Lyophilized Extract of Hibiscus sabdariffa L. Induces Cytotoxicity in Breast Cancer Cell Lines †
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemical
2.2. Plant Material
2.3. Preparation of the HSE Solution
- -
- Preparation of DMSO 0.5% in culture medium as a vehicle.
- -
- Preparation of Hibiscus Sabdariffa stock solution (1 mg/mL).
- -
- HSE 1.7 mg + Medium + DMSO 0.5%: 1700 mg.
2.4. Cell Lines and Cell Culture
2.4.1. Cell Viability Assay and Cell Count
2.4.2. Cytotoxicity Test (MTT Test)
2.5. Statistical Analysis
3. Results and Discussion
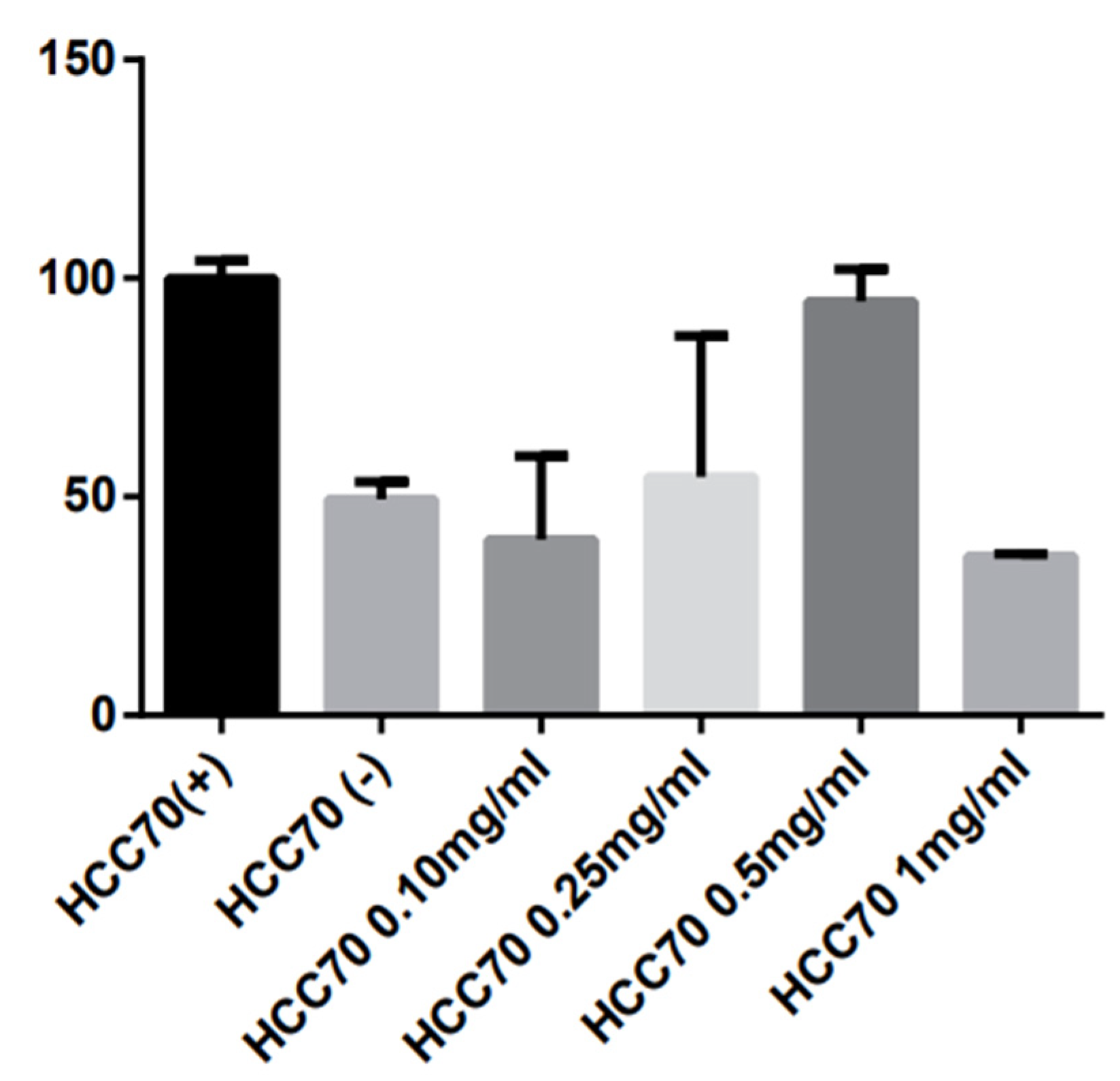
3.1. Cell Viability Assay
3.2. Cytotoxicity
Acknowledgments
References
- Meza-Junco, J.; Montaño-Loza, A.; Aguayo-González, Á. Bases moleculares del cáncer. Rev. Investig. Clin. 2006, 58, 56–70. [Google Scholar]
- Vallejo-Zamudio, E.; Rojas-Velázquez, A.; Torres-Bugarín, O.; Torres Bugarín, O.; Una Poderosa Herramienta en la Medicina Preventiva del Cáncer: Los Antioxidantes. El Resid. 2017, p. 104. Available online: www.medigraphic.org.mx (accessed on 10 October 2020).
- Aldaco-Sarvide, F.; Pérez-Pérez, P.; Cervantes-Sánchez, G.; Torrecillas-Torres, L.; Erazo-Valle-Solís, A.A.; Cabrera-Galeana, P.; Motola-Kuba, D.; Anaya, P.; Rivera-Rivera, S.; Cárdenas-Cárdenas, E.; et al. Mortality from cancer in Mexico: 2015 update. Gac. Mex. Oncol. 2018, 17, 28–34. [Google Scholar] [CrossRef]
- IARC. New Global Cancer Data: GLOBOCAN 2018|UICC. International Agency for Research on Cancer. 2018. Available online: https://www.uicc.org/new-global-cancer-data-globocan-2018 (accessed on 2 October 2020).
- OPS/OMS|Cáncer de Mama. Available online: https://www.paho.org/hq/index.php?option=com_content&view=article&id=5041:2011-breast-cancer&Itemid=3639&lang=es (accessed on 7 October 2020).
- Perou, C.M. Molecular Stratification of Triple-Negative Breast Cancers. Available online: www.TheOncologist.com (accessed on 8 October 2020).
- Zaharia, M.; Gómez, H. Cáncer de mama triple negativo: Una enfermedad de difícil diagnóstico y tratamiento. Rev. Peru. Med. Exp. Salud Publica 2014, 30, 2–8. [Google Scholar] [CrossRef]
- OMS. Nuevas Directrices de la OMS para Fomentar el uso Adecuado de las Medicinas Tradicionales; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- García, B.; Alberto Saldaña, L.S.; El Estrés Oxidativo y los Antioxidantes en la Prevención del Cáncer. Revista Habanera de Cienicas Médicas. 2013. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1729-519X2013000200005 (accessed on 7 October 2020).
- Tsuda, H.; Ohshima, Y.; Nomoto, H.; Fujita, K.; Matsuda, E.; Iigo, M.; Takasuka, N.; Malcolm, A. Cancer Prevention by Natural Compounds. Drug Metab. Pharmacokinet. 2004, 19, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Chen, J.; Wang, C. Chemopreventive Properties and Molecular Mechanisms of the Bioactive Compounds in Hibiscus sabdariffa Linne. Curr. Med. Chem. 2011, 18, 1245–1254. [Google Scholar] [CrossRef]
- Chiu, C.T.; Hsuan, S.W.; Lin, H.H.; Hsu, C.C.; Chou, F.P.; Chen, J.H. Hibiscus sabdariffa Leaf polyphenolic extract induces human melanoma cell death, apoptosis, and autophagy. J. Food Sci. 2015, 80, H649–H658. [Google Scholar] [CrossRef]
- Lin, H.H.; Chan, K.C.; Sheu, J.Y.; Hsuan, S.W.; Wang, C.J.; Chen, J.H. Hibiscus sabdariffa leaf induces apoptosis of human prostate cancer cells in vitro and in vivo. Food Chem. 2012, 132, 880–891. [Google Scholar] [CrossRef]
- Wu, C.; Huang, C.; Hung, C.; Yao, F.; Wang, C.; Chang, Y. Delphinidin-rich extracts of Hibiscus sabdariffa L. trigger mitochondria-derived autophagy and necrosis through reactive oxygen species in human breast cancer cells. J. Funct. Foods 2016, 25, 279–290. [Google Scholar] [CrossRef]
- Cell Viability and Proliferation Assays|Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/technical-documents/articles/biofiles/cell-viability-and-proliferation.html (accessed on 13 October 2020).
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Khaghani, S.; Razi, F.; Yajloo, M.M.; Paknejad, M.; Shariftabrizi, A.; Pasalar, P. Selective Cytotoxicity and Apoptogenic Activity of Hibiscus sabdariffa Aqueous Extract Against MCF-7 Human Breast Cancer Cell Line. J. Cancer Ther. 2011, 2, 394–400. [Google Scholar] [CrossRef]
- Ahirwar, B.; Ahirwar, D. In vivo and in vitro investigation of cytotoxic and antitumor activities of polyphenolic leaf extract of Hibiscus sabdariffa against breast cancer cell lines. Res. J. Pharm. Technol. 2020, 13, 615–620. [Google Scholar] [CrossRef]
- Chiu, C.T.; Chen, J.H.; Chou, F.P.; Lin, H.H. Hibiscus sabdariffa leaf extract inhibits human prostate cancer cell invasion via down-regulation of Akt/NF-κB/MMP-9 pathway. Nutrients 2015, 7, 5065–5087. [Google Scholar] [CrossRef] [PubMed]
- Olvera-García, V.; Castaño-Tostado, E.; Rezendiz-Lopez, R.I.; Reynoso-Camacho, R.; González de Mejía, E.; Elizondo, G.; Loarca-Piña, G. Hibiscus sabdariffa L. extracts inhibit the mutagenicity in microsuspension assay and the proliferation of HeLa cells. J. Food Sci. 2008, 73, T75–T81. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millan, Z.E.; García-Garcia, M.R.; Leyva, J.G.; Martinez, R.F.; Vital, J.A.V.; García, E.H.; Iglesias, T.G. Lyophilized Extract of Hibiscus sabdariffa L. Induces Cytotoxicity in Breast Cancer Cell Lines. Proceedings 2020, 61, 22. https://doi.org/10.3390/IECN2020-07002
Millan ZE, García-Garcia MR, Leyva JG, Martinez RF, Vital JAV, García EH, Iglesias TG. Lyophilized Extract of Hibiscus sabdariffa L. Induces Cytotoxicity in Breast Cancer Cell Lines. Proceedings. 2020; 61(1):22. https://doi.org/10.3390/IECN2020-07002
Chicago/Turabian StyleMillan, Zyanya Escobar, Maritza Roxana García-Garcia, Juan Gómez Leyva, Ricardo Figueroa Martinez, Jorge Alfredo Vargas Vital, Eduardo Huerta García, and Trinidad García Iglesias. 2020. "Lyophilized Extract of Hibiscus sabdariffa L. Induces Cytotoxicity in Breast Cancer Cell Lines" Proceedings 61, no. 1: 22. https://doi.org/10.3390/IECN2020-07002
APA StyleMillan, Z. E., García-Garcia, M. R., Leyva, J. G., Martinez, R. F., Vital, J. A. V., García, E. H., & Iglesias, T. G. (2020). Lyophilized Extract of Hibiscus sabdariffa L. Induces Cytotoxicity in Breast Cancer Cell Lines. Proceedings, 61(1), 22. https://doi.org/10.3390/IECN2020-07002





