Integration of a Bead-Based Immunoassay on a Commercial PCR-Performing POC Device †
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
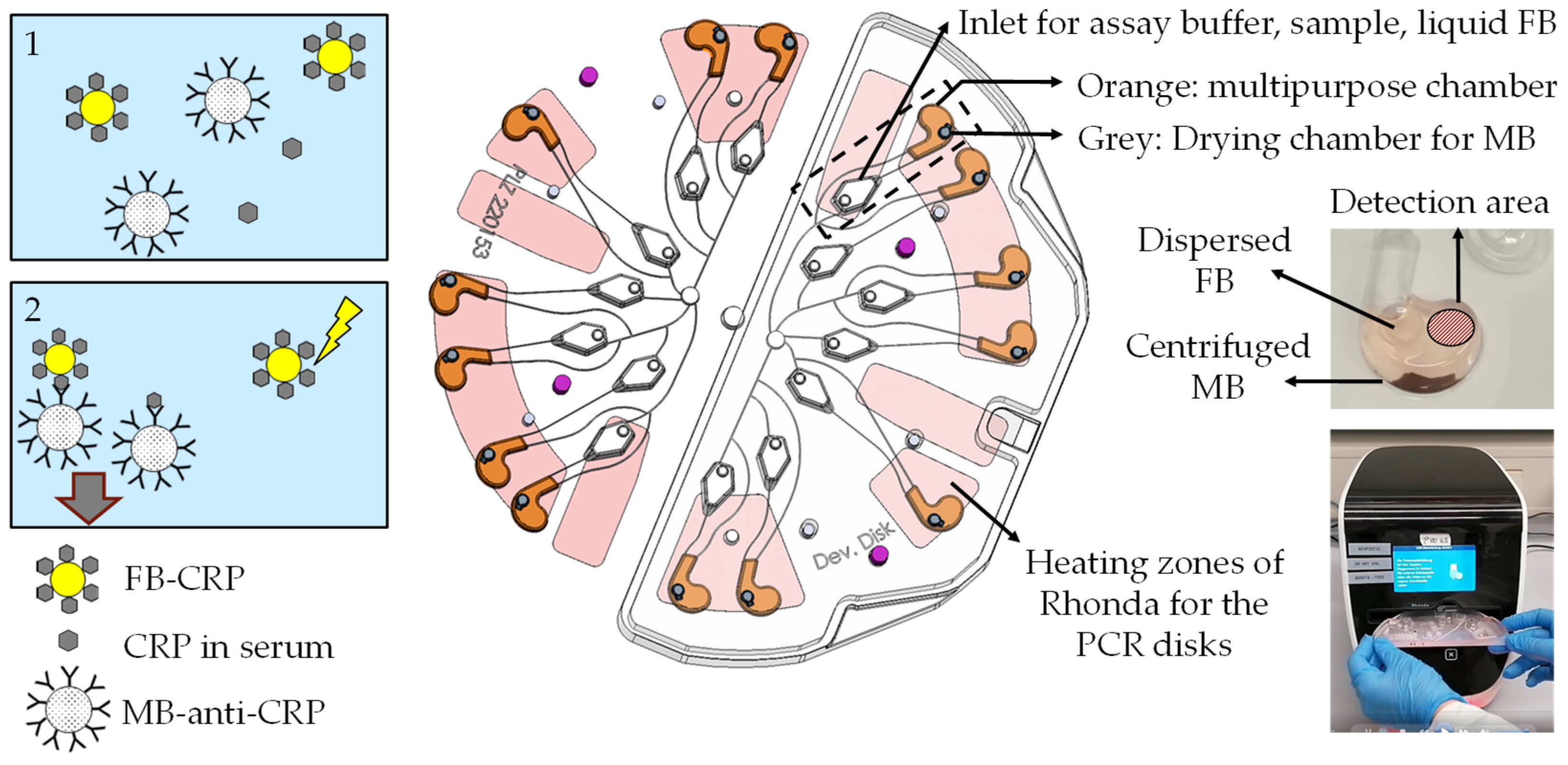
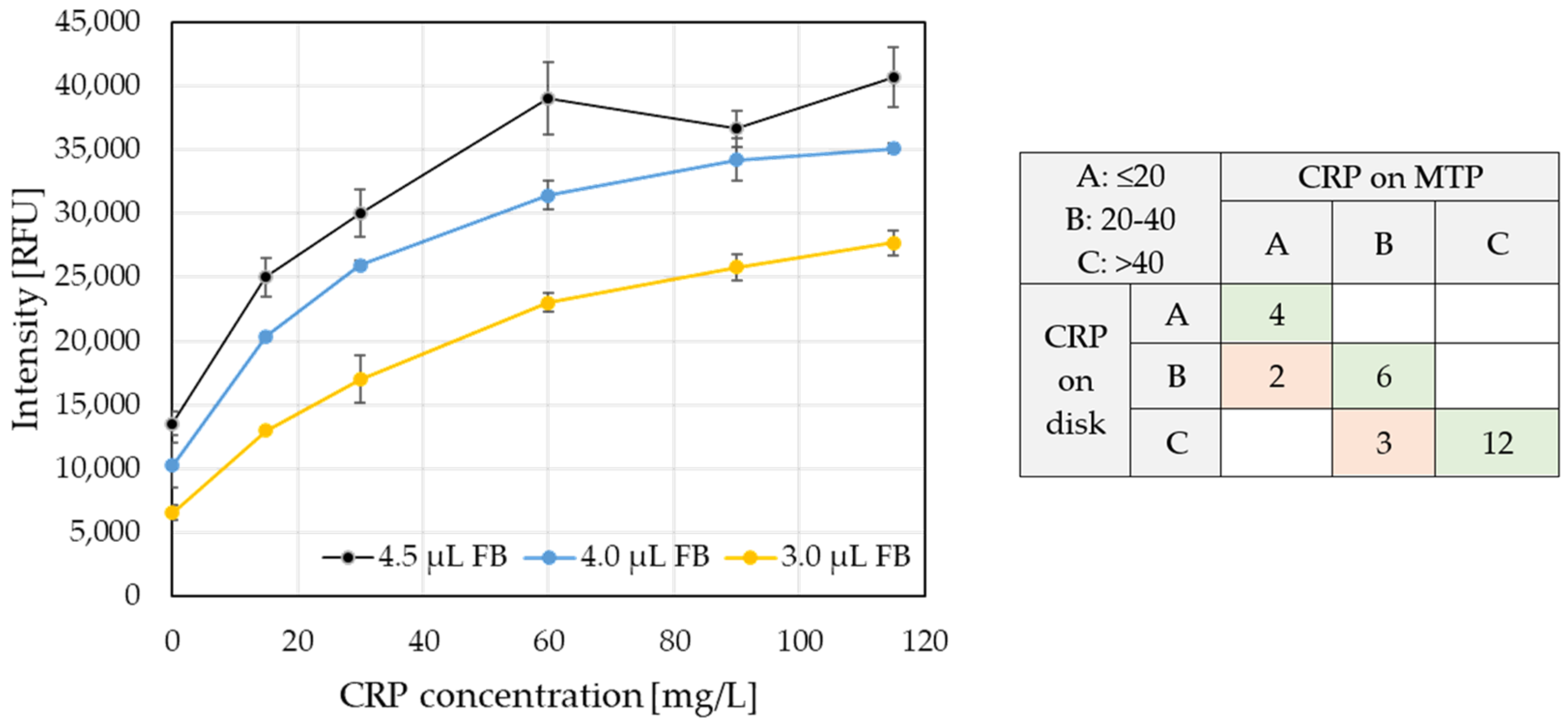
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gentile, I.; Schiano Moriello, N.; Hopstaken, R.; Llor, C.; Melbye, H.; Senn, O. The Role of CRP POC Testing in the Fight against Antibiotic Overuse in European Primary Care: Recommendations from a European Expert Panel. Diagnostics 2023, 13, 320. [Google Scholar] [CrossRef]
- Johannsen, B.; Baumgartner, D.; Karpíšek, M.; Stejskal, D.; Boillat-Blanco, N.; Knüsli, J.; Panning, M.; Paust, N.; Zengerle, R.; Mitsakakis, K. Patient Stratification for Antibiotic Prescriptions Based on the Bound-Free Phase Detection Immunoassay of C-Reactive Protein in Serum Samples. Biosensors 2023, 13, 1009. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, B.; Baumgartner, D.; Karkossa, L.; Paust, N.; Karpíšek, M.; Bostanci, N.; Zengerle, R.; Mitsakakis, K. ImmunoDisk-A Fully Automated Bead-Based Immunoassay Cartridge with All Reagents Pre-Stored. Biosensors 2022, 12, 413. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.C.; Gillespie, D.; White, P.; Bates, J.; Lowe, R.; Thomas-Jones, E.; Wootton, M.; Hood, K.; Phillips, R.; Melbye, H.; et al. C-Reactive Protein Testing to Guide Antibiotic Prescribing for COPD Exacerbations. N. Engl. J. Med. 2019, 381, 111–120. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johannsen, B.; Baumgartner, D.; Karpíšek, M.; Stejskal, D.; Paust, N.; Zengerle, R.; Mitsakakis, K. Integration of a Bead-Based Immunoassay on a Commercial PCR-Performing POC Device. Proceedings 2024, 97, 166. https://doi.org/10.3390/proceedings2024097166
Johannsen B, Baumgartner D, Karpíšek M, Stejskal D, Paust N, Zengerle R, Mitsakakis K. Integration of a Bead-Based Immunoassay on a Commercial PCR-Performing POC Device. Proceedings. 2024; 97(1):166. https://doi.org/10.3390/proceedings2024097166
Chicago/Turabian StyleJohannsen, Benita, Desirée Baumgartner, Michal Karpíšek, David Stejskal, Nils Paust, Roland Zengerle, and Konstantinos Mitsakakis. 2024. "Integration of a Bead-Based Immunoassay on a Commercial PCR-Performing POC Device" Proceedings 97, no. 1: 166. https://doi.org/10.3390/proceedings2024097166
APA StyleJohannsen, B., Baumgartner, D., Karpíšek, M., Stejskal, D., Paust, N., Zengerle, R., & Mitsakakis, K. (2024). Integration of a Bead-Based Immunoassay on a Commercial PCR-Performing POC Device. Proceedings, 97(1), 166. https://doi.org/10.3390/proceedings2024097166






