Abstract
Tungsten oxide is one of the most commonly used materials for metal oxide-based gas sensors. In order to tune the sensing behavior, small clusters of noble metals are often added to the surface of WO3. Previously, it has been found that in the case of oxidized metal clusters, e.g., Rh and Pt additives, the Fermi-level pinning mechanism dominates. Unlike other noble metal surface clusters, gold seems to remain metallic under sensor operation. As a result, the behavior of WO3-based sensors was found to be significantly enhanced for all reducing gases and decreased for NO2.
1. Introduction
Metal oxide-based gas sensors are an attractive option for numerous applications as they are robust, compact, and inexpensive. The major drawback, however, is their inherent lack of selectivity. Now that computing power is widely accessible and miniaturization has matured, the use of tiny arrays is a feasible solution for increased selectivity. In order to create suitable arrays, materials with complementary sensing behaviors are needed. The sensor response of metal oxides is commonly tuned through the addition of noble metal surface additives. In the case of oxidized noble metal additives, e.g., Pt, Pd, and Rh, on common n-type semiconductors, the Fermi-level pinning mechanism has been found to be dominant [1,2,3,4]. The test gas reacts with the oxidized noble metal cluster, changing the junction between the additive and the metal oxide, resulting in a detectable resistance change. Au additives are different as the metal state is stable under operational conditions, making the mechanism different from other noble metals and resulting in desirable sensor behavior.
2. Materials and Methods
WO3 lamella was prepared according to Kida et al. [5]. Firstly, 191 mL of conc. H2SO4 was added to 409 mL of DI water. Then, 16.40 g of Na2WO4*2H2O was dissolved in 100 mL of DI-Wasser and slowly dropped into the sulfuric acid solution. The solution was stirred overnight at 30 °C, and afterwards, the precipitate was washed with DI water until the wash water had a pH of 5.3 (centrifuged at 10,000 rpm for 5 min). The precipitate was then dried for 18 h at 80 °C and calcined for 2 h at 500 °C. In order to add the Au surface clusters, the method of Zanella et al. [6] was used. Firstly, 0.12 g of urea, 0.088 g of HAuCl4*4H2O, and 1 g of WO3 were dispersed in 100 mL of DI water. The mixture was stirred for 16 h at 80 °C and washed 4 times with DI water (centrifuged at 10,000 rpm for 10 min). The precipitate was dried at 50 °C overnight and calcined at 500 °C for 2 h. This resulted in 5 wt% Au loading. The WO3 powders were ground with 1,2-propane diol (Sigma Aldrich; 99.5+% A.C.S. Reagent) into a paste that was screen-printed onto alumina substrates with interdigitated Pt electrodes and a backside Pt heater (CeramTec Gmbh). After drying at RT, the sensor was calcined for 10 min at 400 °C, 10 min at 500 °C, and 10 min at 400 °C. Five chemically different and application-relevant gases were tested [3]. The gases were supplied from bottles (Westfalen AG, Münster, Germany) at a constant flowrate of 200 mL/min using an automated gas-mixing system. The sensors were heated to 300 °C, and the resistance was measured using a Keithley 199 electrometer. The following relationship was used to calculate the signal for reducing gases (the inverse was used for NO2):
3. Results and Discussion
The WO3-based samples with the Au additive showed higher sensor signals for all of the tested reducing gases, see Figure 1a. This stands in stark contrast to the results attained with other oxidized noble metal additives, where the sensor signal for all the test gases, except ethanol, became negligible at 5 wt% loading [1,2,3,4]. Additionally, unlike other noble metal additives that cause a significant increase in the sensor’s resistance to nitrogen, the values for the sensor with Au additives were similar to those of the pristine sample, see Figure 1b.
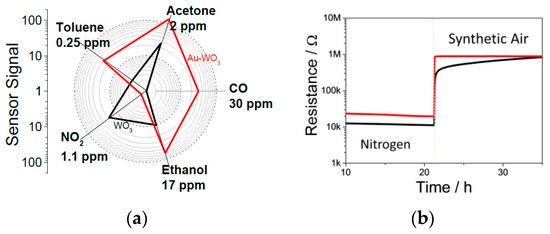
Figure 1.
(a) Sensor profile and (b) response to oxygen of the pristine and the 5 wt% WO3 sensor with Au additive.
It is clear from these results that Au additives in WO3 follow a different mechanism than other noble metal additives. Catalytic sensitization seems likely. In fuel cells, gold is known to be a poor catalyst for the oxygen reduction reaction. It could therefore be possible that the oxidation reaction of the reducing gases is markedly catalyzed, while the enhancement of the oxidation kinetics is lower. Additional characterization will be carried out to elucidate the mechanism.
Author Contributions
Conceptualization, A.S. and N.B.; methodology, A.S.; formal analysis, A.S.; investigation, A.S.; resources, U.W. and N.B.; data curation, A.S.; writing—original draft preparation, A.S., writing—review and editing, N.B.; visualization, A.S.; supervision, U.W.; project administration, U.W.; funding acquisition, U.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data to this study will be made available upon request.
Acknowledgments
This work was supported by Tetsuya Kida (Kumamoto University).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Morrison, S.R.; Estrup, P.J. The Chemical Physics of Surfaces. Phys. Today 1978, 31, 52–53. [Google Scholar] [CrossRef]
- Yamazoe, N. New Approaches for Improving Semiconductor Gas Sensors. Sens. Actuators B 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Staerz, A.; Boehme, I.; Degler, D.; Bahri, M.; Doronkin, D.; Zimina, A.; Brinkmann, H.; Herrmann, S.; Junker, B.; Ersen, O.; et al. Rhodium Oxide Surface-Loaded Gas Sensors. Nanomaterials 2018, 8, 892. [Google Scholar] [CrossRef]
- Staerz, A.; Liu, Y.; Geyik, U.; Brinkmann, H.; Weimar, U.; Zhang, T. The Effect of Platinum Loading on WO3 Based Sensors. Sens. Actuators B Chem. 2019, 291, 378–384. [Google Scholar] [CrossRef]
- Hua, Z.; Yuasa, M.; Kida, T.; Yamazoe, N.; Shimanoe, K. H2 Sensing Mechanism of Pd-Loaded WO3 Nanoparticles Gas Sensors. Chem. Lett. 2014, 2, 3–6. [Google Scholar] [CrossRef]
- Zanella, R.; Giorgio, S.; Henry, C.R.; Louis, C. Alternative Methods for the Preparation of Gold Nanoparticles Supported on TiO2. J. Phys. Chem. B 2002, 106, 7634–7642. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).