Comparison of Different Methods of Extraction for Pomegranate Seeds †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Oil from Seeds
2.2.1. Soxhlet Extraction
2.2.2. Cold Extraction
2.2.3. Accelerated Solvent Extraction
2.3. Fatty Acid Composition
2.4. Positional Distribution of Fatty Acids in the sn-2 and sn-1,3 Positions of TAG
2.5. Determination of Sterols
2.6. PDSC Measurements
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahesar, S.A.; Kori, A.H.; Sherazi, S.T.H.; Kandhro, A.A.; Laghari, Z.H. Pomegranate (Punica granatum) Seed Oil. In Fruit Oils: Chemistry and Functionality; Ramadan, M., Ed.; Springer: Cham, Switzerland, 2019; pp. 691–710. [Google Scholar] [CrossRef]
- Białek, A.; Stawarska, A.; Bodecka, J.; Białek, M.; Tokarz, A. Pomegranate seed oil influences the fatty acids profile and reduces the activity of desaturases in livers of Sprague-Dawley rats. Prostaglandins Other Lipid Mediat. 2017, 131, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Caligiani, A.; Bonzanini, F.; Palla, G.; Martina, C.; Renato, B. Characterization of a Potential Nutraceutical Ingredient: Pomegranate (Punica granatum L.) Seed Oil Unsaponifiable Fraction. Plant Foods Hum. Nutr. 2010, 65, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Natolino, A.; Da Porto, C. Supercritical carbon dioxide extraction of pomegranate (Punica granatum L.) seed oil: Kinetic modelling and solubility evaluation. J. Supercrit. Fluids 2019, 151, 30–39. [Google Scholar] [CrossRef]
- Yekdane, N.; Goli, S.A.H. Effect of Pomegranate Juice on Characteristics and Oxidative Stability of Microencapsulated Pomegranate Seed Oil Using Spray Drying. Food Bioprocess. Technol. 2019, 12, 1614–1625. [Google Scholar] [CrossRef]
- Drinić, Z.; Mudrić, J.; Zdunić, G.; Bigović, D.; Menković, N.; Šavikin, K. Effect of pomegranate peel extract on the oxidative stability of pomegranate seed oil. Food Chem. 2020, 333, 127501. [Google Scholar] [CrossRef] [PubMed]
- Kraujalis, P.; Venskutonis, R.P.; Pukalskas, A.; Kazernaviciut, R. Accelerated solvent extraction of lipids from Amaranthus spp. seeds and characterization of their composition. J. Food Sci. Technol. 2013, 54, 528–534. [Google Scholar] [CrossRef]
- Chena, W.; Liu, Y.; Song, L.; Sommerfeld, M.; Hu, Q. Automated accelerated solvent extraction method for total lipid analysis of microalgae. Algal Res. 2020, 51, 102080. [Google Scholar] [CrossRef]
- Liu, X.; Fan, K.; Song, W.; Wang, Z. Optimization of accelerated solvent extraction of fatty acids from Coix seeds using chemometrics methods. Food Meas. 2019, 13, 1773–1780. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, N.; Aljamea, A.; Abuthayn, S.; Aqeel, M. Evaluation of solvent and temperature effect on green accelerated solvent extraction (ASE) and UHPLC quantification of phenolics in fresh olive fruit (Olea europaea). Food Chem. 2020, 128248. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. ISO 665: Oilseeds—Determination of Moisture and Volatile Matter Content; International Organization for Standardization: Geneva, Switzerland, 2000. [Google Scholar]
- International Organization for Standardization. ISO 659: Oilseeds—Determination of Oil Content (Reference Method); International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Lohani, U.C.; Fallahi, P.; Muthukumarappan, K. Comparison of Ethyl Acetate with Hexane for Oil Extraction from Various Oilseeds. J. Am. Oil Chem. Soc. 2015, 92, 743–754. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 5509: Animal and Vegetable Fats and Oils—Preparation of Methyl Esters of Fatty Acids; International Organization for Standardization: Geneva, Switzerland, 2001. [Google Scholar]
- International Organization for Standardization. ISO 5508: Animal and Vegetable Fats and Oils—Analysis by Gas Chromatography of Methyl Esters of Fatty Acids; International Organization for Standardization: Geneva, Switzerland, 1996. [Google Scholar]
- Bryś, J.; Vaz Flores, I.F.; Górska, A.; Wirkowska-Wojdyła, M.; Ostrowska-Ligęza, E.; Bryś, A. Use of GC and PDSC methods to characterize human milk fat substitutes obtained from lard and milk thistle oil mixtures. J. Ther. Anal. Calorim. 2017, 130, 319–327. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 12228-1: Determination of Individual and Total Sterols Contents—Gas Chromatographic Method—Part 1: Animal and Vegetable Fats and Oils; International Organization for Standardization: Geneva, Switzerland, 2014. [Google Scholar]
- Habibnia, M.; Ghavami, M.; Ansaripour, M.; Vosough, S. Chemical evaluation of oils extracted from five different varieties of iranian pomegranate seeds. J. Food Biosci. Technol. 2012, 2, 35–40. [Google Scholar]
- Fernandes, L.; Pereira, J.A.; Lope’z-Corte, I.; Salazar, D.M.; Ramalhosa, E.; Casal, S. Fatty acid, vitamin E and sterols composition of seed oils from nine different pomegranate (Punica granatum L.) cultivars grown in Spain. J. Food Compos. Anal. 2015, 39, 13–22. [Google Scholar] [CrossRef]
- Silva, L.O.; Ranquine, L.G.; Monteiro, M.; Torresa, A.G. Pomegranate (Punica granatum L.) seed oil enriched with conjugated linolenic acid (cLnA), phenolic compounds and tocopherols: Improved extraction of a specialty oil by supercritical CO2. J. Supercrit. Fluids 2019, 147, 126–137. [Google Scholar] [CrossRef]
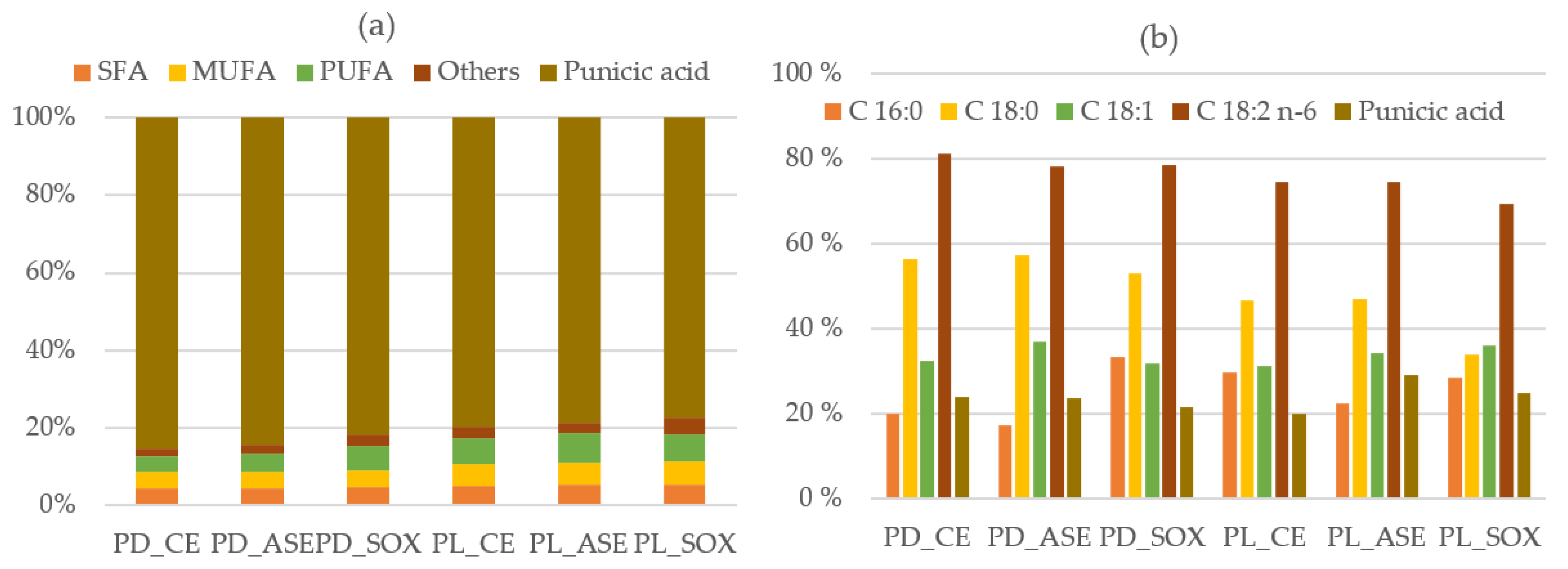
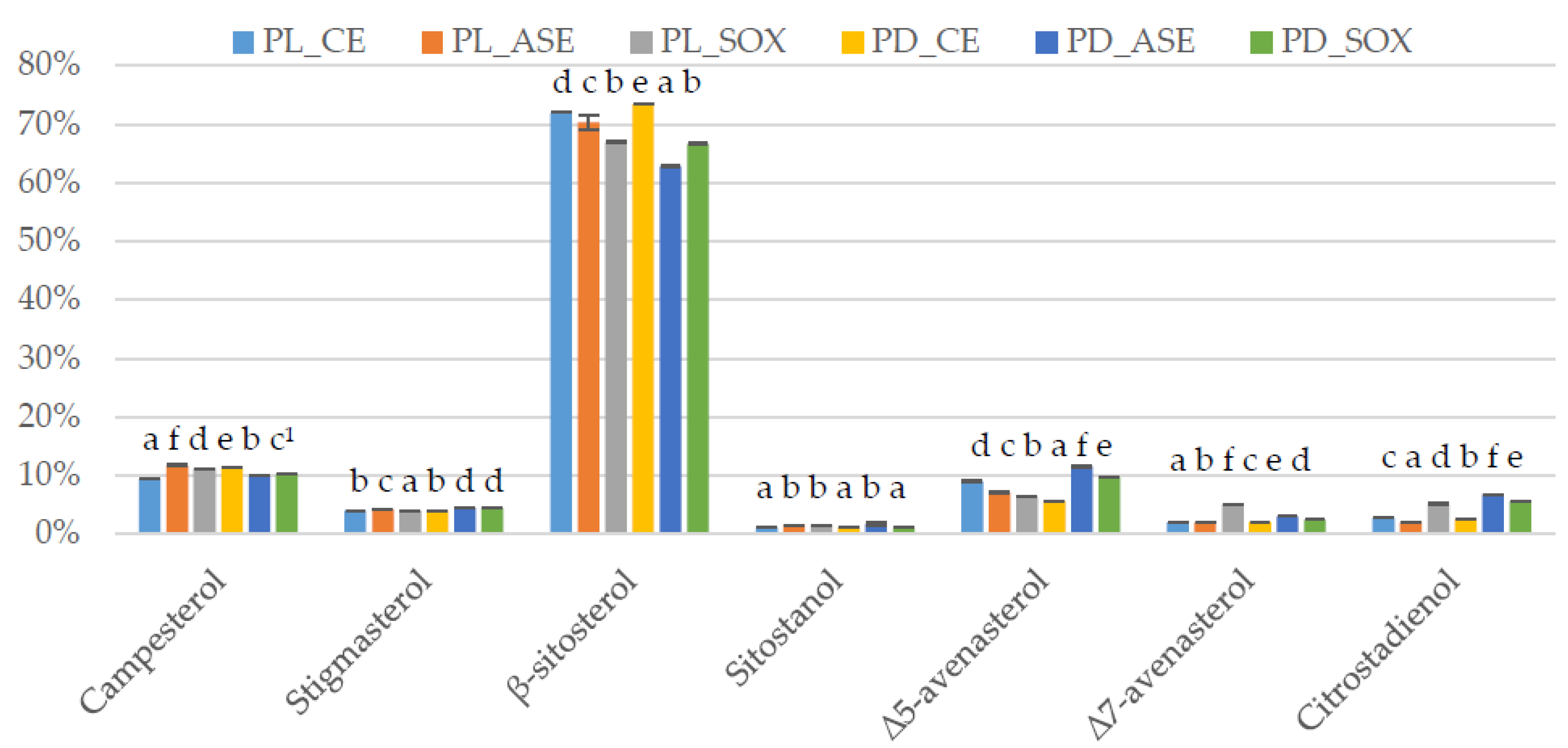
| Type of Sample | Extraction Yield 1 | Induction Time 1 |
|---|---|---|
| PD_CE | 16.95 ± 0.59 d | 4.55 ± 0.15 e |
| PD_ASE | 12.05 ± 0.13 b | 3.75 ± 0.06 d |
| PD_SOX | 16.31 ± 0.67 d | 0.71 ± 0.03 b |
| PL_CE | 13.34 ± 0.30 c | 3.81 ± 0.25 d |
| PL_ASE | 10.99 ± 0.22 a | 2.63 ± 0.08 c |
| PL_SOX | 14.02 ± 0.17 c | 0.32 ± 0.11 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryś, J.; Obranović, M.; Repajić, M.; Kraljić, K.; Škevin, D.; Bryś, A.; Górska, A.; Ostrowska-Ligęza, E.; Wirkowska-Wojdyła, M. Comparison of Different Methods of Extraction for Pomegranate Seeds. Proceedings 2021, 70, 91. https://doi.org/10.3390/foods_2020-07657
Bryś J, Obranović M, Repajić M, Kraljić K, Škevin D, Bryś A, Górska A, Ostrowska-Ligęza E, Wirkowska-Wojdyła M. Comparison of Different Methods of Extraction for Pomegranate Seeds. Proceedings. 2021; 70(1):91. https://doi.org/10.3390/foods_2020-07657
Chicago/Turabian StyleBryś, Joanna, Marko Obranović, Maja Repajić, Klara Kraljić, Dubravka Škevin, Andrzej Bryś, Agata Górska, Ewa Ostrowska-Ligęza, and Magdalena Wirkowska-Wojdyła. 2021. "Comparison of Different Methods of Extraction for Pomegranate Seeds" Proceedings 70, no. 1: 91. https://doi.org/10.3390/foods_2020-07657
APA StyleBryś, J., Obranović, M., Repajić, M., Kraljić, K., Škevin, D., Bryś, A., Górska, A., Ostrowska-Ligęza, E., & Wirkowska-Wojdyła, M. (2021). Comparison of Different Methods of Extraction for Pomegranate Seeds. Proceedings, 70(1), 91. https://doi.org/10.3390/foods_2020-07657











