Characterization of Methanosarcina mazei JL01 Isolated from Holocene Arctic Permafrost and Study of the Archaeon Cooperation with Bacterium Sphaerochaeta associata GLS2T †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Enrichment and Isolation
2.2. Phenotypic, Physiological and Biochemical Characterization
2.3. Cooperation Study
2.4. Molecular and Phylogenetic Analyses
2.5. Genomes Analysis
3. Results and Discussions
3.1. Enrichment and Pure Culture Isolation
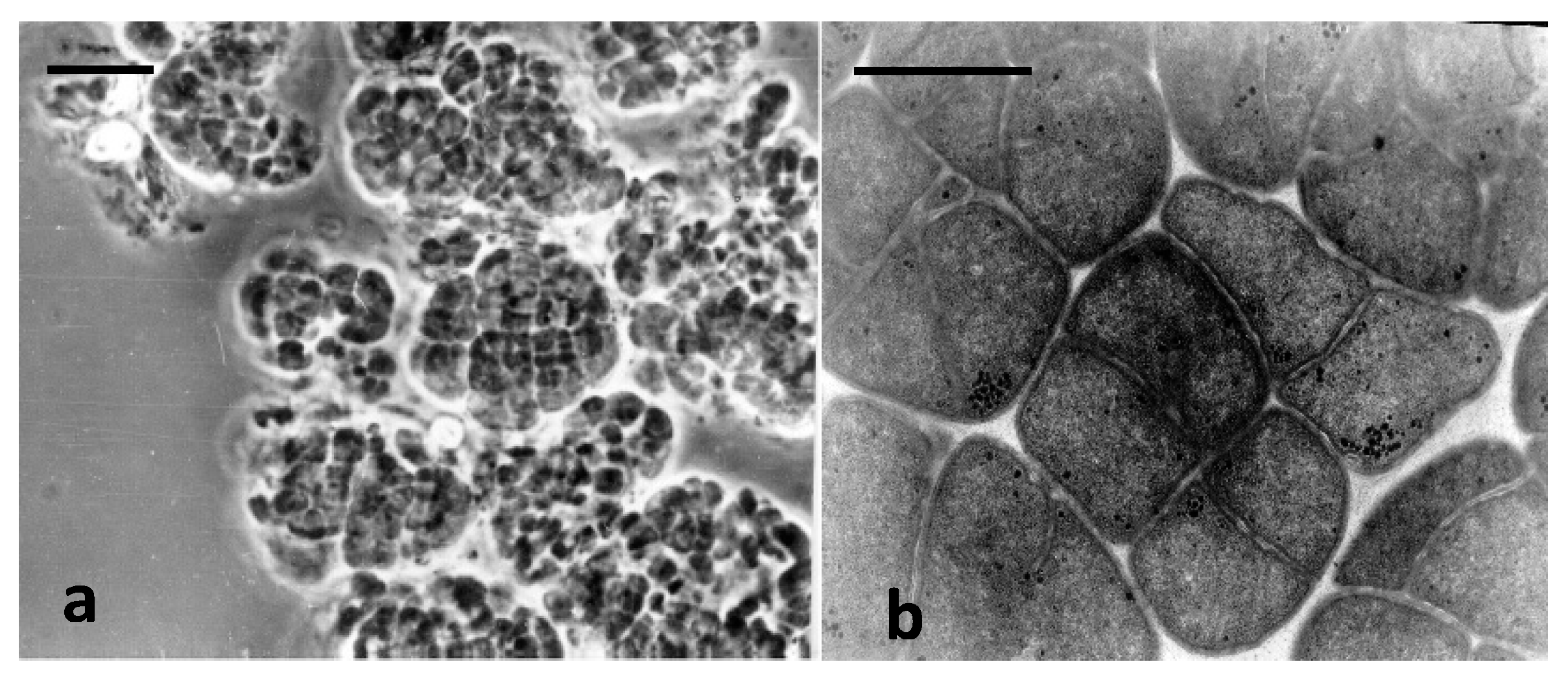
3.2. Characterization of Novel Methanogenic Strain
3.3. Phylogenetic Analyses
3.4. Genome Organization of Strain JL01
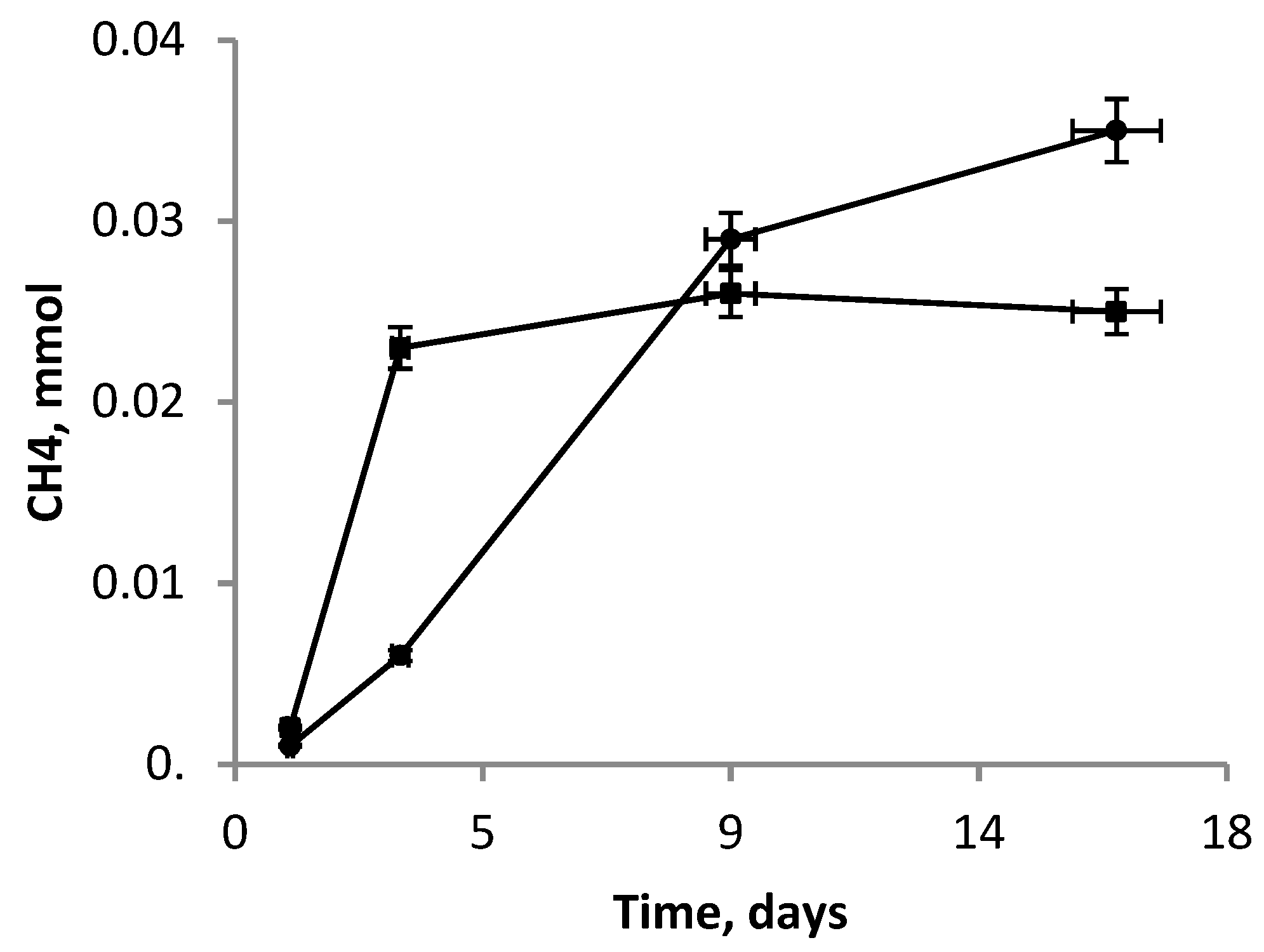
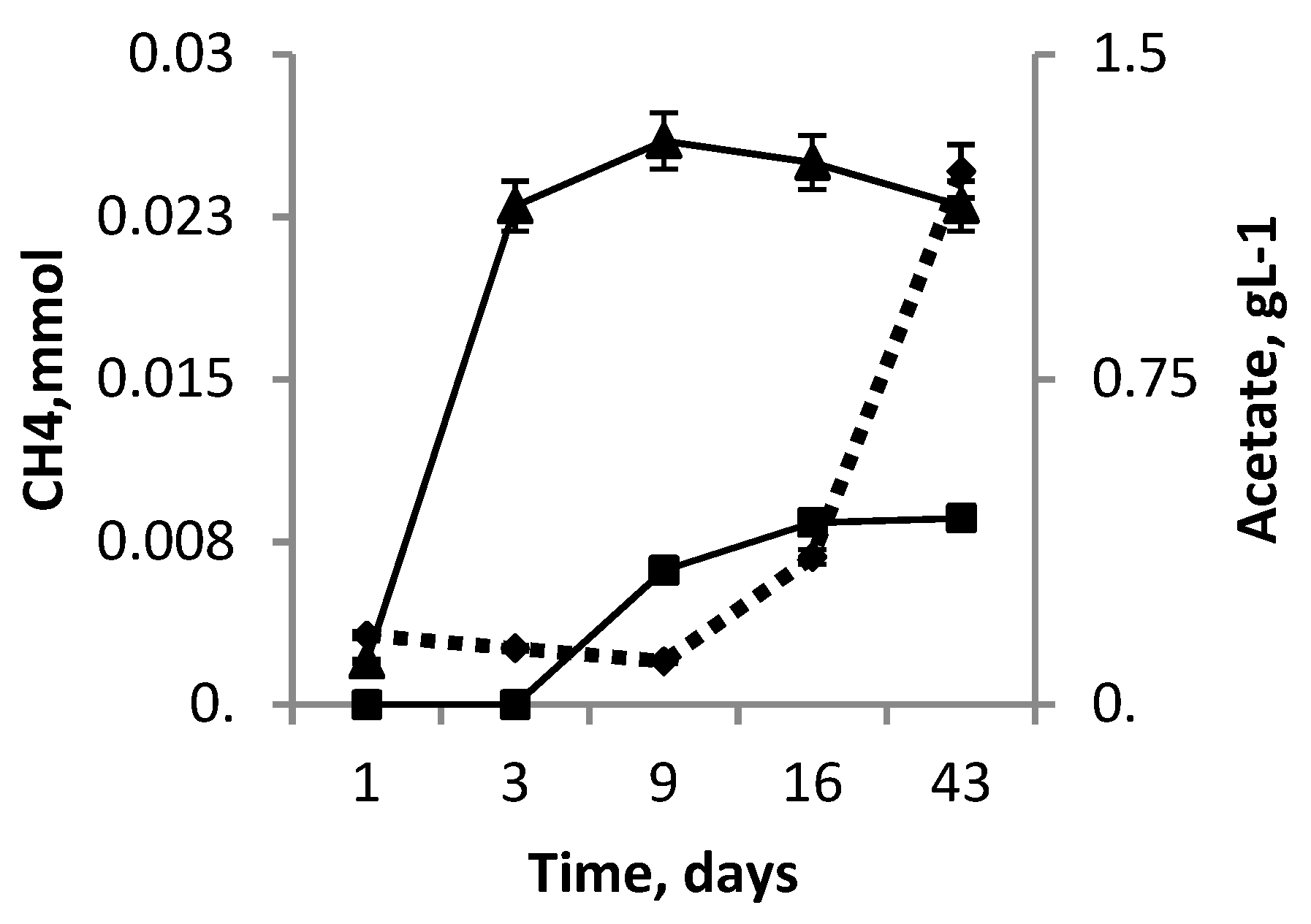
3.5. Co-Cultivation M. mazei JL01 and S. associata GLS2T
3.6. Genomic Data Support Possibility of the Cooperation
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Rivkina, E.M.; Shcherbakova, V.; Laurinavichius, K.; Petrovskaya, L.; Krivushin, K.; Kraev, G.; Pecheritsina, S.; Gilichinsky, D. Biogeochemistry of methane and methanogenic archaea in permafrost. FEMS Microbiol. Ecol. 2007, 61, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shcherbakova, V.; Yoshimura, Y.; Ryzhmanova, Y.; Taguchi, Y.; Segawa, T.; Oshurkova, V.; Rivkina, E. Archaeal communities of Arctic methane-containing permafrost. FEMS Microbiol. Ecol. 2016, 92, fiw135. [Google Scholar] [CrossRef] [PubMed]
- Rivkina, E.M.; Laurinavichius, K.; McGrath, J.; Tiedje, J.; Shcherbakova, V.; Gilichinsky, D. Microbial life in permafrost. Adv. Space Res. 2004, 33, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Krivushin, K.V.; Shcherbakova, V.A.; Petrovskaya, L.E.; Rivkina, E.M. Methanobacterium veterum sp. nov., from ancient Siberian permafrost. Int. J. Syst. Evol. Microbiol. 2010, 60, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Shcherbakova, V.; Rivkina, E.; Pecheritsyna, S.; Laurinavichius, K.; Suzina, N.; Gilichinsky, D. Methanobacterium arcticum sp. nov., a methanogenic archaeon from Holocene Arctic permafrost. Int. J. Syst. Evol. Microbiol. 2011, 61, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Rivkina, E.; Petrovskaya, L.; Vishnivetskaya, T.; Krivushin, K.; Shmakova, L.; Tutukina, M.; Meyers, A.; Kondrashov, F. Metagenomic analyses of the late Pleistocene permafrost—Additional tools for reconstruction of environmental conditions. Biogeosciences 2016, 13, 2207–2219. [Google Scholar] [CrossRef]
- Morris, B.E.; Henneberger, R.; Huber, H.; Moissl-Eichinger, C. Microbial syntrophy: Interaction for the common good. FEMS Microbiol. Rev. 2013, 37, 384–406. [Google Scholar] [CrossRef] [PubMed]
- Boone, D.R.; Whitman, W.B. Proposal of Minimal Standards for Describing New Taxa of Methanogenic Bacteria. Int. J. Syst. Bacteriol. 1988, 38, 212–219. [Google Scholar] [CrossRef]
- Troshina, O.; Oshurkova, V.; Suzina, N.; Machulin, A.; Ariskina, E.; Vinokurova, N.; Kopitsyn, D.; Novikov, A.; Shcherbakova, V. Sphaerochaeta associata sp. nov., a spherical spirochaete isolated from cultures of Methanosarcina mazei JL01. Int. J. Syst. Evol. Microbiol. 2015, 65, 4315–4322. [Google Scholar] [CrossRef] [PubMed]
- Marmur, J.; Doty, P. Thermal renaturation of deoxyribonucleic acids. J. Mol. Biol. 1961, 3, 585–594. [Google Scholar] [CrossRef]
- Delong, E.F. Archaea in coastal marine environments. Proc. Natl. Acad. Sci. USA 1992, 89, 5685–5689. [Google Scholar] [CrossRef] [PubMed]
- Kittelmann, S.; Seedorf, H.; Walters, W.A.; Clemente, J.C.; Knight, R.; Gordon, J.I.; Janssen, P.H. Simultaneous Amplicon Sequencing to Explore Co-Occurrence Patterns of Bacterial, Archaeal and Eukaryotic Microorganisms in Rumen Microbial Communities. PLoS ONE 2013, 8, e47879. [Google Scholar] [CrossRef]
- Springer, E.; Sachs, M.S.; Woese, C.R.; Boone, D.R. Partial gene sequences for the A subunit of methyl-coenzyme M reductase (mcrA) a phylogenetic tool for the family Methanosarcinaceae. Int. J. Syst. Bacteriol. 1995, 45, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Jiang, N.; Liu, X.; Dong, X. Methanogenesis from Methanol at Low Temperatures by a Novel Psychrophilic Methanogen, “Methanolobus psychrophilus” sp. nov., Prevalent in Zoige Wetland of the Tibetan Plateau. Appl. Environ. Microbiol. 2008, 74, 6114–6120. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oshurkova, V.; Troshina, O.; Trubitsyn, V.; Ryzhmanova, Y.; Bochkareva, O.; Shcherbakova, V. Characterization of Methanosarcina mazei JL01 Isolated from Holocene Arctic Permafrost and Study of the Archaeon Cooperation with Bacterium Sphaerochaeta associata GLS2T. Proceedings 2020, 66, 4. https://doi.org/10.3390/proceedings2020066004
Oshurkova V, Troshina O, Trubitsyn V, Ryzhmanova Y, Bochkareva O, Shcherbakova V. Characterization of Methanosarcina mazei JL01 Isolated from Holocene Arctic Permafrost and Study of the Archaeon Cooperation with Bacterium Sphaerochaeta associata GLS2T. Proceedings. 2020; 66(1):4. https://doi.org/10.3390/proceedings2020066004
Chicago/Turabian StyleOshurkova, Viktoriia, Olga Troshina, Vladimir Trubitsyn, Yana Ryzhmanova, Olga Bochkareva, and Viktoria Shcherbakova. 2020. "Characterization of Methanosarcina mazei JL01 Isolated from Holocene Arctic Permafrost and Study of the Archaeon Cooperation with Bacterium Sphaerochaeta associata GLS2T" Proceedings 66, no. 1: 4. https://doi.org/10.3390/proceedings2020066004
APA StyleOshurkova, V., Troshina, O., Trubitsyn, V., Ryzhmanova, Y., Bochkareva, O., & Shcherbakova, V. (2020). Characterization of Methanosarcina mazei JL01 Isolated from Holocene Arctic Permafrost and Study of the Archaeon Cooperation with Bacterium Sphaerochaeta associata GLS2T. Proceedings, 66(1), 4. https://doi.org/10.3390/proceedings2020066004





