Amygdalin as a Plant-Based Bioactive Constituent: A Mini-Review on Intervention with Gut Microbiota, Anticancer Mechanisms, Bioavailability, and Microencapsulation †
Abstract
:1. Introduction
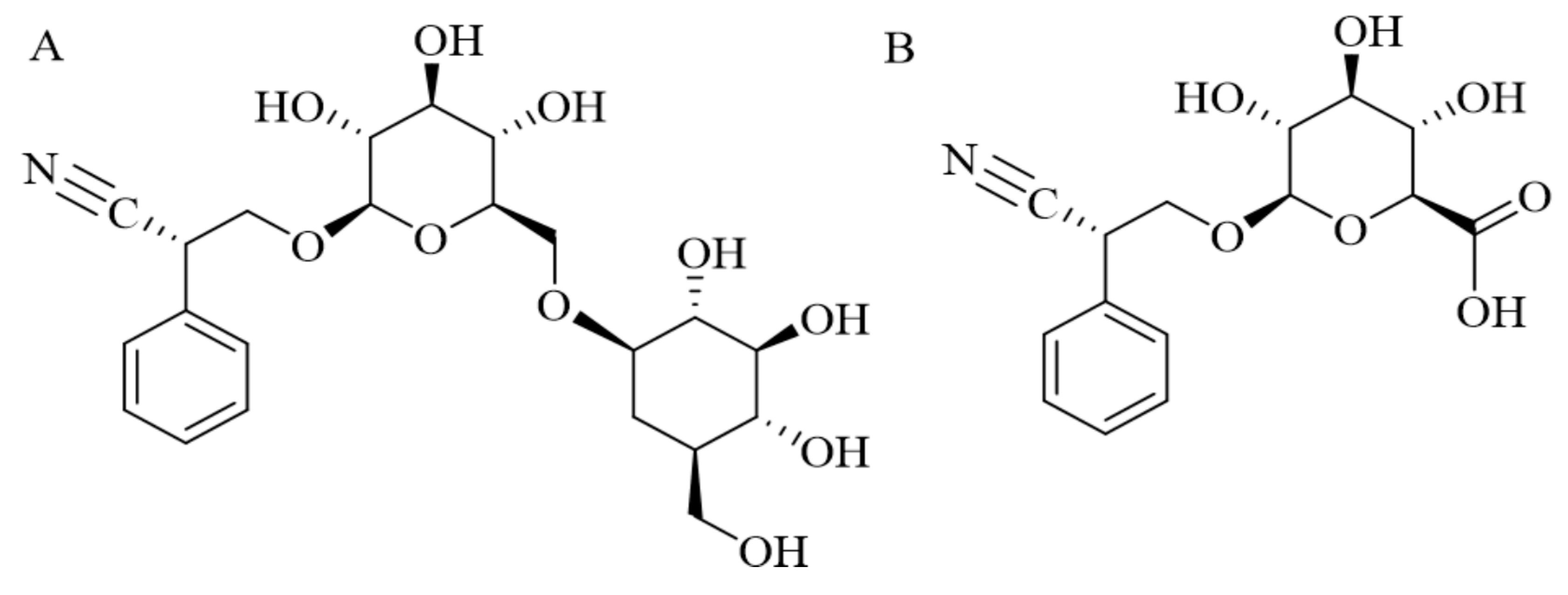
2. The Microbiome and Oral Amygdalin Administration: Intervention with Gut Microbiota
3. Anti-Cancer Mechanisms of Amygdalin: A Molecular Approach
4. Microencapsulation and Bioavailability
5. Future Perspectives and Conclusions
Funding
References
- Jaswal, V.; Palanivelu, J.; Ramalingam, C. Effects of the gut microbiota on amygdalin and its use as an anti-cancer therapy: substantial review on the key components involved in altering dose efficacy and toxicity. Biochem. Biophys. Rep. 2018, 14, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Toomey, V.M.; Nickum, E.A.; Flurer, C.L. Cyanide and amygdalin as indicators of the presence of bitter almonds in imported raw almonds. J. Forensic Sci. 2012, 57, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Orlikova, B.; Legrand, N.; Panning, J.; Dicato, M.; Diederich, M. Anti-inflammatory and anticancer drugs from nature. In Advances in Nutrition and Cancer; Springer: Berlin/Heidelberg, Germany, 2014; pp. 123–143. [Google Scholar]
- Qadir, M.; Fatima, K. Review on pharmacological activity of amygdalin. Arch. Cancer Res. 2017, 5, 160. [Google Scholar] [CrossRef]
- Li, X.B.; Liu, C.H.; Zhang, R.; Huang, X.T.; Li, Y.Y.; Han, L.; Xu, M.L.; Mi, S.Q.; Wang, N.S. Determination and pharmacokinetics of amygdalin in rats by LC–MS-MS. J. Chromatogr. Sci. 2014, 52, 476–481. [Google Scholar] [CrossRef]
- Song, Z.; Xu, X. Advanced research on anti-tumor effects of amygdalin. J. Cancer Res. Ther. 2014, 10, 3. [Google Scholar] [PubMed]
- Chang, H.K.; Shin, M.S.; Yang, H.Y.; Lee, J.W.; Kim, Y.S.; Lee, M.H.; Kim, J.; Kim, K.H.; Kim, C.J. Amygdalin induces apoptosis through regulation of Bax and Bcl-2 expressions in human DU145 and LNCaP prostate cancer cells. Biol. Pharm. Bull. 2006, 29, 1597–1602. [Google Scholar] [CrossRef]
- Zhou, C.; Qian, L.; Ma, H.; Yu, X.; Zhang, Y.; Qu, W.; Zhang, X.; Xia, W. Enhancement of amygdalin activated with β-D-glucosidase on HepG2 cells proliferation and apoptosis. Carbohydr. Polym. 2012, 90, 516–523. [Google Scholar] [CrossRef]
- Park, H.J.; Yoon, S.H.; Han, L.S.; Zheng, L.T.; Jung, K.H.; Uhm, Y.K.; Lee, J.H.; Jeong, J.S.; Joo, W.S.; Yim, S.V. Amygdalin inhibits genes related to cell cycle in SNU-C4 human colon cancer cells. World J. Gastroenterol. WJG 2005, 11, 5156. [Google Scholar]
- Chen, Y.; Ma, J.; Wang, F.; Hu, J.; Cui, A.; Wei, C.; Yang, Q.; Li, F. Amygdalin induces apoptosis in human cervical cancer cell line HeLa cells. Immunopharmacol. Immunotoxicol. 2013, 35, 43–51. [Google Scholar] [CrossRef]
- Ushakova, N.; Nekrasov, R.; Pravdin, I.; Sverchkova, N.; Kolomiyets, E.; Pavlov, D. Mechanisms of the effects of probiotics on symbiotic digestion. Biol. Bull. 2015, 42, 394–400. [Google Scholar] [CrossRef]
- Blaheta, R.A.; Nelson, K.; Haferkamp, A.; Juengel, E. Amygdalin, quackery or cure? Phytomedicine 2016, 23, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Tokpohozin, S.E.; Fischer, S.; Sacher, B.; Becker, T. β-d-Glucosidase as “key enzyme” for sorghum cyanogenic glucoside (dhurrin) removal and beer bioflavouring. Food Chem. Toxicol. 2016, 97, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, N.; Qian, C.; Wang, Q.; Wang, Q.; Long, Y.; Huang, Y.; Zhou, Z.; Yan, X. Phylogenetic and functional analysis of gut microbiota of a fungus-growing higher termite: Bacteroidetes from higher termites are a rich source of β-glucosidase genes. Microb. Ecol. 2014, 68, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Sorbo, B. On the properties of rhodanese: Partial Purification, Inhibitors and Intracellular Distribution. Acta Chem. Scand. 1951, 5, 724–726. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Greenberg, D.M. The vitamin fraud in cancer quackery. West. J. Med. 1975, 122, 345. [Google Scholar]
- Chong, E.S.L. A potential role of probiotics in colorectal cancer prevention: Review of possible mechanisms of action. World J. Microbiol. Biotechnol. 2014, 30, 351–374. [Google Scholar] [CrossRef]
- Kuugbee, E.D.; Shang, X.; Gamallat, Y.; Bamba, D.; Awadasseid, A.; Suliman, M.A.; Zang, S.; Ma, Y.; Chiwala, G.; Xin, Y. Structural change in microbiota by a probiotic cocktail enhances the gut barrier and reduces cancer via TLR2 signaling in a rat model of colon cancer. Dig. Dis. Sci. 2016, 61, 2908–2920. [Google Scholar] [CrossRef]
- Bromley, J.; Hughes, B.G.; Leong, D.C.; Buckley, N.A. Life-threatening interaction between complementary medicines: Cyanide toxicity following ingestion of amygdalin and vitamin C. Ann. Pharmacother. 2005, 39, 1566–1569. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Sun, A.Y.; Chen, Q.; Espey, M.G.; Drisko, J.; Levine, M. Vitamin C: Intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS ONE 2010, 5, e11414. [Google Scholar] [CrossRef]
- Richards, E. The politics of therapeutic evaluation: The vitamin C and cancer controversy. Soc. Stud. Sci. 1988, 18, 653–701. [Google Scholar] [CrossRef]
- Calabrese, E.J. Conjoint use of laetrile and megadoses of ascorbic acid in cancer treatment: Possible side effects. Med. Hypotheses 1979, 5, 995–997. [Google Scholar] [CrossRef]
- Ward, E.; Jemal, A.; Cokkinides, V.; Singh, G.K.; Cardinez, C.; Ghafoor, A.; Thun, M. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J. Clin. 2004, 54, 78–93. [Google Scholar] [CrossRef]
- Holstege, C.P.; Forrester, J.D.; Borek, H.A.; Lawrence, D.T. A case of cyanide poisoning and the use of arterial blood gas analysis to direct therapy. Hosp. Pract. 2010, 38, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Kleessen, B.; Sykura, B.; Zunft, H.J.; Blaut, M. Effects of inulin and lactose on fecal microflora, microbial activity, and bowel habit in elderly constipated persons. Am. J. Clin. Nutr. 1997, 65, 1397–1402. [Google Scholar] [CrossRef]
- Borron, S.W.; Baud, F.J.; Mégarbane, B.; Bismuth, C. Hydroxocobalamin for severe acute cyanide poisoning by ingestion or inhalation. Am. J. Emerg. Med. 2007, 25, 551–558. [Google Scholar] [CrossRef]
- Oyewole, O.; Olayinka, E. Hydroxocobalamin (vit B12a) effectively reduced extent of cyanide poisoning arising from oral amygdalin ingestion in rats. J. Toxicol. Environ. Health Sci. 2009, 1, 008–011. [Google Scholar]
- Chan, T.Y. A probable case of amygdalin-induced peripheral neuropathy in a vegetarian with vitamin B12 deficiency. Ther. Drug Monit. 2006, 28, 140–141. [Google Scholar] [CrossRef]
- Liczbiński, P.; Bukowska, B. Molecular mechanism of amygdalin action in vitro: Review of the latest research. Immunopharmacol. Immunotoxicol. 2018, 40, 212–218. [Google Scholar] [CrossRef]
- Ying, J.; Ge, Q.; Hu, S.; Luo, C.; Lu, F.; Yu, Y.; Xu, T.; Lv, S.; Zhang, L.; Shen, J.; et al. Amygdalin promotes fracture healing through TGF-beta/Smad signaling in mesenchymal stem cells. Stem Cells Int. 2020, 2020, 8811963. [Google Scholar] [CrossRef]
- He, X.Y.; Wu, L.J.; Wang, W.X.; Xie, P.J.; Chen, Y.H.; Wang, F. Amygdalin—A pharmacological and toxicological review. J. Ethnopharmacol. 2020, 254, 112717. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Chang, H.K.; Lee, J.W.; Kim, Y.S.; Kim, H.; Lee, M.H.; Shin, M.S.; Ham, D.H.; Park, H.K.; Lee, H.; et al. Amygdalin suppresses lipopolysaccharide-induced expressions of cyclooxygenase-2 and inducible nitric oxide synthase in mouse BV2 microglial cells. Neurol. Res. 2007, 29, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Lee, H.J.; Kim, C.J.; Shim, I.; Hahm, D.H.J.J.M.B. Inhibitory effect of amygdalin on lipopolysaccharide-inducible TNF-alpha and IL-1beta mRNA expression and carrageenan-induced rat arthritis. J. Microbiol. Biotechnol. 2008, 18, 1641–1647. [Google Scholar] [PubMed]
- Luo, D.; Shan, Z.; Jinlian, G.; Liu, Q.; Luo, L. Effects of amygdalin on TNF-α and sICAM-1 of rats with type I collagen-induced arthritis. Chin. J. Inf. Tradit. Chin. Med. 2015, 7, 75–77. [Google Scholar]
- Zhang, A.; Pan, W.; Lv, J.; Wu, H. Protective effect of amygdalin on LPS-induced acute lung injury by inhibiting NF-κB and NLRP3 signaling pathways. Inflammation 2017, 40, 745–751. [Google Scholar] [CrossRef]
- Zhong, X.Q.; Li, L.; Lu, C.J.; Lu, Y.; Wei, J.A.; Han, L. Anti-inflammation effect of amygdalin on macrophage 264.7 Cells Stimulated by Lipopolysaccharide. Tradit. Chin. Drug Res. Clin. Pharmacol. 2018, 29, 257–263. [Google Scholar]
- Sohail, R.; Abbas, S.R. Evaluation of amygdalin-loaded alginate-chitosan nanoparticles as biocompatible drug delivery carriers for anticancerous efficacy. Int. J. Biol. Macromol. 2020, 153, 36–45. [Google Scholar] [CrossRef]
- Sarmento, B.; Ribeiro, A.; Veiga, F.; Ferreira, D.; Neufeld, R. Insulin-loaded nanoparticles are prepared by alginate ionotropic pre-gelation followed by chitosan polyelectrolyte complexation. J. Nanosci. Nanotechnol. 2007, 7, 2833–2841. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef]
- Zhou, J.; Hou, J.; Rao, J.; Zhou, C.; Liu, Y.; Gao, W. Magnetically directed enzyme/prodrug prostate cancer therapy based on beta-Glucosidase/amygdalin. Int. J. Nanomed. 2020, 15, 4639–4657. [Google Scholar] [CrossRef] [PubMed]
| Model | Dose/Method/Period | Activity | Refs. |
|---|---|---|---|
| BV2 glial cells | 1, 10, 100, 1000 μg mL−1; culture; 24 h |
COX-2 mRNA, iNOS mRNA ↓ the synthesis of prostaglandin E2 ↓ the production of nitric oxide ↓ | [33] |
| RAW 264.7 cells | 1, 10, 100 mmol L−1; culture; 6 h |
At a concentration of 1 mM, TNF-α and IL-1β mRNA ↓ Amygdalin does not inhibit TNF-α and IL-1β mRNA expressioniiin a dose-dependent manner. | [34] |
| Arthritis pain model (Carrageenan-induced), SD male rats | 0.005, 0.05, and 0.1 mg kg−1; i.m; 8 h |
At a concentration of 0.005 mg/kg, Fos, TNF-α and IL-1β ↓; However, no analgesic effect of amygdalin was observed at doses greater than 0.005 mg/kg. ↓ | [35] |
| Pain model (plantar injection of formalin), SD male rats | 0.1, 0.5, 1.0, and 10.0 mg kg−1; Plantar injection |
c-Fos, TNF-α, IL-1β Laetrile reduces pain in a dose-dependent manner in a dose range of less than 1 mg/kg. | [34] |
| CIA rat model (type II collagen-induced), Wistar rats | 120 mg kg−1; gavage; 28 days | TNF-α and sICAM-1 ↓ | [35] |
| BALB/c mice | 0.5, 1, and 2 mg kg−1; ip; 7 h |
NF-κB ↓ Reduced pulmonary edema in a dose-dependent manner. | [36] |
| RAW 264.7 cells | 6.25, 12.5, 25, 50, 100, 200, 400 µmol L−1; culture; 24 h |
IL-17A, IL-23, CCL2, and CCL5 mRNA ↓ p-p38 ↓ the viability of RAW264.7 cell ↓ | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barakat, H. Amygdalin as a Plant-Based Bioactive Constituent: A Mini-Review on Intervention with Gut Microbiota, Anticancer Mechanisms, Bioavailability, and Microencapsulation. Proceedings 2020, 61, 15. https://doi.org/10.3390/IECN2020-06984
Barakat H. Amygdalin as a Plant-Based Bioactive Constituent: A Mini-Review on Intervention with Gut Microbiota, Anticancer Mechanisms, Bioavailability, and Microencapsulation. Proceedings. 2020; 61(1):15. https://doi.org/10.3390/IECN2020-06984
Chicago/Turabian StyleBarakat, Hassan. 2020. "Amygdalin as a Plant-Based Bioactive Constituent: A Mini-Review on Intervention with Gut Microbiota, Anticancer Mechanisms, Bioavailability, and Microencapsulation" Proceedings 61, no. 1: 15. https://doi.org/10.3390/IECN2020-06984
APA StyleBarakat, H. (2020). Amygdalin as a Plant-Based Bioactive Constituent: A Mini-Review on Intervention with Gut Microbiota, Anticancer Mechanisms, Bioavailability, and Microencapsulation. Proceedings, 61(1), 15. https://doi.org/10.3390/IECN2020-06984





