Magnetic Nanoparticles and Magnetic Sensors for Ultrasensitive and Fast Diagnostics †
Abstract
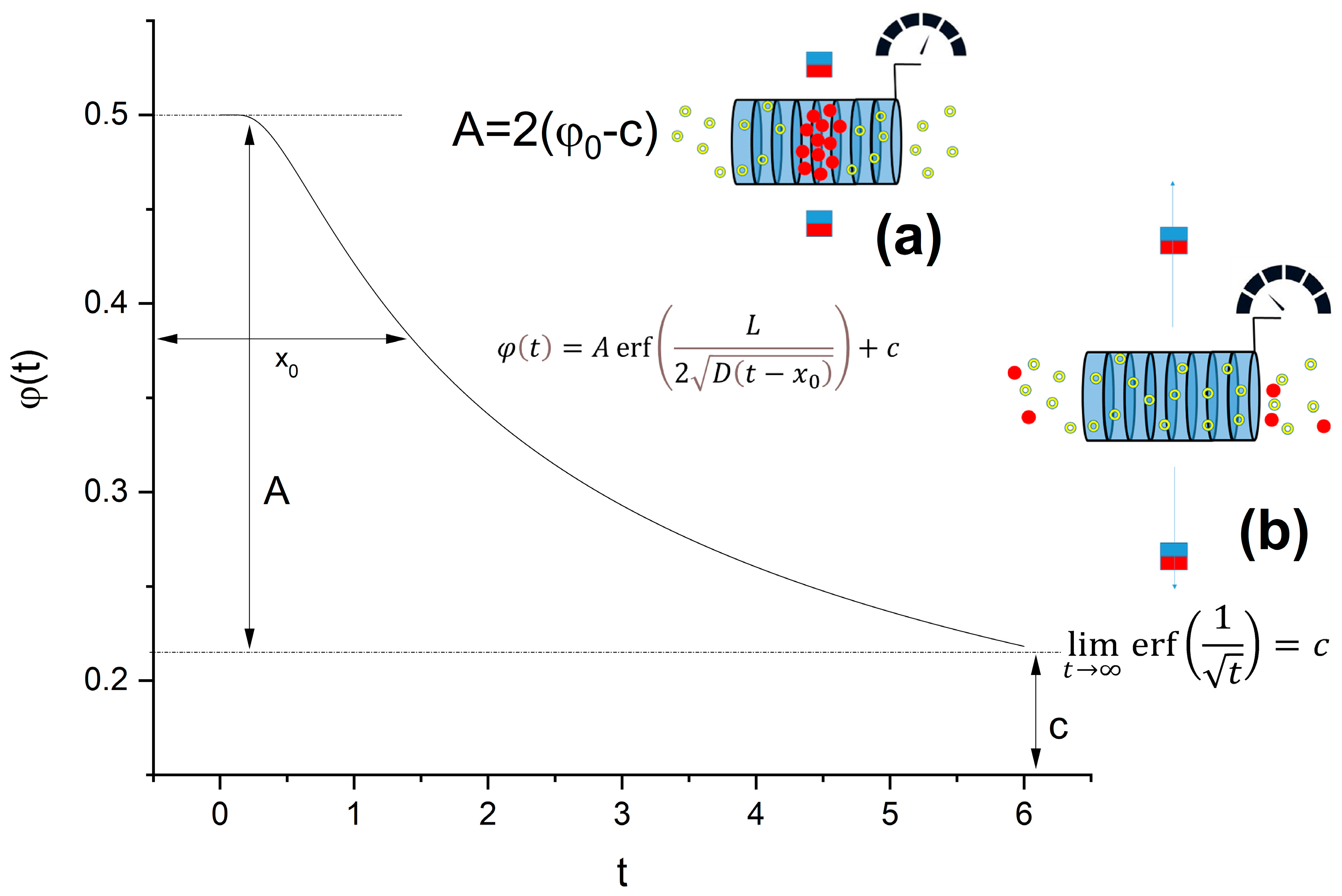
1. Introduction
2. Materials and Methods
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Oronzo, S.; Brown, J.; Coleman, R. The role of biomarkers in the management of bone-homing malignancies. J. Bone Oncol. 2017, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sarhadi, V.K.; Armengol, G. Molecular Biomarkers in Cancer. Biomolecules 2022, 12, 1021. [Google Scholar]
- Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. 2021, 27, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Surpi, A.; Shelyakova, T.; Murgia, M.; Rivas, J.; Piñeiro, Y.; Greco, P.; Dediu, V.A. Versatile magnetic configuration for the control and manipulation of superparamagnetic nanoparticles. Sci. Rep. 2023, 13, 5301. [Google Scholar] [CrossRef] [PubMed]
- Surpi, A.; Murgia, M.; López, S.; González, M.; Piñeiro, Y.; Rivas, J.; Perugini, P.; Santin, M.; Sobrino, T.; Greco, G.; et al. Magnetic separation and concentration of Aβ 1-42 molecules dispersed at the threshold concentration for Alzheimer’s disease diagnosis in clinically-relevant volumes of sample. J. Nanobiotechnol. 2023, 21, 329. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surpi, A.; Gnoli, L.; Shelykova, T.; Giavaresi, G.; González-Gómez, M.A.; Piñeiro, Y.; Rivas, J.; Dediu, V.A. Magnetic Nanoparticles and Magnetic Sensors for Ultrasensitive and Fast Diagnostics. Proceedings 2024, 97, 219. https://doi.org/10.3390/proceedings2024097219
Surpi A, Gnoli L, Shelykova T, Giavaresi G, González-Gómez MA, Piñeiro Y, Rivas J, Dediu VA. Magnetic Nanoparticles and Magnetic Sensors for Ultrasensitive and Fast Diagnostics. Proceedings. 2024; 97(1):219. https://doi.org/10.3390/proceedings2024097219
Chicago/Turabian StyleSurpi, Alessandro, Luca Gnoli, Tatiana Shelykova, Gianluca Giavaresi, Manuel A. González-Gómez, Yolanda Piñeiro, José Rivas, and Valentin Alek Dediu. 2024. "Magnetic Nanoparticles and Magnetic Sensors for Ultrasensitive and Fast Diagnostics" Proceedings 97, no. 1: 219. https://doi.org/10.3390/proceedings2024097219
APA StyleSurpi, A., Gnoli, L., Shelykova, T., Giavaresi, G., González-Gómez, M. A., Piñeiro, Y., Rivas, J., & Dediu, V. A. (2024). Magnetic Nanoparticles and Magnetic Sensors for Ultrasensitive and Fast Diagnostics. Proceedings, 97(1), 219. https://doi.org/10.3390/proceedings2024097219








