Sensing Material Temperature Effect on the Multiple Gas Sensor Sensing Response †
Abstract
1. Introduction
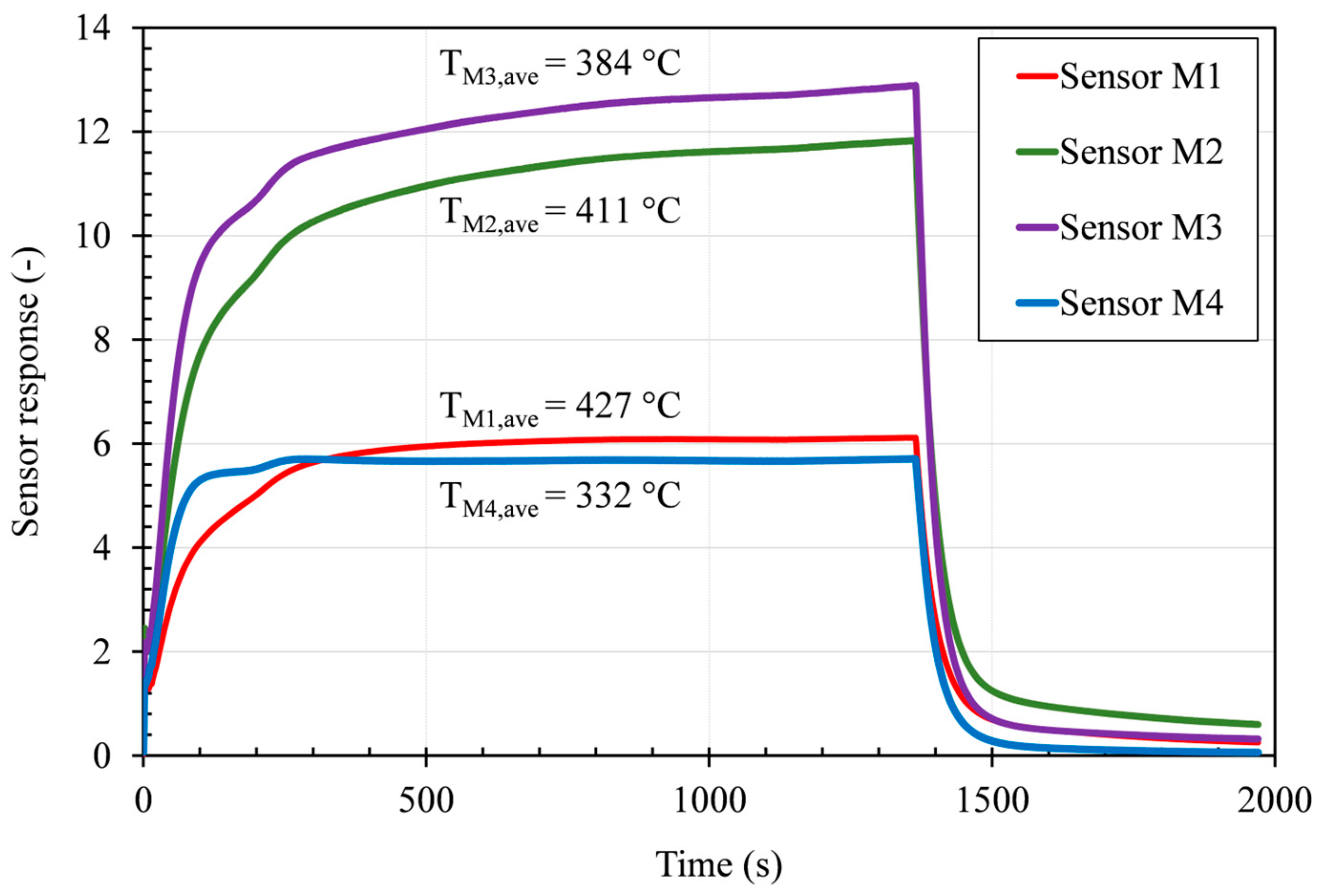
2. Materials and Methods
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitar, A.; Scattolo, E.; Valt, M.; Bagolini, A.; Tosato, P.; Gaiardo, A. Sensing Material Temperature Effect on the Multiple Gas Sensor Sensing Response. Proceedings 2024, 97, 130. https://doi.org/10.3390/proceedings2024097130
Sitar A, Scattolo E, Valt M, Bagolini A, Tosato P, Gaiardo A. Sensing Material Temperature Effect on the Multiple Gas Sensor Sensing Response. Proceedings. 2024; 97(1):130. https://doi.org/10.3390/proceedings2024097130
Chicago/Turabian StyleSitar, Anze, Elia Scattolo, Matteo Valt, Alvise Bagolini, Pietro Tosato, and Andrea Gaiardo. 2024. "Sensing Material Temperature Effect on the Multiple Gas Sensor Sensing Response" Proceedings 97, no. 1: 130. https://doi.org/10.3390/proceedings2024097130
APA StyleSitar, A., Scattolo, E., Valt, M., Bagolini, A., Tosato, P., & Gaiardo, A. (2024). Sensing Material Temperature Effect on the Multiple Gas Sensor Sensing Response. Proceedings, 97(1), 130. https://doi.org/10.3390/proceedings2024097130





