Indication of the Coronavirus Model Using a Nanowire Biosensor †
Abstract
:1. Introduction
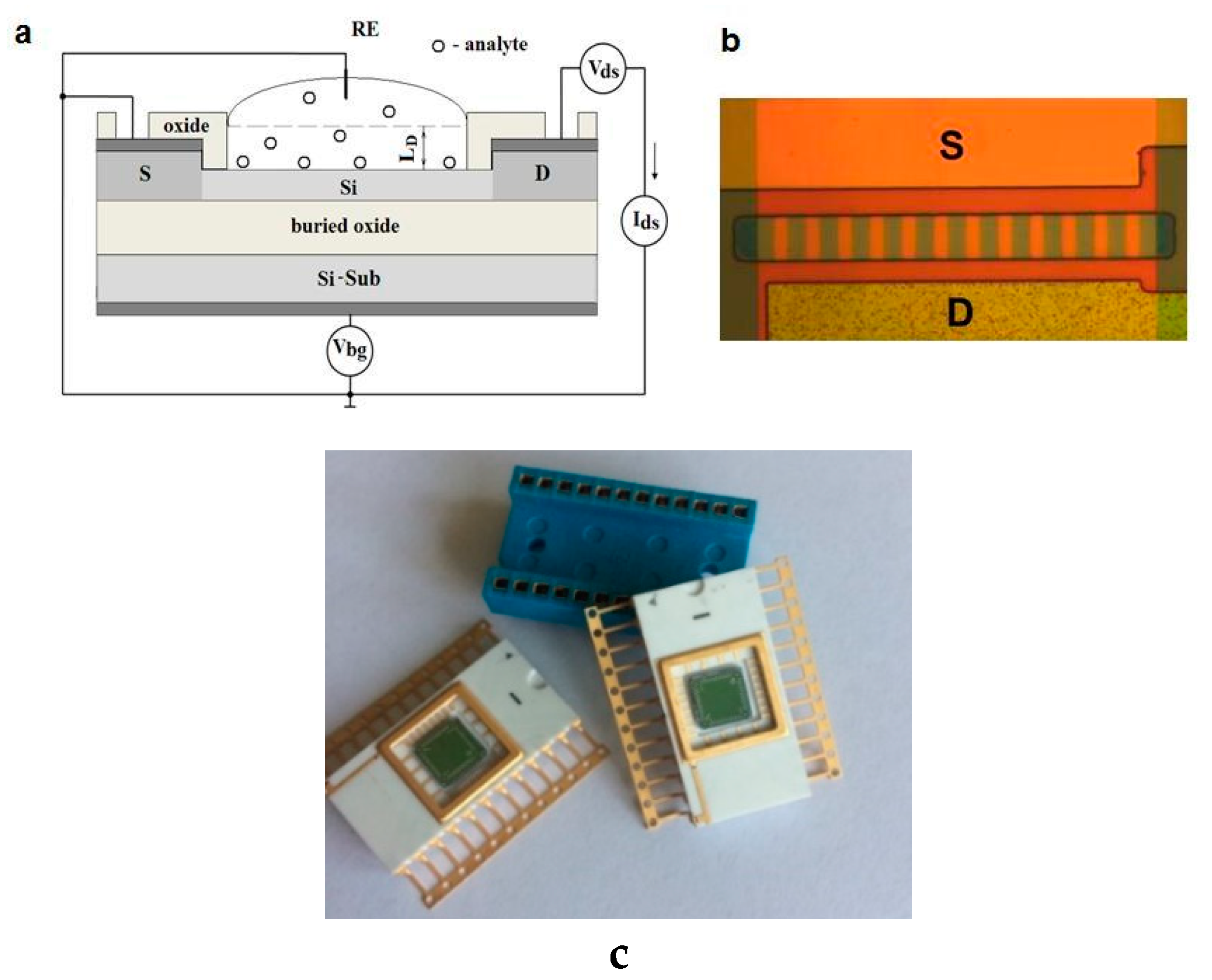
2. Materials and Methods
- The procedure for cleaning the surface of the Si-NW FET was carried out as follows:
- -
- 96% ethanol solution with a volume of V = 10 µL was applied and removed with a pipette from the surface of the SI-NW FET 20–30 times, then the surface was washed with distilled water according to the same scheme. The procedure was repeated 3 times. Afterwards the SI-NW FET was totally dried.
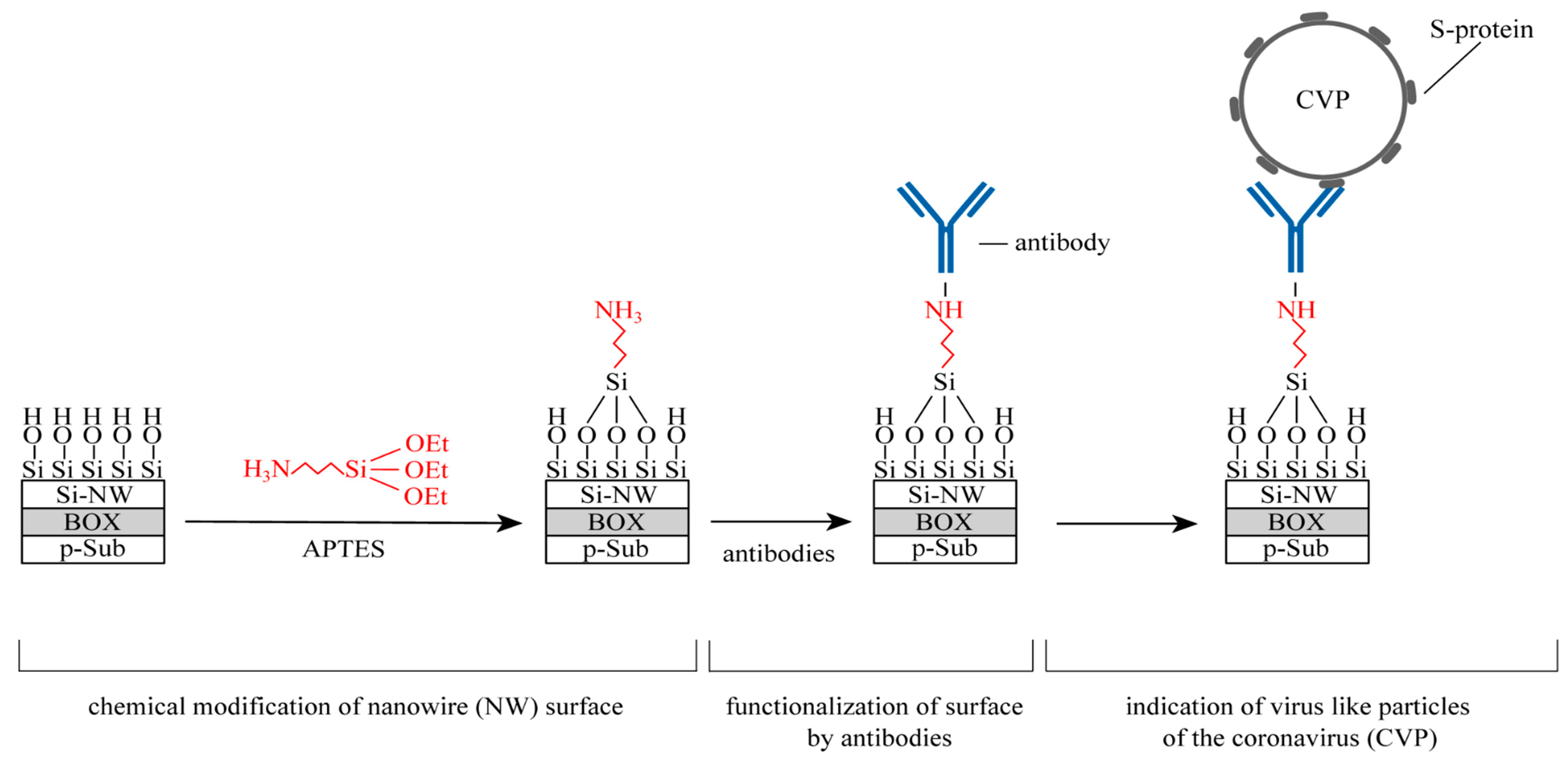
- The cleaned and dried surface of the SI-NW FET was modified by 25% ethanol solution APTES with a volume of V = 5–10 µL. The SI-NW FET was placed in a Petri dish and covered with a lid. A 25% ethanol solution of APTES was previously added to the Petri dish to create the vapors of the specified analyte. The SI-NW FET was in the specified pairs for 5 h, at T = 20 °C. The fill level of the analyte did not exceed the surface height of the SI-NW FET.
- After 5 h, the surface of the SI-NW FET was washed with distilled water. The procedure was repeated 3 times. Afterwards the SI-NW FET was totally dried.
- A suspension of diluted antibodies with a volume of V = 5–10 µL was applied to the dry surface of the SI-NW FET. Then the SI-NW FET was placed in a Petri dish with water. The cup was covered with a lid and placed in the refrigerator. SI-NW FET was in the specified water vapor for 2 h, at T = 4 °C. The water fill level did not exceed the surface height of the SI-NW FET. The antibodies were chemically bound to APTES and held on the surface.
- After 2 h, antibodies were removed from the surface of the SI-NW FET that did not bind to it. The surface was washed with distilled water using a pipette. V = 10 µL of distilled water was applied and removed 20–30 times from the surface of the SI-NW FET. The procedure was repeated 3 times. Next, the surface of the SI-NW FET was dried. Drying was carried out in closed Petri dishes.
- After this first part, the experimental SI-NW FET as the biosensor was ready for use.
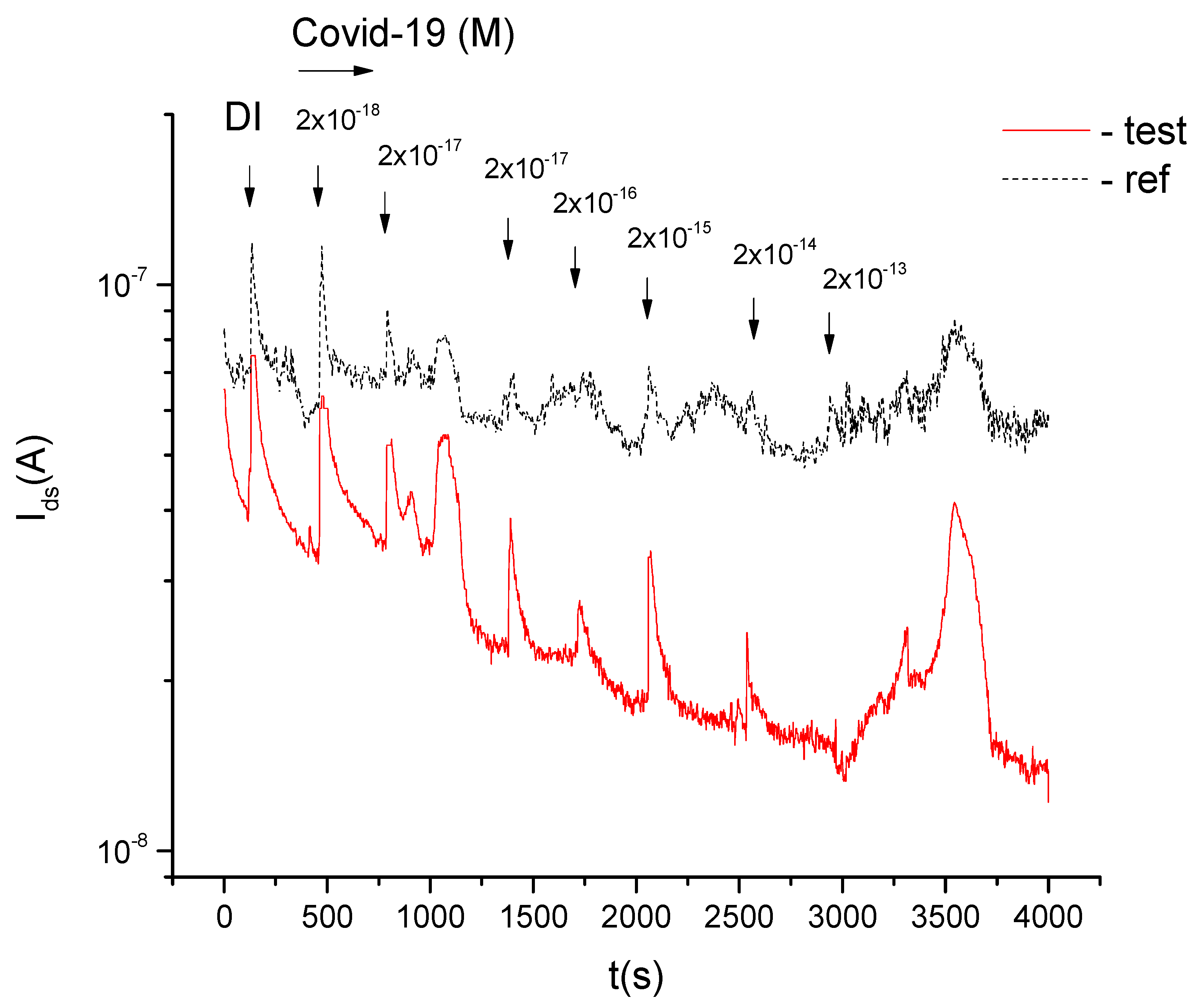
3. Results
- -
- antibody + CVP complexes, taking into account specific proteins in the suspension at the phase section with the surface of the nanowire, modulate the current of the biosensor field-effect transistor;
- -
- HPVCS have an electrically positive charge at the phase section “nanowire surface viral suspension»;
- -
- antibody + HPV complexes have an electrically negative charge on the phase section “surface of the nanowire viral suspension»;
- -
- the sensitivity of the biosensor is made up of 10−18 M;
- -
- the display time was 200–300 s.
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/ru/emergencies/diseases/novel-coronavirus-2019 (accessed on 2 November 2020).
- WHO Director-General’s Opening Remarks at the Media Briefing on COVID-1919 11 March 2020 r. Available online: https://www.who.int/ru/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 2 November 2020).
- Coronavirus. Available online: https://www.who.int/ru/health-topics/coronavirus/coronavirus (accessed on 2 November 2020).
- Surveillance Strategies for COVID-19 Human Infection: Interim Guidance, 10 May 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/332051/WHO-2019-nCoV-National_Surveillance-2020.1-rus.pdf (accessed on 2 November 2020).
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019-Novel Coronavirus (2019-nCoV) Pneumonia in Wuhan, China. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Rospotrebnadzor Research Center Has Started Testing a Vaccine Against a New Coronavirus. Available online: http://www.vector.nsc.ru/news/571/ (accessed on 2 November 2020).
- Ancelevich, L.M.; Yagudin, L.A. Practical application of enzyme immunoassay in the diagnosis of diseases. Pract. Med. 2014, 3, 28–34. (In Russia) [Google Scholar]
- Naumova, O.V.; Fomin, B.I.; Nasimov, D.A.; Dudchenko, N.V.; Devyatova, S.F.; Zhanaev, E.D.; Popov, V.P.; Latyshev, A.V.; Aseev, A.L.; Ivanov, Y.D.; et al. SOI nanowires as sensors for charge detection. Semicond. Sci. Technol. 2010, 25, 055004. [Google Scholar] [CrossRef]
- Prabakaran, P.; Gan, J.; Feng, Y.; Zhu, Z.; Choudhry, V.; Xiao, X.; Ji, X.; Dimitrov, D.S. Structure of severe acute respiratory syndrome coronavirus receptor-binding domain complexed with neutralizing antibody. J. Biol. Chem. 2006, 281, 15829–15836. [Google Scholar] [CrossRef] [PubMed]
- Parka, J.; Nguyena, H.H.; Woubitc, A.; Kima, M. Applications of field-effect transistor (FET)-type biosensors. Appl. Sci. Converg. Technol. 2014, 23, 61–71. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, G.-J. Silicon nanowire-transistor biosensor for study of molecule-molecule interactions. Rev. Anal. Chem. 2014, 33, 95–10. [Google Scholar] [CrossRef]
- Dmitrienko, E.; Naumova, O.; Fomin, B.; Kupryushkin, M.; Volkova, A.; Amirkhanov, N.; Semenov, D.; Pyshnaya, I.; Pyshnyi, D. Surface modification of SOI-FET sensors for label-free and specific detection of short RNA analyte. Nanomedicine 2016, 11, 2073–2082. [Google Scholar] [CrossRef] [PubMed]
- Stern, E.; Wagner, R.; Sigworth, F.J.; Breaker, R.; Fahmy, T.M.; Reed, M.A. Importance of the Debye screening length on nanowire field effect transistor sensors. Nano Lett. 2007, 7, 3405–3409. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Generalov, V.; Naumova, O.; Shcherbakov, D.; Safatov, A.; Zaitsev, B.; Zaitseva, E.; Buryak, G.; Shcheglov, D.; Cheremiskina, A.; Merkuleva, I.; et al. Indication of the Coronavirus Model Using a Nanowire Biosensor. Proceedings 2020, 60, 50. https://doi.org/10.3390/IECB2020-07228
Generalov V, Naumova O, Shcherbakov D, Safatov A, Zaitsev B, Zaitseva E, Buryak G, Shcheglov D, Cheremiskina A, Merkuleva I, et al. Indication of the Coronavirus Model Using a Nanowire Biosensor. Proceedings. 2020; 60(1):50. https://doi.org/10.3390/IECB2020-07228
Chicago/Turabian StyleGeneralov, Vladimir, Olga Naumova, Dmitry Shcherbakov, Alexander Safatov, Boris Zaitsev, Elza Zaitseva, Galina Buryak, Dmitry Shcheglov, Anastasiya Cheremiskina, Iuliia Merkuleva, and et al. 2020. "Indication of the Coronavirus Model Using a Nanowire Biosensor" Proceedings 60, no. 1: 50. https://doi.org/10.3390/IECB2020-07228
APA StyleGeneralov, V., Naumova, O., Shcherbakov, D., Safatov, A., Zaitsev, B., Zaitseva, E., Buryak, G., Shcheglov, D., Cheremiskina, A., Merkuleva, I., & Aseyev, A. (2020). Indication of the Coronavirus Model Using a Nanowire Biosensor. Proceedings, 60(1), 50. https://doi.org/10.3390/IECB2020-07228





