene) Block Copolymer on Thermal Behavior and Morphology. Macromol. Chem. Phys. 2004, 205, 946–950. [Google Scholar] [CrossRef]
- Jeong, H.; Rho, J.; Shin, J.; Lee, D.Y.; Hwang, T.; Kim, K.J. Mechanical properties and cytotoxicity of PLA/PCL films. Biomed. Eng. Lett. 2018, 8, 267–272. [Google Scholar] [CrossRef]
- Ye, G.; Gu, T.; Chen, B.; Bi, H.; Hu, Y. Mechanical, thermal properties and shape memory behaviors of PLA/PCL/PLA-g-GMA blends. Polym. Eng. Sci. 2023, 63, 2084–2092. [Google Scholar] [CrossRef]
- Jeon, J.S.; Han, D.H.; Shin, B.Y. Improvements in the Rheological Properties, Impact Strength, and the Biodegradability of PLA/PCL Blend Compatibilized by Electron-Beam Irradiation in the Presence of a Reactive Agent. Adv. Mater. Sci. Eng. 2018, 2018, 5316175. [Google Scholar] [CrossRef]
- Carvalho, J.R.G.; Conde, G.; Antonioli, M.L.; Dias, P.P.; Vasconcelos, R.O.; Taboga, S.R.; Canola, P.A.; Chinelatto, M.A.; Pereira, G.T.; Ferraz, G.C. Biocompatibility and biodegradation of poly(lactic acid) (PLA) and an immiscible PLA/poly(ε-caprolactone) (PCL) blend compatibilized by poly(ε-caprolactone-b-tetrahydrofuran) implanted in horses. Polym. J. 2020, 52, 629–643. [Google Scholar] [CrossRef]
- Patrício, T.; Bártolo, P. Thermal Stability of PCL/PLA Blends Produced by Physical Blending Process. Procedia Eng. 2013, 59, 292–297. [Google Scholar] [CrossRef]
- Anakabe, J.; Zaldua Huici, A.M.; Eceiza, A.; Arbelaiz, A. The effect of the addition of poly(styrene-co-glycidyl methacrylate) copolymer on the properties of polylactide/poly(methyl methacrylate) blend. J. Appl. Polym. Sci. 2016, 133, 43935. [Google Scholar] [CrossRef]
- Gao, H.; Li, J.; Li, Z.; Li, Y.; Wang, X.; Jiang, J.; Li, Q. Enhancing interfacial interaction of immiscible PCL/PLA blends by in-situ crosslinking to improve the foamability. Polym. Test. 2023, 124, 108063. [Google Scholar] [CrossRef]
- Matumba, K.I.; Motloung, M.P.; Ojijo, V.; Ray, S.S.; Sadiku, E.R. Investigation of the Effects of Chain Extender on Material Properties of PLA/PCL and PLA/PEG Blends: Comparative Study between Polycaprolactone and Polyethylene Glycol. Polymers 2023, 15, 2230. [Google Scholar] [CrossRef] [PubMed]
- Othman, R.; Vladisavljević, G.T.; Nagy, Z.K. Preparation of biodegradable polymeric nanoparticles for pharmaceutical applications using glass capillary microfluidics. Chem. Eng. Sci. 2015, 137, 119–130. [Google Scholar] [CrossRef]
- Fortelny, I.; Ujcic, A.; Fambri, L.; Slouf, M. Phase Structure, Compatibility, and Toughness of PLA/PCL Blends: A Review. Front. Mater. 2019, 6, 206. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Kolloid-Z. Z. Für Polym. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- Arrighi, V.; Cowie, J.M.G.; Fuhrmann, S.; Youssef, A. Miscibility Criterion in Polymer Blends and its Determination. In Anonymous Encyclopedia of Polymer Blends; Wiley: Hoboken, NJ, USA, 2010; pp. 153–198. [Google Scholar]
- Ross, S.; Topham, P.D.; Tighe, B.J. Identification of optically clear regions of ternary polymer blends using a novel rapid screening method. Polym. Int. 2014, 63, 44–51. [Google Scholar] [CrossRef]
- Coleman, M.M.; Serman, C.J.; Bhagwagar, D.E.; Painter, P.C. A practical guide to polymer miscibility. Polymer 1990, 31, 1187–1203. [Google Scholar] [CrossRef]
- Shuai, X.; He, Y.; Asakawa, N.; Inoue, Y. Miscibility and phase structure of binary blends of poly(L-lactide) and poly(vinyl alcohol). J. Appl. Polym. Sci. 2001, 81, 762–772. [Google Scholar] [CrossRef]
- Urquijo, J.; Guerrica-Echevarría, G.; Eguiazábal, J.I. Melt processed PLA/PCL blends: Effect of processing method on phase structure, morphology, and mechanical properties. J. Appl. Polym. Sci. 2015, 132, 42641. [Google Scholar] [CrossRef]
- Mofokeng, J.P.; Luyt, A.S. Dynamic mechanical properties of PLA/PHBV, PLA/PCL, PHBV/PCL blends and their nanocomposites with TiO2 as nanofiller. Thermochim. Acta 2015, 613, 41–53. [Google Scholar] [CrossRef]
- Luyt, A.S.; Gasmi, S. Influence of blending and blend morphology on the thermal properties and crystallization behaviour of PLA and PCL in PLA/PCL blends. J. Mater. Sci. 2016, 51, 4670–4681. [Google Scholar] [CrossRef]
- Anakabe, J.; Zaldua Huici, A.M.; Eceiza, A.; Arbelaiz, A.; Avérous, L. Combined effect of nucleating agent and plasticizer on the crystallization behaviour of polylactide. Polym. Bull. 2017, 74, 4857–4886. [Google Scholar] [CrossRef]
- Pan, P.; Kai, W.; Zhu, B.; Dong, T.; Inoue, Y. Polymorphous Crystallization and Multiple Melting Behavior of Poly(l-lactide): Molecular Weight Dependence. Macromolecules 2007, 40, 6898–6905. [Google Scholar] [CrossRef]
- Matta, A.K.; Rao, R.U.; Suman, K.; Rambabu, V. Preparation and characterization of biodegradable PLA/PCL polymeric blends. Procedia Mater. Sci. 2014, 6, 1266–1270. [Google Scholar] [CrossRef]
- Starkweather, H.W.J.; Avakian, P.; Fontanella, J.J.; Wintersgill, M.C. Internal motions in polylactide and related polymers. Macromolecules 1993, 26, 5084–5087. [Google Scholar] [CrossRef]
- Hernández-Alamilla, M.; Valadez-Gonzalez, A. The effect of two commercial melt strength enhancer additives on the thermal, rheological and morphological properties of polylactide. J. Polym. Eng. 2016, 36, 31–41. [Google Scholar] [CrossRef]
- Wachirahuttapong, S.; Thongpin, C.; Sombatsompop, N. Effect of PCL and Compatibility Contents on the Morphology, Crystallization and Mechanical Properties of PLA/PCL Blends. Energy Procedia 2016, 89, 198–206. [Google Scholar] [CrossRef]
- Ostafinska, A.; Fortelný, I.; Hodan, J.; Krejčíková, S.; Nevoralová, M.; Kredatusová, J.; Kruliš, Z.; Kotek, J.; Šlouf, M. Strong synergistic effects in PLA/PCL blends: Impact of PLA matrix viscosity. J. Mech. Behav. Biomed. Mater. 2017, 69, 229–241. [Google Scholar] [CrossRef]
- Aliotta, L.; Gigante, V.; Geerinck, R.; Coltelli, M.; Lazzeri, A. Micromechanical analysis and fracture mechanics of Poly(lactic acid) (PLA)/Polycaprolactone (PCL) binary blends. Polym. Test. 2023, 121, 107984. [Google Scholar] [CrossRef]
- Finotti, P.F.M.; Costa, L.C.; Chinelatto, M.A. Effect of the Chemical Structure of Compatibilizers on the Thermal, Mechanical and Morphological Properties of Immiscible PLA/PCL Blends. Macromol. Symp. 2016, 368, 24–29. [Google Scholar] [CrossRef]
- Cheng, H.; Li, Y.; Zhang, Y.; Yu, Y.; Yu, M.; Han, C.; Shi, H. Ternary blends from biodegradable poly(L-lactic acid), poly(ε-caprolactone) and poly(vinyl acetate) with balanced properties. J. Polym. Res. 2023, 30, 184. [Google Scholar] [CrossRef]
- Correa-Pacheco, Z.N.; Jimenez-Perez, J.L.; Sabino, M.A.; Cruz-Orea, A.; Loaiza, M. Photothermal and morphological characterization of PLA/PCL polymer blends. Appl. Phys. A 2015, 120, 1323–1329. [Google Scholar]
- Zhou, Z.; Wang, E.; Liang, Y.; Miao, Y.; Chen, H.; Ling, M.; Li, W.; Huang, J.; Zhang, W. Bio-based PA-grafted bamboo charcoal for improving the flame retardancy of PLA/PCL film without damaging mechanical properties and degradability. Ind. Crops Prod. 2024, 210, 118182. [Google Scholar] [CrossRef]
- Shin, B.Y.; Han, D.H. Viscoelastic properties of PLA/PCL blends compatibilized with different methods. Korea-Aust. Rheol. J. 2017, 29, 295–302. [Google Scholar] [CrossRef]
- Jaffar Al-Mulla, E.A.; Ibrahim, N.A.B.; Shameli, K.; Ahmad, M.B.; Zin Wan Yunus, W.M. Effect of epoxidized palm oil on the mechanical and morphological properties of a PLA–PCL blend. Res. Chem. Intermed. 2014, 40, 689–698. [Google Scholar] [CrossRef]
- Kiattipornpithak, K.; Thajai, N.; Kanthiya, T.; Rachtanapun, P.; Leksawasdi, N.; Phimolsiripol, Y.; Rohindra, D.; Ruksiriwanich, W.; Sommano, S.R.; Jantanasakulwong, K. Reaction Mechanism and Mechanical Property Improvement of Poly(Lactic Acid) Reactive Blending with Epoxy Resin. Polymers 2021, 13, 2429. [Google Scholar] [CrossRef] [PubMed]
- Pereira Barros, J.J.; Dayane dos Santos Silva Ingridy Jaques, N.G.; Lia Fook, M.V.; Ramos Wellen, R.M. Influence of PCL on the epoxy workability, insights from thermal and spectroscopic analyses. Polym. Test. 2020, 89, 106679. [Google Scholar] [CrossRef]
- Liu, J.; Lou, L.; Yu, W.; Liao, R.; Li, R.; Zhou, C. Long chain branching polylactide: Structures and properties. Polymer 2010, 51, 5186–5197. [Google Scholar] [CrossRef]



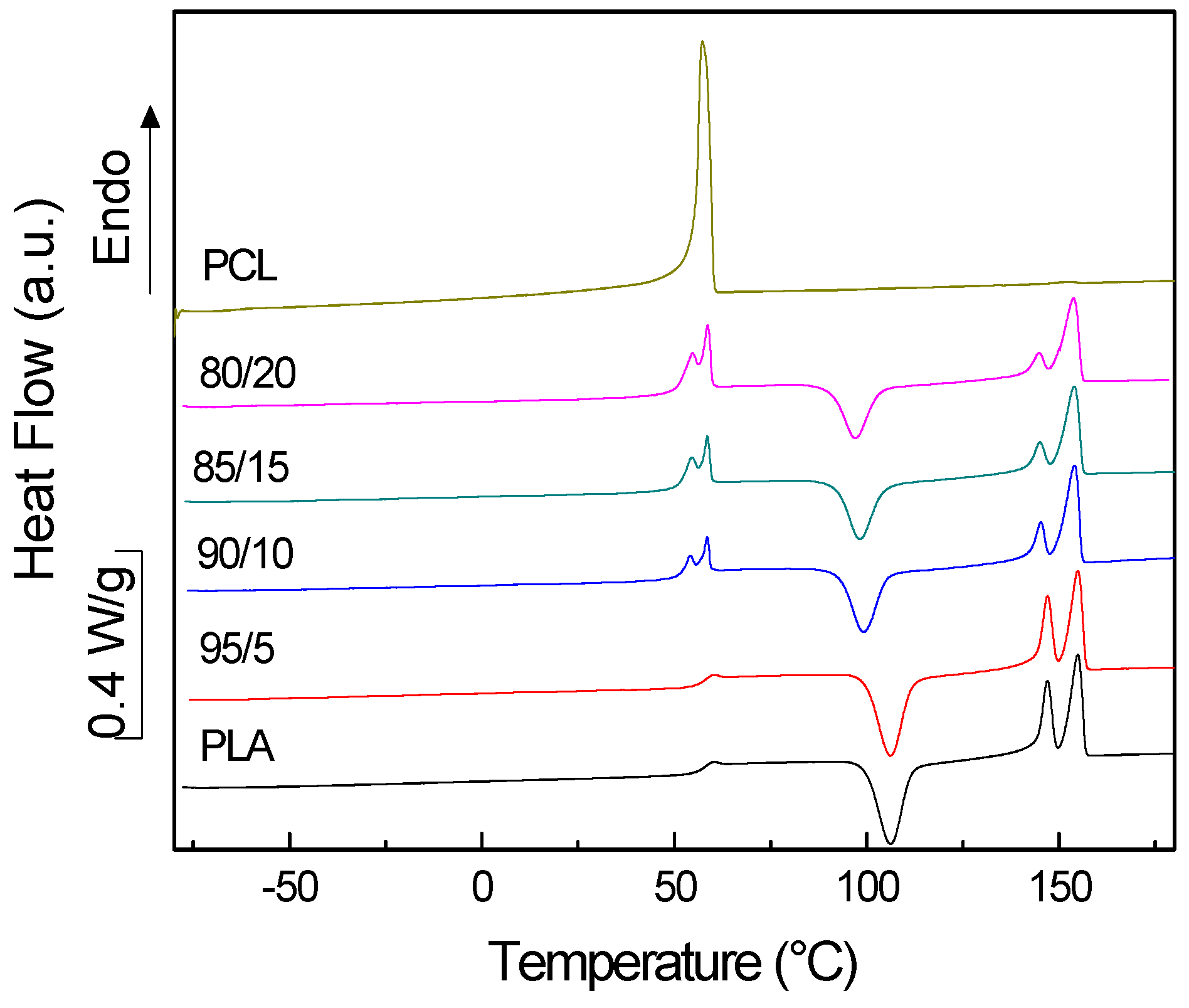

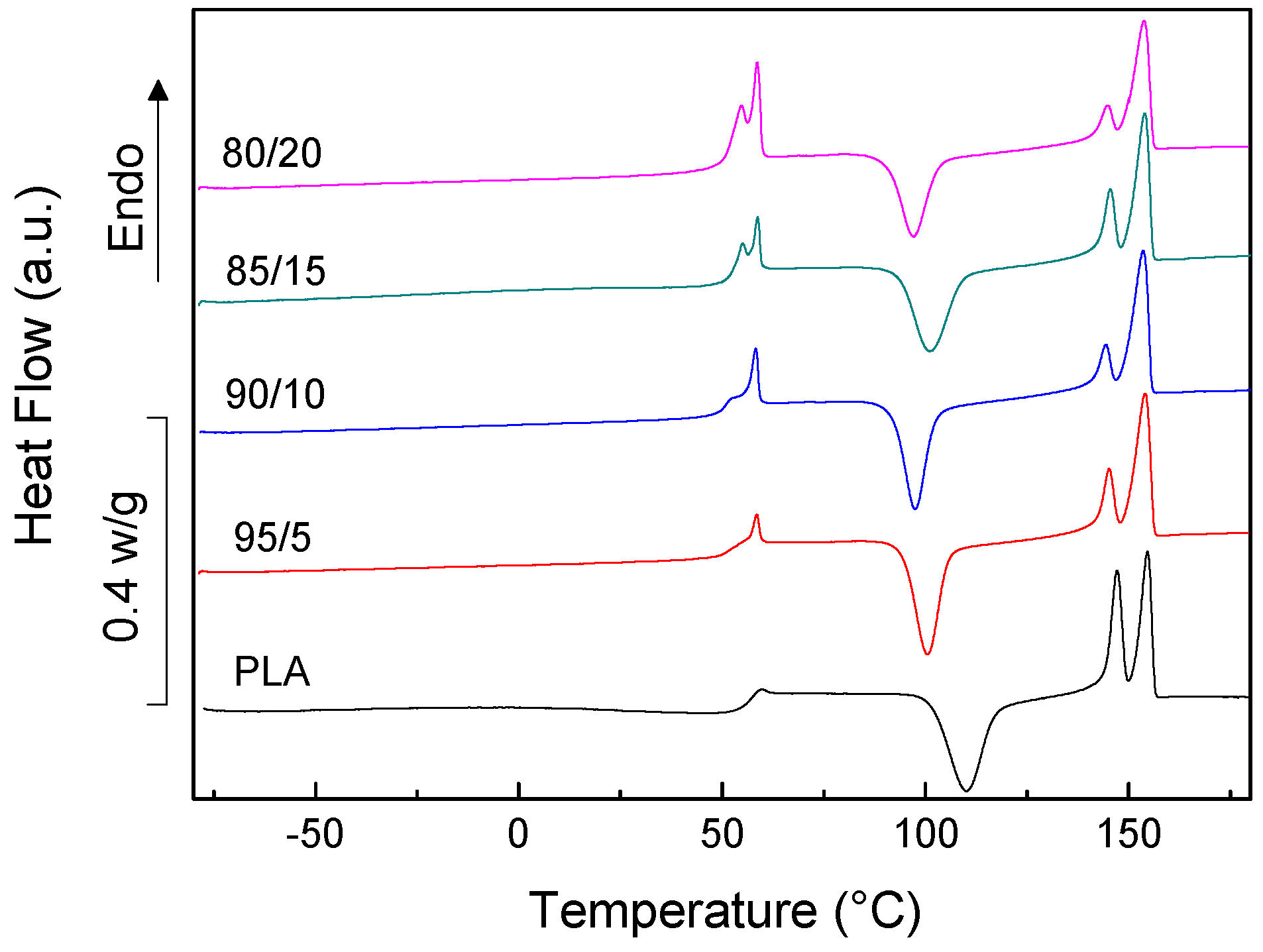

| System | TgA (°C) | TgB (°C) | TmA (°C) | ∆HmA (W/g) | TcB (°C) | ∆HcB (W/g) | TmB1 (°C) | TmB2 (°C) | ∆HmB (W/g) | XcA (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Neat PLA | - | 55.0 | - | - | 106.2 | −1.24 | 147.0 | 155.0 | 1.35 | 0.1 |
| 95/5 | n.a. | 55.3 | n.a. | n.a. | 106.2 | −1.28 | 147.0 | 155.0 | 1.33 | 0.1 |
| 90/10 | n.a. | 51.1 | 58.6 | overlapped | 99.2 | −1.13 | 145.3 | 154.0 | 1.21 | 0.1 |
| 85/15 | n.a. | 50.5 | 58.6 | overlapped | 98.2 | −1.01 | 145.1 | 154.1 | 1.12 | 0.1 |
| 80/20 | n.a. | 50.1 | 58.7 | overlapped | 97.0 | −0.91 | 144.8 | 153.9 | 1.11 | 0.3 |
| Neat PCL | −59.6 | - | 57.4 | 2.63 | - | - | - | - | - | - |
| System | TgA (°C) | TgB (°C) | TmA (°C) | ∆HmA (W/g) | TcB (°C) | ∆HcB (W/g) | TmB1 (°C) | TmB2 (°C) | ∆HmB (W/g) | XcA (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| PLA + DGEBA | - | 53.2 | -- | -- | 110.1 | −1.22 | 149.2 | 154.6 | 1.39 | 0.2 |
| 95/5 | n.a. | 48.7 | 58.5 | overlapped | 100.5 | −1.22 | 145.2 | 154.0 | 1.39 | 0.2 |
| 90/10 | n.a. | 48.5 | 58.3 | overlapped | 97.5 | −1.03 | 144.4 | 153.6 | 1.30 | 0.3 |
| 85/15 | n.a. | 51.2 | 58.7 | overlapped | 101.1 | −1.03 | 145.5 | 154.0 | 1.24 | 0.3 |
| 80/20 | n.a. | 49.1 | 58.6 | overlapped | 97.1 | −0.98 | 144.8 | 153.8 | 1.16 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arbelaiz, A.; Landa, B.; Peña-Rodriguez, C. The Preparation and Characterization of Poly(lactic Acid)/Poly(ε-caprolactone) Polymer Blends: The Effect of Bisphenol A Diglycidyl Ether Addition as a Compatibilizer. J. Manuf. Mater. Process. 2025, 9, 38. https://doi.org/10.3390/jmmp9020038
Arbelaiz A, Landa B, Peña-Rodriguez C. The Preparation and Characterization of Poly(lactic Acid)/Poly(ε-caprolactone) Polymer Blends: The Effect of Bisphenol A Diglycidyl Ether Addition as a Compatibilizer. Journal of Manufacturing and Materials Processing. 2025; 9(2):38. https://doi.org/10.3390/jmmp9020038
Chicago/Turabian StyleArbelaiz, Aitor, Beñat Landa, and Cristina Peña-Rodriguez. 2025. "The Preparation and Characterization of Poly(lactic Acid)/Poly(ε-caprolactone) Polymer Blends: The Effect of Bisphenol A Diglycidyl Ether Addition as a Compatibilizer" Journal of Manufacturing and Materials Processing 9, no. 2: 38. https://doi.org/10.3390/jmmp9020038
APA StyleArbelaiz, A., Landa, B., & Peña-Rodriguez, C. (2025). The Preparation and Characterization of Poly(lactic Acid)/Poly(ε-caprolactone) Polymer Blends: The Effect of Bisphenol A Diglycidyl Ether Addition as a Compatibilizer. Journal of Manufacturing and Materials Processing, 9(2), 38. https://doi.org/10.3390/jmmp9020038






