Optically Induced Dielectrophoresis and Machine Learning Algorithms for the Identification of the Circulating Tumor Cells †
Abstract
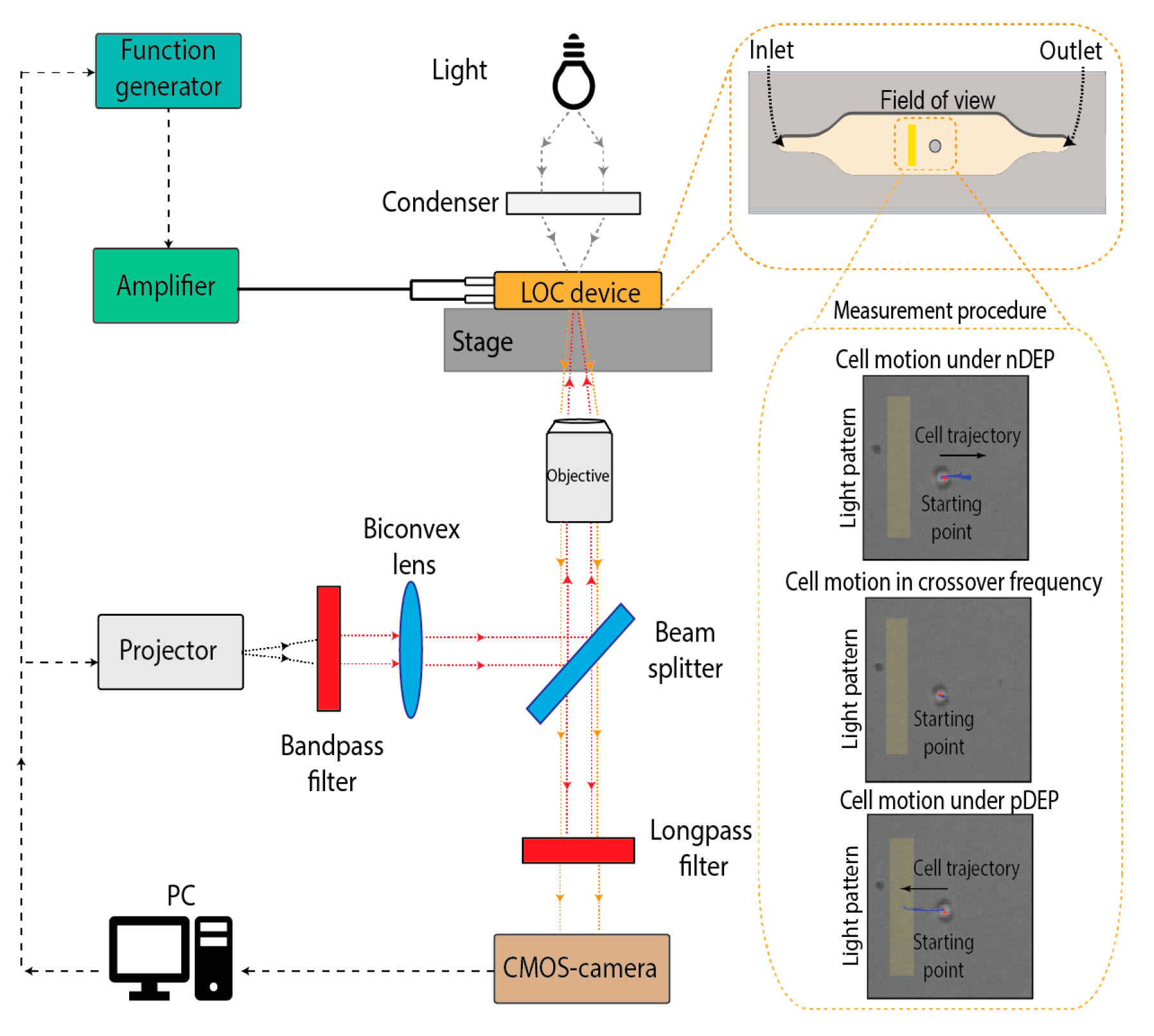
1. Introduction
2. Materials and Methods
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plaks, V.; Koopman, C.D.; Werb, Z. Circulating Tumor Cells. Science 2013, 341, 1186–1188. [Google Scholar] [CrossRef]
- Chen, H.-H.; Lin, M.-W.; Tien, W.-T.; Lai, C.-P.; Weng, K.-Y.; Ko, C.-H.; Lin, C.-C.; Chen, J.-C.; Tiao, K.-T.; Chen, T.-C.; et al. High-purity separation of cancer cells by optically induced dielectrophoresis. J. Biomed. Opt. 2014, 19, 1. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.; Vreeburg, R.A.M.; Savelkoul, H.F.J.; Wichers, H.J. Transcription profiles of LPS-stimulated THP-1 monocytes and macrophages: A tool to study inflammation modulating effects of food-derived compounds. Food Funct. 2010, 1, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Filippi, J.; Di Giuseppe, D.; Casti, P.; Mencattini, A.; Antonelli, G.; D’Orazio, M.; Corsi, F.; Della-Morte Canosci, D.; Ghibelli, L.; Witte, C.; et al. Exploiting spectral information in Opto-Electronic Tweezers for cell classification and drug response evaluation. Sens. Actuators B Chem. 2022, 368, 132200. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippi, J.; Corsi, F.; Casti, P.; Antonelli, G.; D’Orazio, M.; Capradossi, F.; Capuano, R.; Curci, G.; Ghibelli, L.; Mencattini, A.; et al. Optically Induced Dielectrophoresis and Machine Learning Algorithms for the Identification of the Circulating Tumor Cells. Proceedings 2024, 97, 71. https://doi.org/10.3390/proceedings2024097071
Filippi J, Corsi F, Casti P, Antonelli G, D’Orazio M, Capradossi F, Capuano R, Curci G, Ghibelli L, Mencattini A, et al. Optically Induced Dielectrophoresis and Machine Learning Algorithms for the Identification of the Circulating Tumor Cells. Proceedings. 2024; 97(1):71. https://doi.org/10.3390/proceedings2024097071
Chicago/Turabian StyleFilippi, Joanna, Francesca Corsi, Paola Casti, Gianni Antonelli, Michele D’Orazio, Francesco Capradossi, Rosamaria Capuano, Giorgia Curci, Lina Ghibelli, Arianna Mencattini, and et al. 2024. "Optically Induced Dielectrophoresis and Machine Learning Algorithms for the Identification of the Circulating Tumor Cells" Proceedings 97, no. 1: 71. https://doi.org/10.3390/proceedings2024097071
APA StyleFilippi, J., Corsi, F., Casti, P., Antonelli, G., D’Orazio, M., Capradossi, F., Capuano, R., Curci, G., Ghibelli, L., Mencattini, A., & Martinelli, E. (2024). Optically Induced Dielectrophoresis and Machine Learning Algorithms for the Identification of the Circulating Tumor Cells. Proceedings, 97(1), 71. https://doi.org/10.3390/proceedings2024097071









