Limited Impact of Drone Transport of Blood on Platelet Activation
Abstract
1. Introduction
2. Materials and Methods
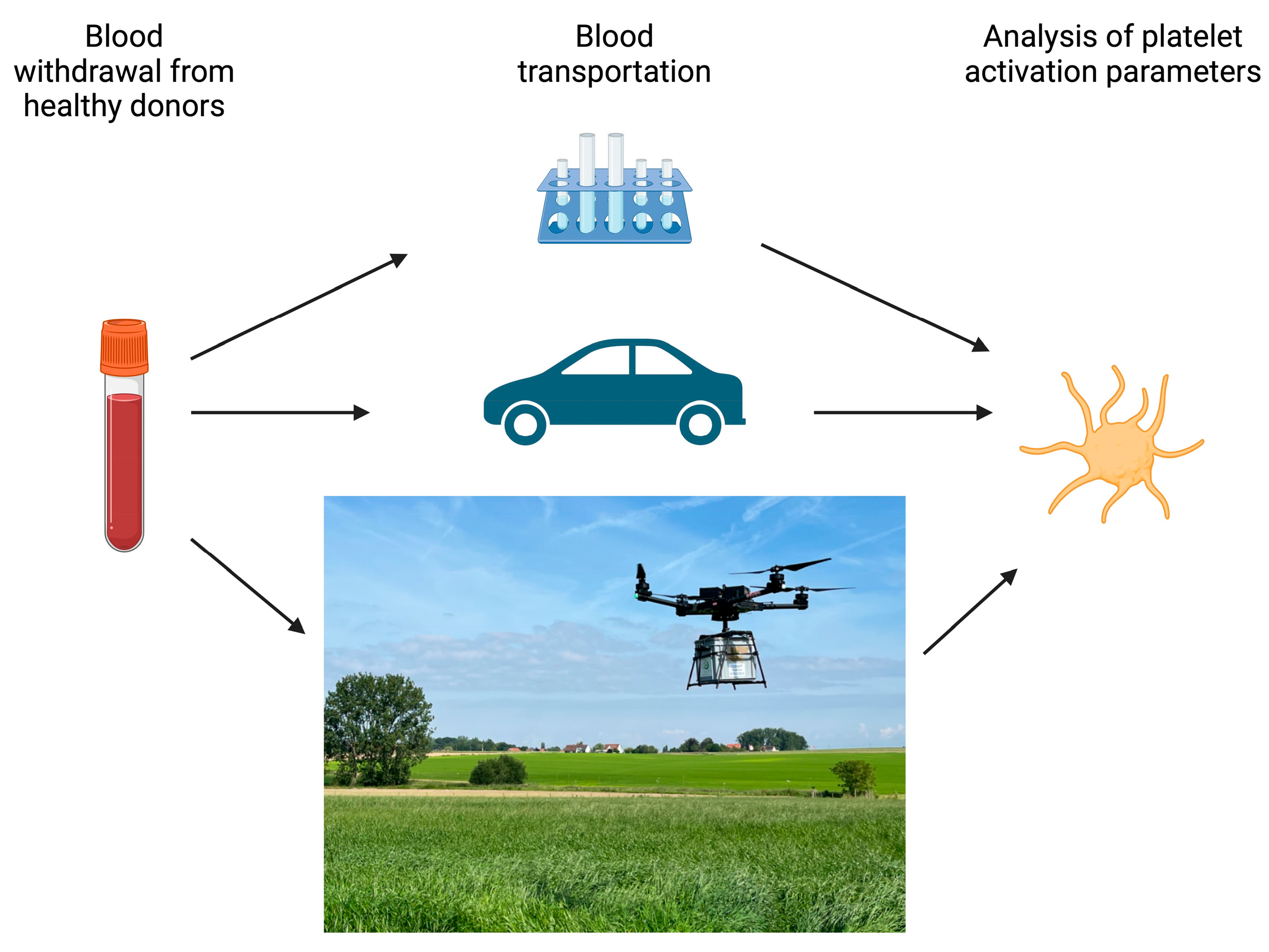
2.1. Study Design
2.2. Clinical Laboratory Analysis
2.3. Platelet Activation Assay
2.4. Platelet Factor 4 (PF4) ELISA
2.5. Statistics
3. Results
3.1. Drone Flights and Car Transport
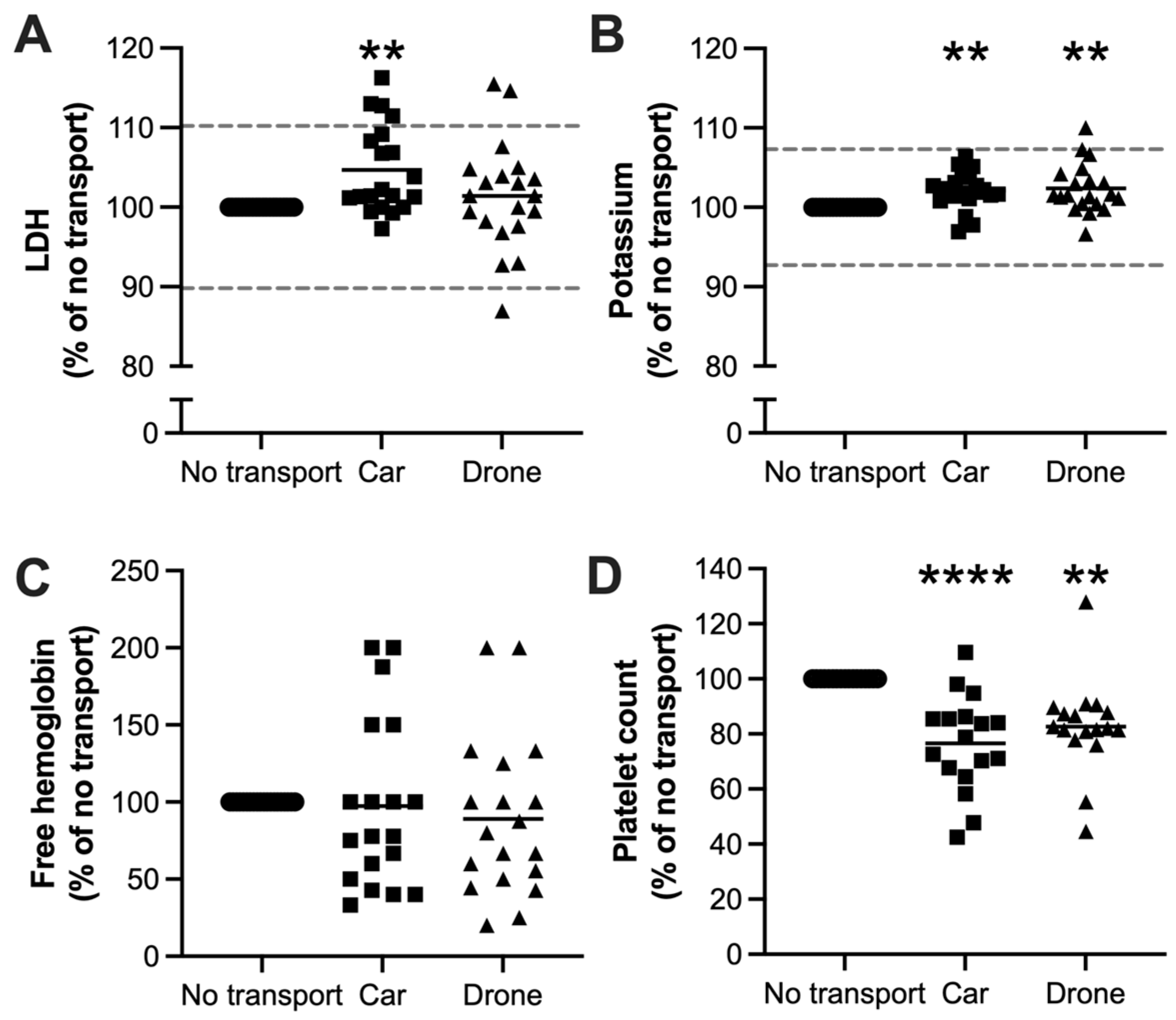
3.2. Clinical Laboratory Parameters
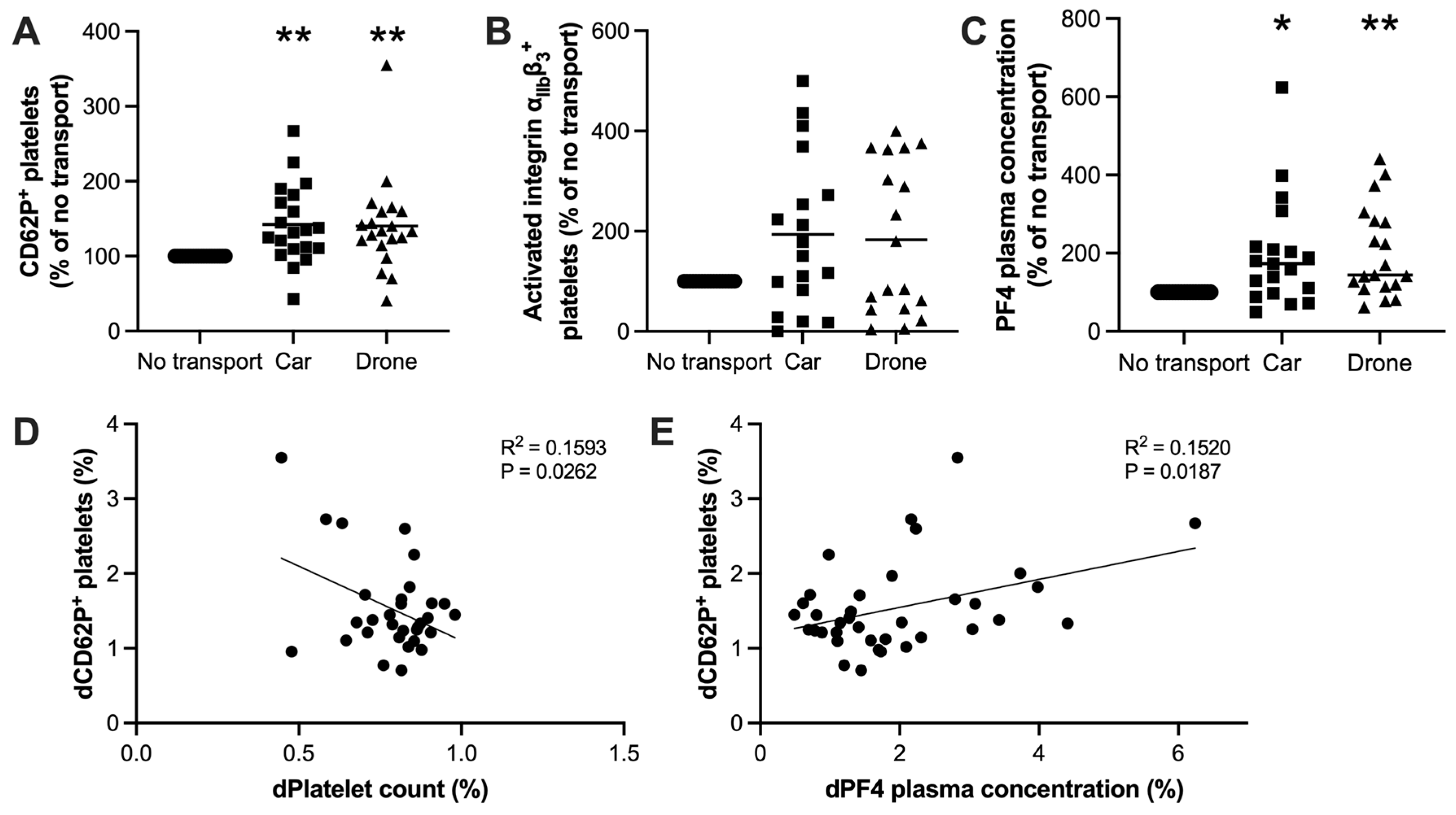
3.3. Platelet Activation During Drone Transportation
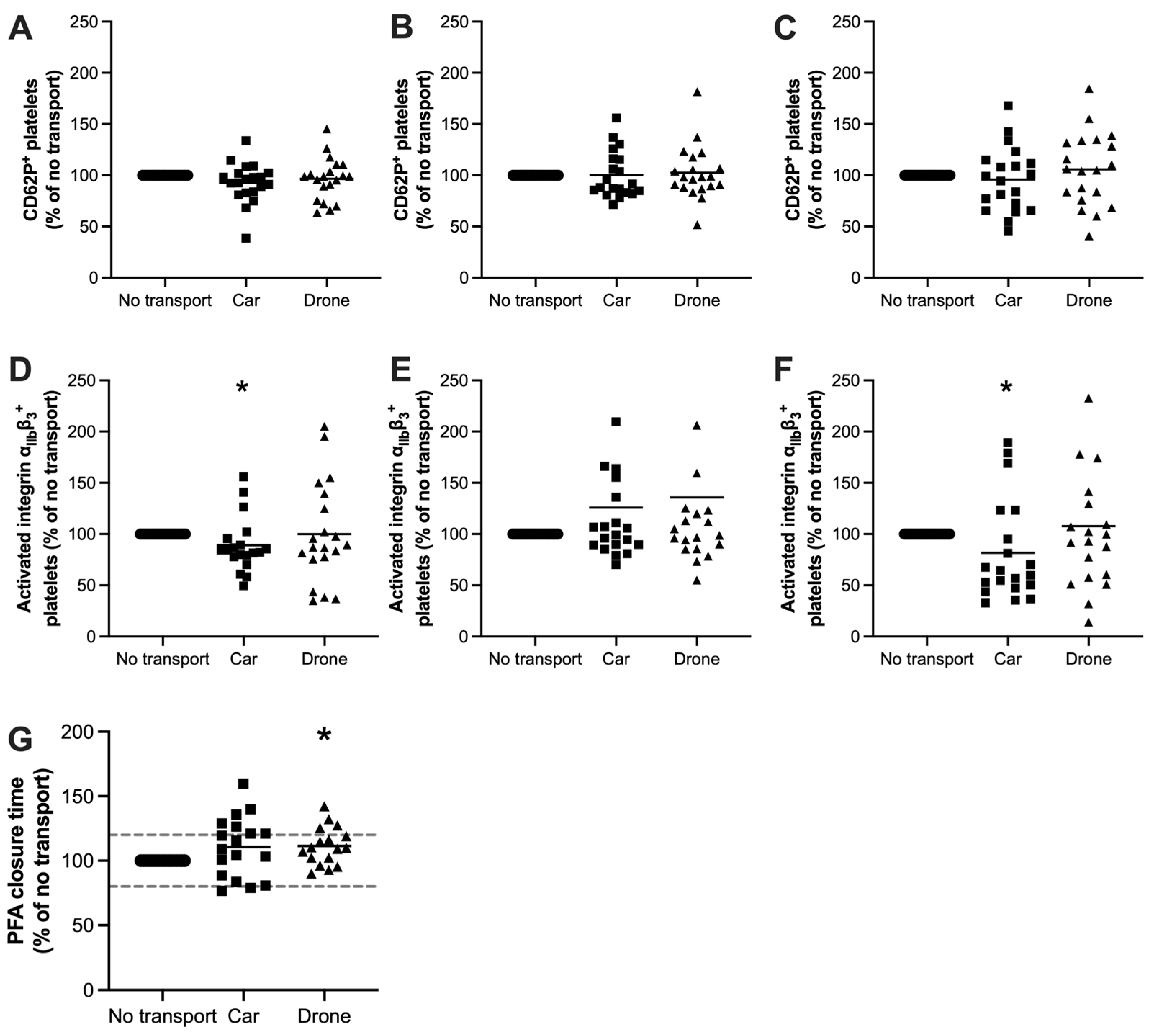
3.4. Drone Transportation Did Not Affect the Platelet Response Toward Strong Agonist Stimulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bael, J.V.; Callewaert, N.; Tersteeg, C. The impact of drone transport on blood quality. Belg. J. Hematol. 2023, 14, 274–277. [Google Scholar]
- Li, Q.; Xia, J.; Ge, F.; Lu, Q.; Zhang, M. Blood delivery by drone: A faltering step in a promising direction. Lancet Glob. Health 2022, 10, e1098. [Google Scholar] [CrossRef] [PubMed]
- Ddungu, H.; Krantz, E.M.; Kajja, I.; Naluzze, S.; Nabbanja, H.; Nalubwama, F.; Phipps, W.; Orem, J.; Wald, A.; Kiwanuka, N. Transfusion Challenges in Patients with Hematological Malignancies in Sub-Saharan Africa: A Prospective Observational Study from the Uganda Cancer Institute. Sci. Rep. 2020, 10, 2825. [Google Scholar] [CrossRef]
- Nisingizwe, M.P.; Ndishimye, P.; Swaibu, K.; Nshimiyimana, L.; Karame, P.; Dushimiyimana, V.; Musabyimana, J.P.; Musanabaganwa, C.; Nsanzimana, S.; Law, M.R. Effect of unmanned aerial vehicle (drone) delivery on blood product delivery time and wastage in Rwanda: A retrospective, cross-sectional study and time series analysis. Lancet Glob. Health 2022, 10, e564–e569. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.matternet.com (accessed on 3 August 2024).
- Amukele, T.K.; Hernandez, J.; Snozek, C.L.; Wyatt, R.G.; Douglas, M.; Amini, R.; Street, J. Drone Transport of Chemistry and Hematology Samples Over Long Distances. Am. J. Clin. Pathol. 2017, 148, 427–435. [Google Scholar] [CrossRef]
- Johannessen, K.A.; Wear, N.K.S.; Toska, K.; Hansbø, M.; Berg, J.P.; Fosse, E. Pathologic Blood Samples Tolerate Exposure to Vibration and High Turbulence in Simulated Drone Flights, but Plasma Samples Should be Centrifuged After Flight. IEEE J. Transl. Eng. Health Med. 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Amukele, T.K.; Sokoll, L.J.; Pepper, D.; Howard, D.P.; Street, J. Can Unmanned Aerial Systems (Drones) Be Used for the Routine Transport of Chemistry, Hematology, and Coagulation Laboratory Specimens? PLoS ONE 2015, 10, e0134020. [Google Scholar] [CrossRef]
- Perlee, D.; van der Steege, K.H.; Besten, G.D. The effect of drone transport on the stability of biochemical, coagulation and hematological parameters in healthy individuals. cclm 2021, 59, 1772–1776. [Google Scholar] [CrossRef]
- Tersteeg, C.; Deckmyn, H. The biochemistry of platelet function regulation. Belg. J. Hematol. 2020, 11, 240–245. [Google Scholar]
- Huskens, D.; Sang, Y.; Konings, J.; van der Vorm, L.; de Laat, B.; Kelchtermans, H.; Roest, M. Standardization and reference ranges for whole blood platelet function measurements using a flow cytometric platelet activation test. PLoS ONE 2018, 13, e0192079. [Google Scholar] [CrossRef]
- Aarsand, A.; Fernandez-Calle, P.; Webster, C.; Coskun, A.; Gonzales-Lao, E.; Diaz-Garzon, J.; Jonker, N.; Simon, M.; Braga, F.; Perich, C.; et al. The EFLM Biological Variation Database. Available online: https://biologicalvariation.eu (accessed on 15 July 2024).
- Božič-Mijovski, M.; Rakuša, M.; Stegnar, M. Variation in Platelet Function Testing Has a Major Influence on Detection of Aspirin Resistance in Healthy Subjects. Pathophysiol. Haemost. Thromb. 2007, 36, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, Q.; Wang, L.; Zhao, X.; Feng, G. Digitalization and third-party logistics performance: Exploring the roles of customer collaboration and government. Int. J. Phys. Distrib. Logist. Manag. 2023, 53, 467–488. [Google Scholar] [CrossRef]
- Yakushiji, F.; Yakushiji, K.; Murata, M.; Hiroi, N.; Takeda, K.; Fujita, H. The Quality of Blood is not Affected by Drone Transport: An Evidential Study of the Unmanned Aerial Vehicle Conveyance of Transfusion Material in Japan. Drones 2020, 4, 4. [Google Scholar] [CrossRef]
- Kundu, S.K.; Heilmann, E.J.; Sio, R.; Garcia, C.; Ostgaard, R.A. Characterization of an In Vitro Platelet Function Analyzer, PFA-100™. Clin. Appl. Thromb. 1996, 2, 241–249. [Google Scholar] [CrossRef]
- Paniccia, R.; Priora, R.; Liotta, A.A.; Abbate, R. Platelet function tests: A comparative review. Vasc. Health Risk Manag. 2015, 11, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Born, G.V.R.; Cross, M.O.J. The Aggregation of Blood Platelets. J. Physiol. 1963, 168, 178–195. [Google Scholar] [CrossRef]
- Femia, E.A.; Scavone, M.; Lecchi, A.; Cattaneo, M. Effect of platelet count on platelet aggregation measured with impedance aggregometry (Multiplate™ analyzer) and with light transmission aggregometry. J. Thromb. Haemost. 2013, 11, 2193–2196. [Google Scholar] [CrossRef]
- Wallin, O.; Söderberg, J.; Grankvist, K.; Jonsson, P.A.; Hultdin, J. Preanalytical effects of pneumatic tube transport on routine haematology, coagulation parameters, platelet function and global coagulation. cclm 2008, 46, 1443–1449. [Google Scholar] [CrossRef]
- Enko, D.; Mangge, H.; Münch, A.; Niedrist, T.; Mahla, E.; Metzler, H.; Prüller, F. Pneumatic tube system transport does not alter platelet function in optical and whole blood aggregometry, prothrombin time, activated partial thromboplastin time, platelet count and fibrinogen in patients on anti-platelet drug therapy. Biochem. Medica 2017, 27, 217–224. [Google Scholar] [CrossRef]
- Bolliger, D.; Seeberger, M.D.; Tanaka, K.A.; Dell-Kuster, S.; Gregor, M.; Zenklusen, U.; Grapow, M.; Tsakiris, D.A.; Filipovic, M. Pre-analytical effects of pneumatic tube transport on impedance platelet aggregometry. Platelets 2009, 20, 458–465. [Google Scholar] [CrossRef]
- Hübner, U.; Böckel-Frohnhöfer, N.; Hummel, B.; Geisel, J. The effect of a pneumatic tube transport system on platelet aggregation using optical aggregometry and the PFA-100. Clin. Lab. 2010, 56, 59–64. [Google Scholar] [PubMed]
- Glas, M.; Mauer, D.; Kassas, H.; Volk, T.; Kreuer, S. Sample transport by pneumatic tube system alters results of multiple electrode aggregometry but not rotational thrombelastometry. Platelets 2013, 24, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Thalén, S.; Forsling, I.; Eintrei, J.; Söderblom, L.; Antovic, J.P. Pneumatic tube transport affects platelet function measured by multiplate electrode aggregometry. Thromb. Res. 2013, 132, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, H.; Frøstrup, A.-B.; Larsen, A.S.; Fenger, M.S.; Dahdouh, S.; Zoel-Gina, R.; Nielsen, L.K. Pneumatic tube transport of blood samples affects global hemostasis and platelet function assays. Int. J. Lab. Hematol. 2021, 43, 1207–1215. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Callewaert, N.; Pareyn, I.; Acke, T.; Desplinter, B.; Van de Pitte, K.; Van Vooren, J.; De Meyer, M.; Seeldraeyers, E.; Peeters, F.; De Meyer, S.F.; et al. Limited Impact of Drone Transport of Blood on Platelet Activation. Drones 2024, 8, 752. https://doi.org/10.3390/drones8120752
Callewaert N, Pareyn I, Acke T, Desplinter B, Van de Pitte K, Van Vooren J, De Meyer M, Seeldraeyers E, Peeters F, De Meyer SF, et al. Limited Impact of Drone Transport of Blood on Platelet Activation. Drones. 2024; 8(12):752. https://doi.org/10.3390/drones8120752
Chicago/Turabian StyleCallewaert, Nico, Inge Pareyn, Tessa Acke, Brian Desplinter, Kyana Van de Pitte, Joke Van Vooren, Mathieu De Meyer, Ellen Seeldraeyers, Frank Peeters, Simon F De Meyer, and et al. 2024. "Limited Impact of Drone Transport of Blood on Platelet Activation" Drones 8, no. 12: 752. https://doi.org/10.3390/drones8120752
APA StyleCallewaert, N., Pareyn, I., Acke, T., Desplinter, B., Van de Pitte, K., Van Vooren, J., De Meyer, M., Seeldraeyers, E., Peeters, F., De Meyer, S. F., Vanhoorelbeke, K., & Tersteeg, C. (2024). Limited Impact of Drone Transport of Blood on Platelet Activation. Drones, 8(12), 752. https://doi.org/10.3390/drones8120752





