Multiple SERS Detection of Phenol Derivatives in Tap Water †
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Methods
SERS Substrates Synthesis
2.3. Experiment
2.4. Data
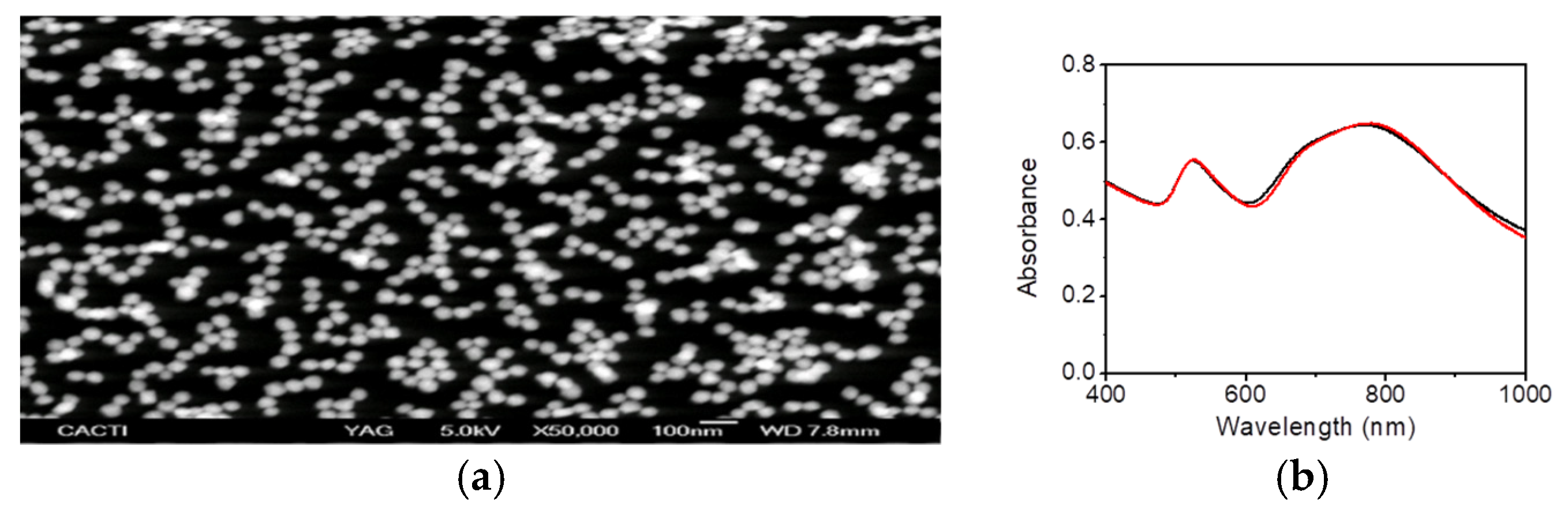
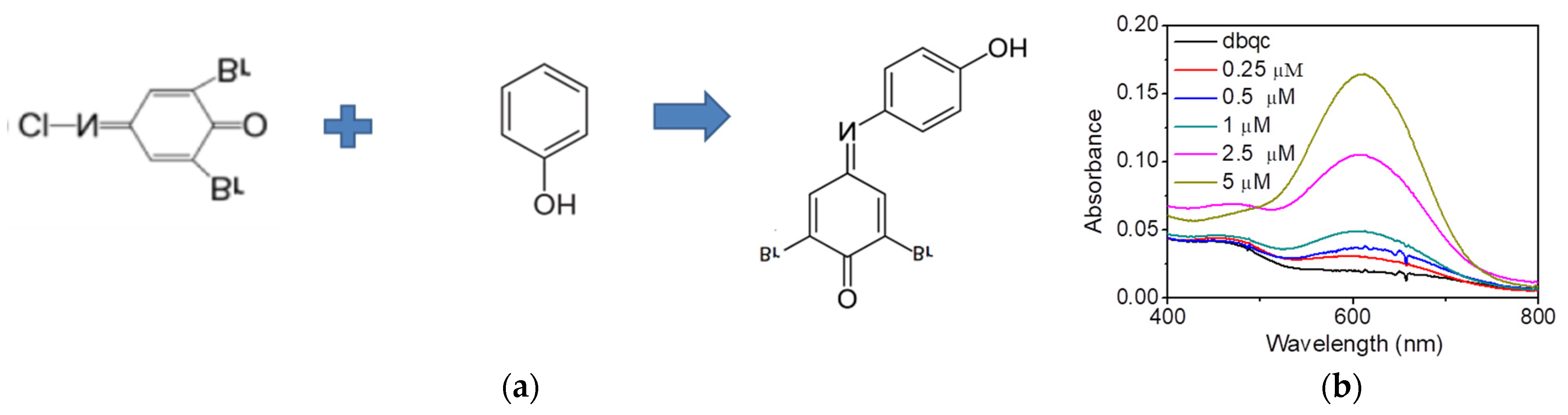
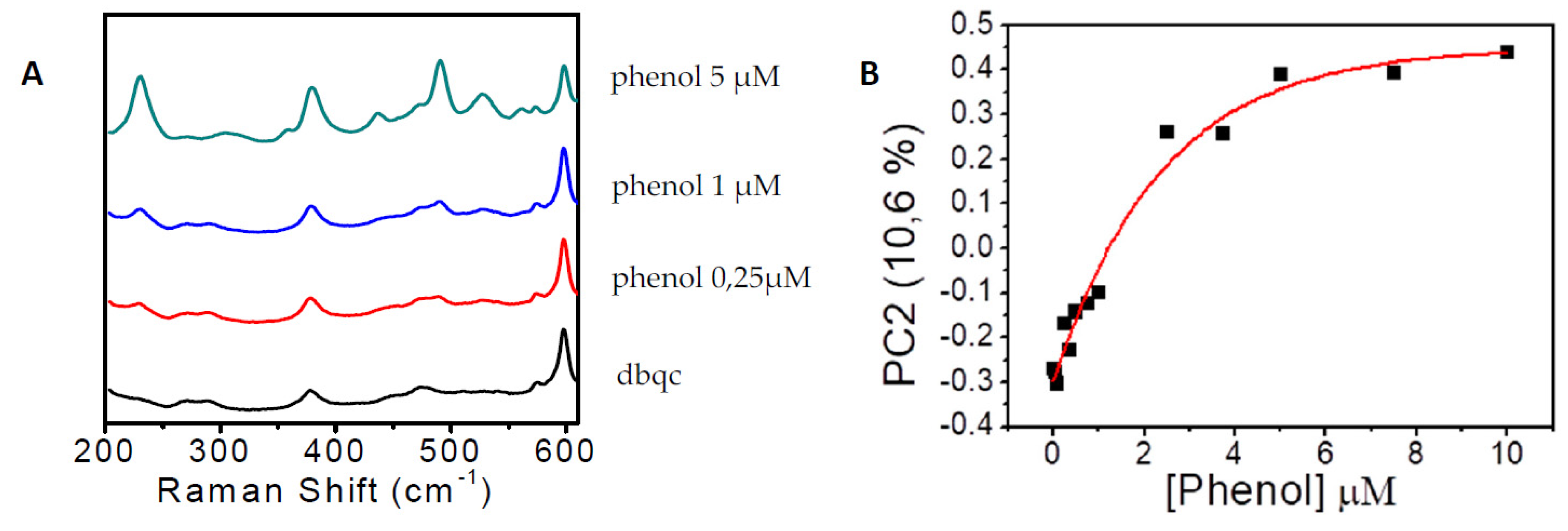
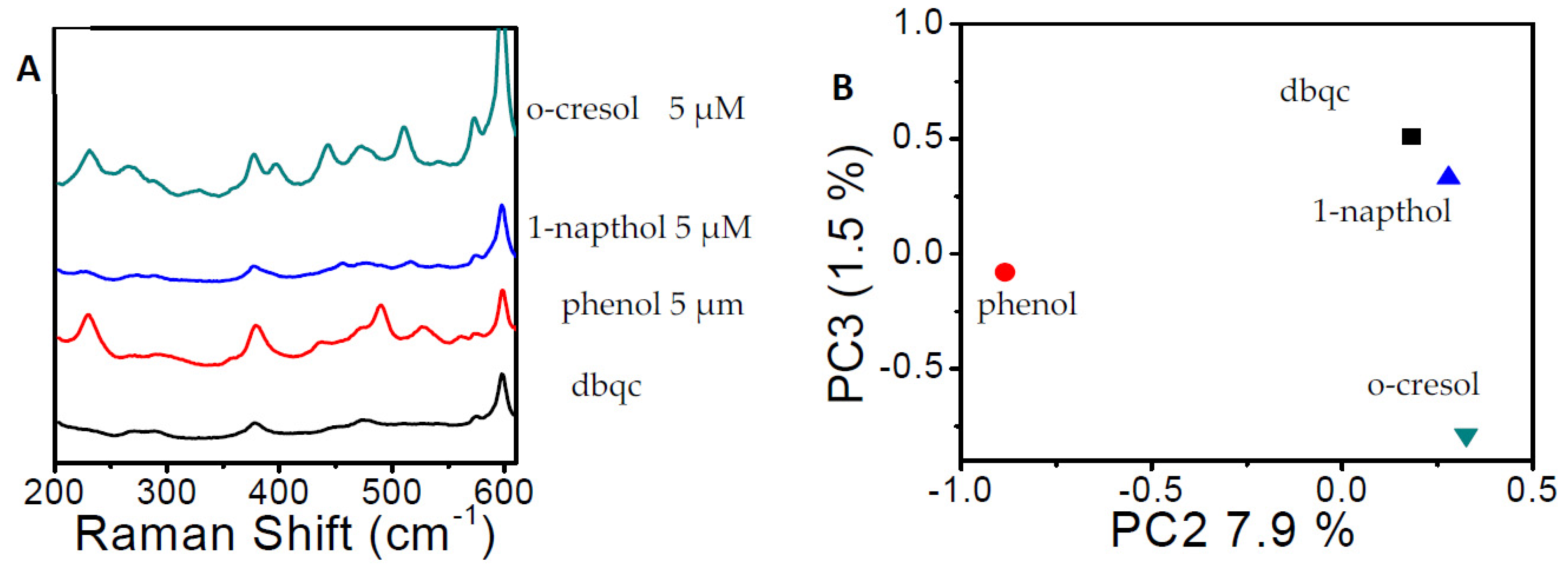
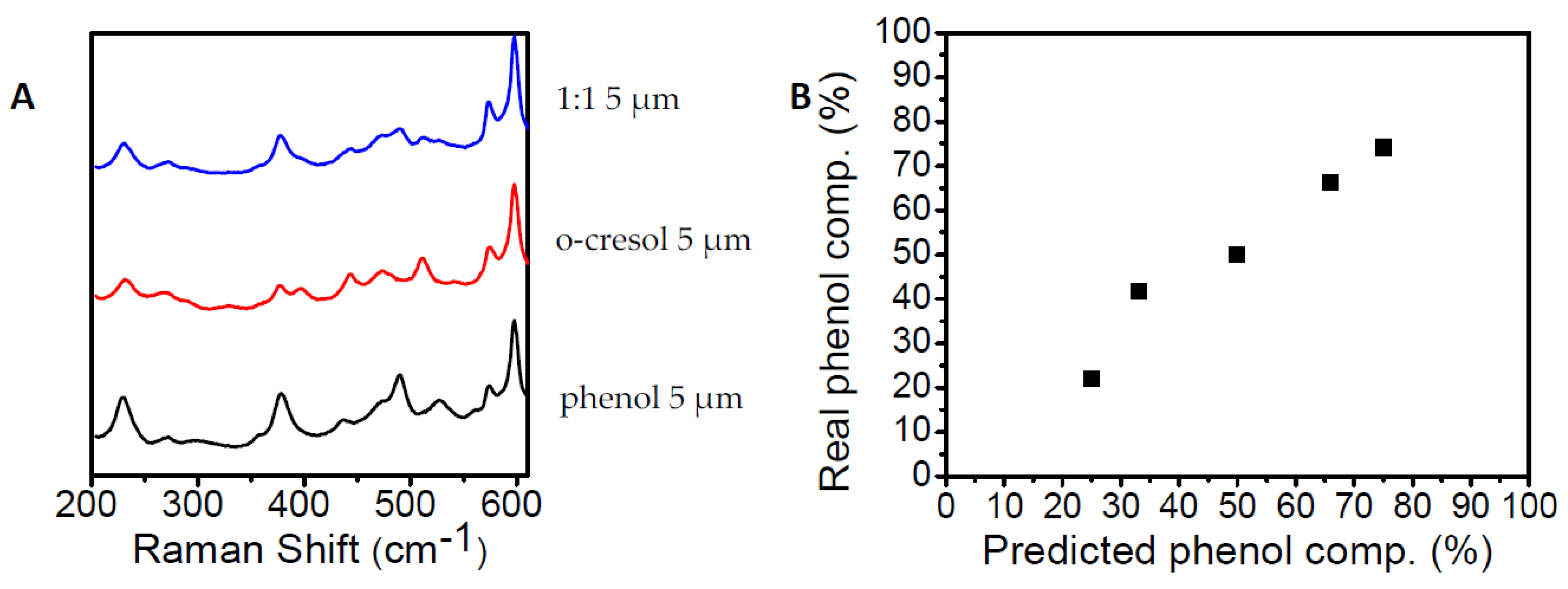
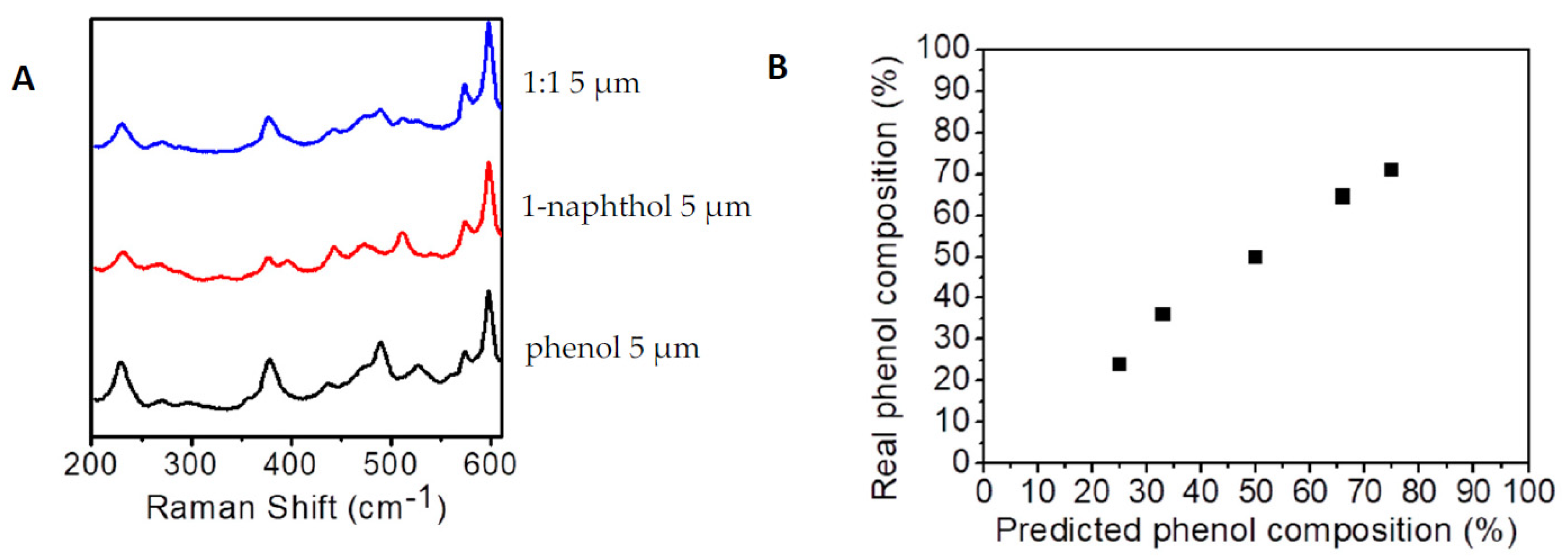
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- US Environmental Protection Agency. Ambient Water Quality Criteria for Pentachlorophenol; US Environmental Protection Agency: Washington, DC, USA, 1980. [Google Scholar]
- Acosta, C.A.; Pasquali, C.E.L.; Paniagua, G.; Garcinuño, R.M.; Hernando, P.F. Evaluation of total phenol pollution in water of San Martin Canal from Santiago del Estero, Argentina. Environ. Pollut. 2018, 236, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Schmauch, L.J.; Grubb, H.M. Determination of Phenols in Waste Waters by Ultraviolet Absorption. Anal. Chem. 1954, 26, 308–311. [Google Scholar] [CrossRef]
- Du, B.; Su, X.; Yang, K.; Pan, L.; Liu, Q.; Gong, L.; Wang, P.; Yang, J.; He, Y. Antibody-Free Colorimetric Detection of Total Aflatoxins in Rice Based on a Simple Two-Step Chromogenic Reaction. Anal. Chem. 2016, 88, 3775–3780. [Google Scholar] [CrossRef] [PubMed]
- Josephy, P.D.; Van Damme, A. Reaction of Gibbs Reagent with Para-Substituted Phenols. Anal. Chem. 1984, 56, 813–814. [Google Scholar] [CrossRef]
- Svobodová, D.; Křenek, P.; Fraenkl, M.; Gasparič, J. Colour reaction of phenols with the gibbs reagent. The reaction mechanism and decomposition and stabilisation of the reagent. Mikrochim. Acta 1977, 67, 251–264. [Google Scholar] [CrossRef]
- Bastús, N.G.; Comenge, J.; Puntes, V. Kinetically controlled seeded growth synthesis of citrate-stabilized gold nanoparticles of up to 200 nm: Size focusing versus ostwald ripening. Langmuir 2011, 27, 11098–11105. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreira-Casais, A.; Montes-García, V.; Pastoriza-Santos, I.; Prieto, M.Á.; Simal-Gandara, J.; Pérez-Juste, J. Multiple SERS Detection of Phenol Derivatives in Tap Water. Proceedings 2021, 70, 88. https://doi.org/10.3390/foods_2020-07755
Carreira-Casais A, Montes-García V, Pastoriza-Santos I, Prieto MÁ, Simal-Gandara J, Pérez-Juste J. Multiple SERS Detection of Phenol Derivatives in Tap Water. Proceedings. 2021; 70(1):88. https://doi.org/10.3390/foods_2020-07755
Chicago/Turabian StyleCarreira-Casais, Anxo, Verónica Montes-García, Isabel Pastoriza-Santos, Miguel Ángel Prieto, Jesus Simal-Gandara, and Jorge Pérez-Juste. 2021. "Multiple SERS Detection of Phenol Derivatives in Tap Water" Proceedings 70, no. 1: 88. https://doi.org/10.3390/foods_2020-07755
APA StyleCarreira-Casais, A., Montes-García, V., Pastoriza-Santos, I., Prieto, M. Á., Simal-Gandara, J., & Pérez-Juste, J. (2021). Multiple SERS Detection of Phenol Derivatives in Tap Water. Proceedings, 70(1), 88. https://doi.org/10.3390/foods_2020-07755






