Microfluidic System with Integrated Electrode Array for High-Throughput Electrochemical Impedance Spectroscopy Analysis of Localised Cells †
Abstract
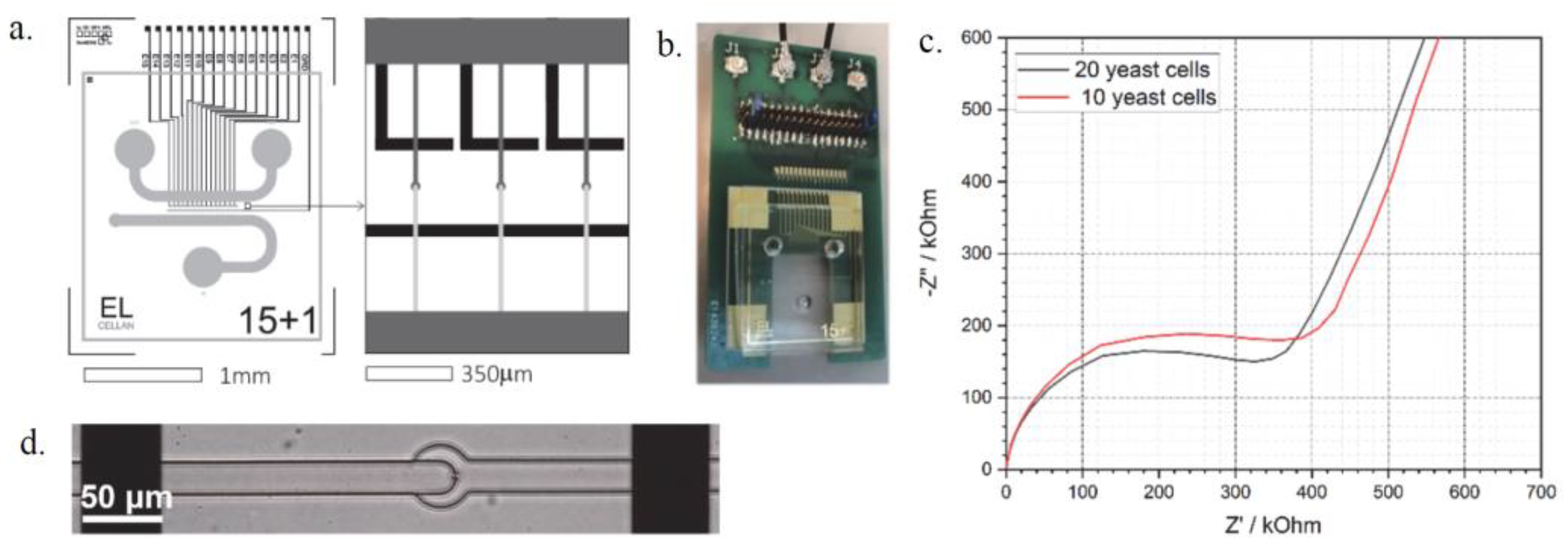
1. Introduction
2. Materials and Methods
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-chips: Into the next decade. Nat. Rev. Drug Discov. 2020, 20, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hui, J.; Yang, P.; Mao, H. Microfluidic organ-on-a-chip system for disease modeling and drug development. Biosensors 2022, 12, 370. [Google Scholar] [CrossRef] [PubMed]
- Asami, K. Characterization of biological cells by dielectric spectroscopy. J. Non-Cryst. Solids 2002, 305, 268–277. [Google Scholar] [CrossRef]
- Taylor, A.M.; Blurton-Jones, M.; Rhee, S.W.; Cribbs, D.H.; Cotman, C.W.; Jeon, N.L. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods 2005, 2, 599–605. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bató, L.; Fürjes, P. Microfluidic System with Integrated Electrode Array for High-Throughput Electrochemical Impedance Spectroscopy Analysis of Localised Cells. Proceedings 2024, 97, 187. https://doi.org/10.3390/proceedings2024097187
Bató L, Fürjes P. Microfluidic System with Integrated Electrode Array for High-Throughput Electrochemical Impedance Spectroscopy Analysis of Localised Cells. Proceedings. 2024; 97(1):187. https://doi.org/10.3390/proceedings2024097187
Chicago/Turabian StyleBató, Lilia, and Péter Fürjes. 2024. "Microfluidic System with Integrated Electrode Array for High-Throughput Electrochemical Impedance Spectroscopy Analysis of Localised Cells" Proceedings 97, no. 1: 187. https://doi.org/10.3390/proceedings2024097187
APA StyleBató, L., & Fürjes, P. (2024). Microfluidic System with Integrated Electrode Array for High-Throughput Electrochemical Impedance Spectroscopy Analysis of Localised Cells. Proceedings, 97(1), 187. https://doi.org/10.3390/proceedings2024097187





