Characteristics of Ammonia/Hydrogen Premixed Combustion in a Novel Linear Engine Generator †
Abstract
:1. Introduction
2. Methodology
2.1. Ammonia Combustion Mechanism Review
2.2. Parametric Study Conditions
2.2.1. Premixed Laminar Flame Speed Modelling
2.2.2. Ignition Delay Time Modelling
2.2.3. Burner-Stabilized Flame Structure Modelling
3. Mechanism Selection for Parametric Study
4. Results and Discussion
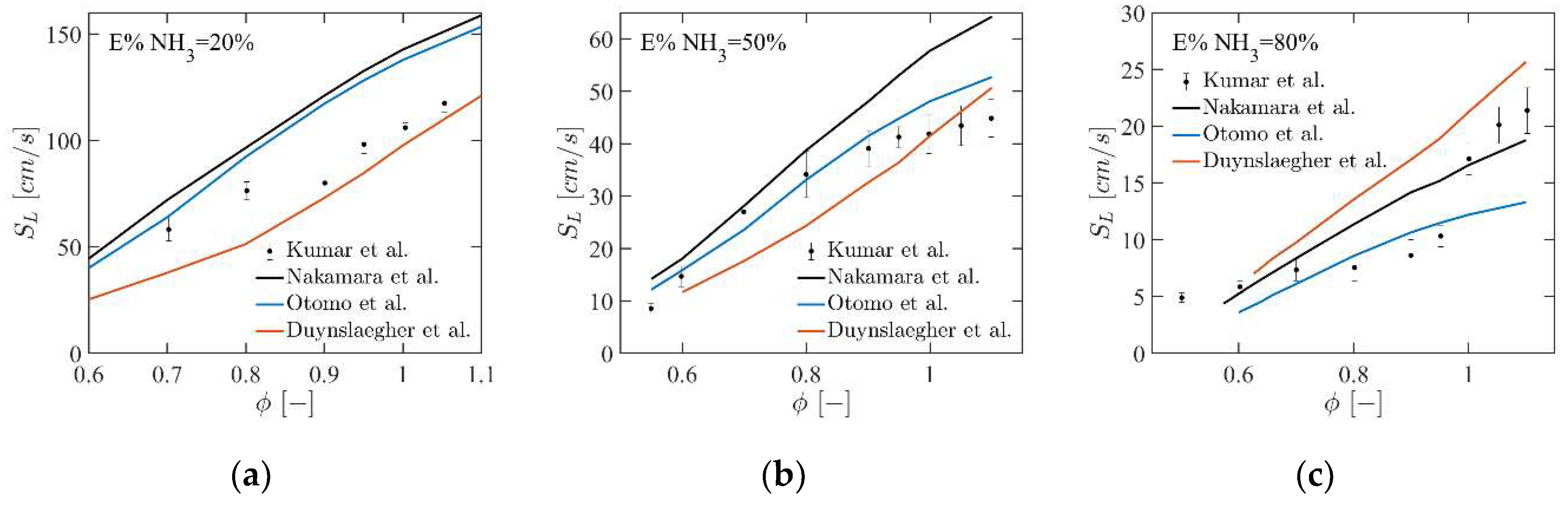
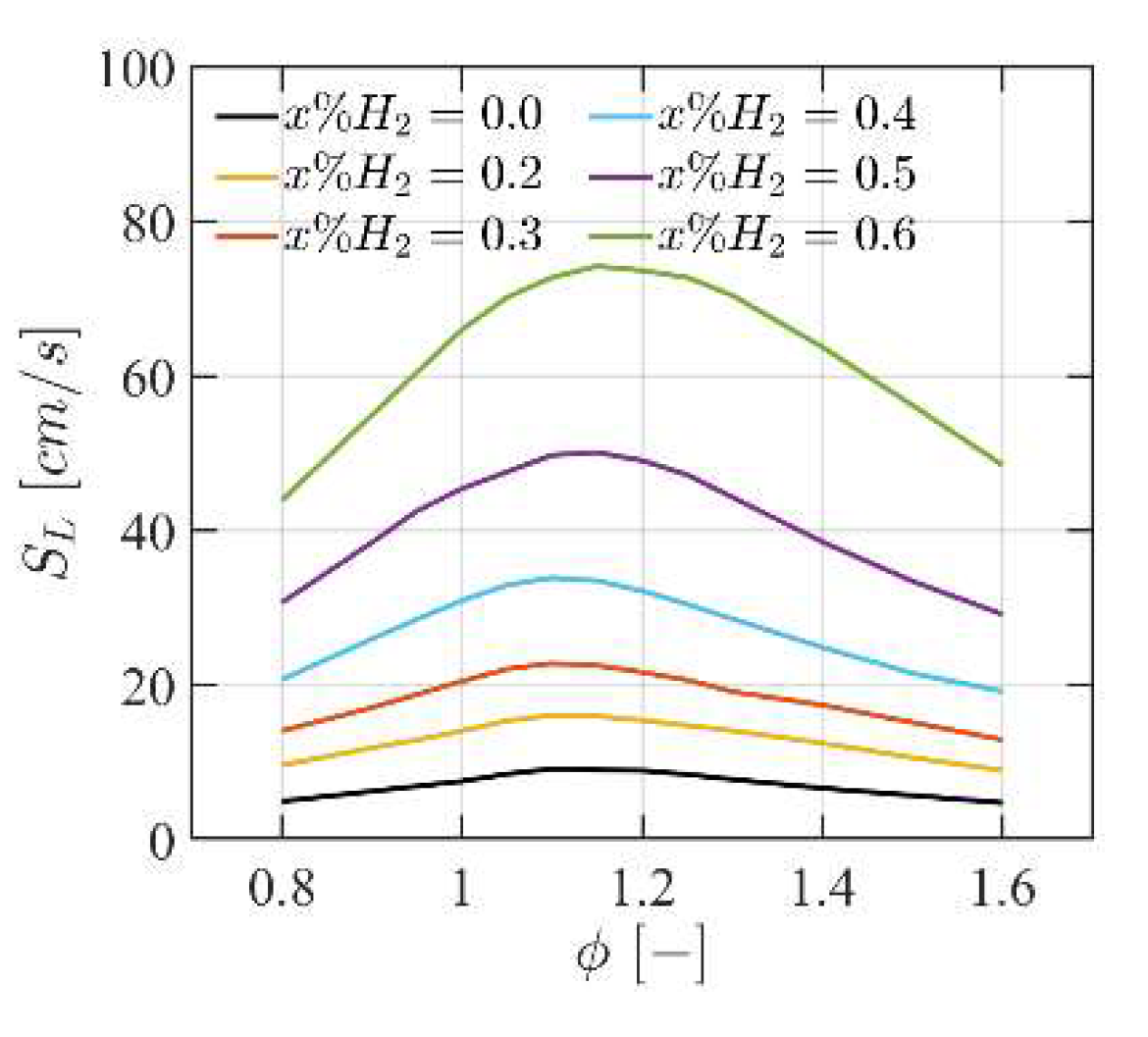
4.1. Premixed Laminar Flame Speed
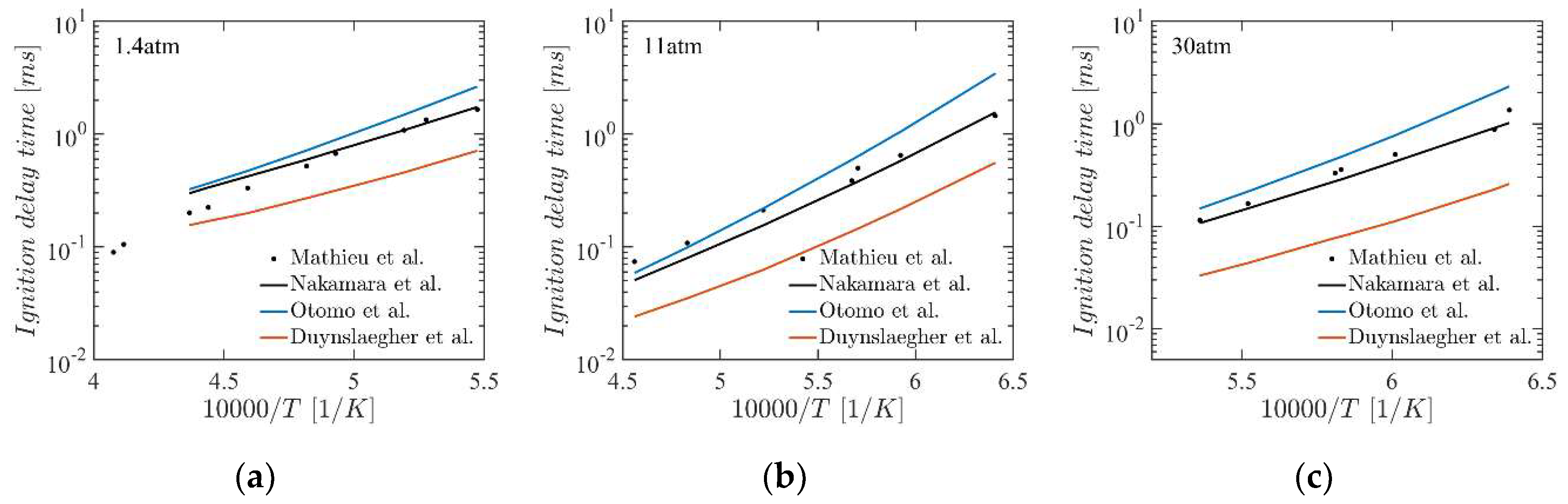
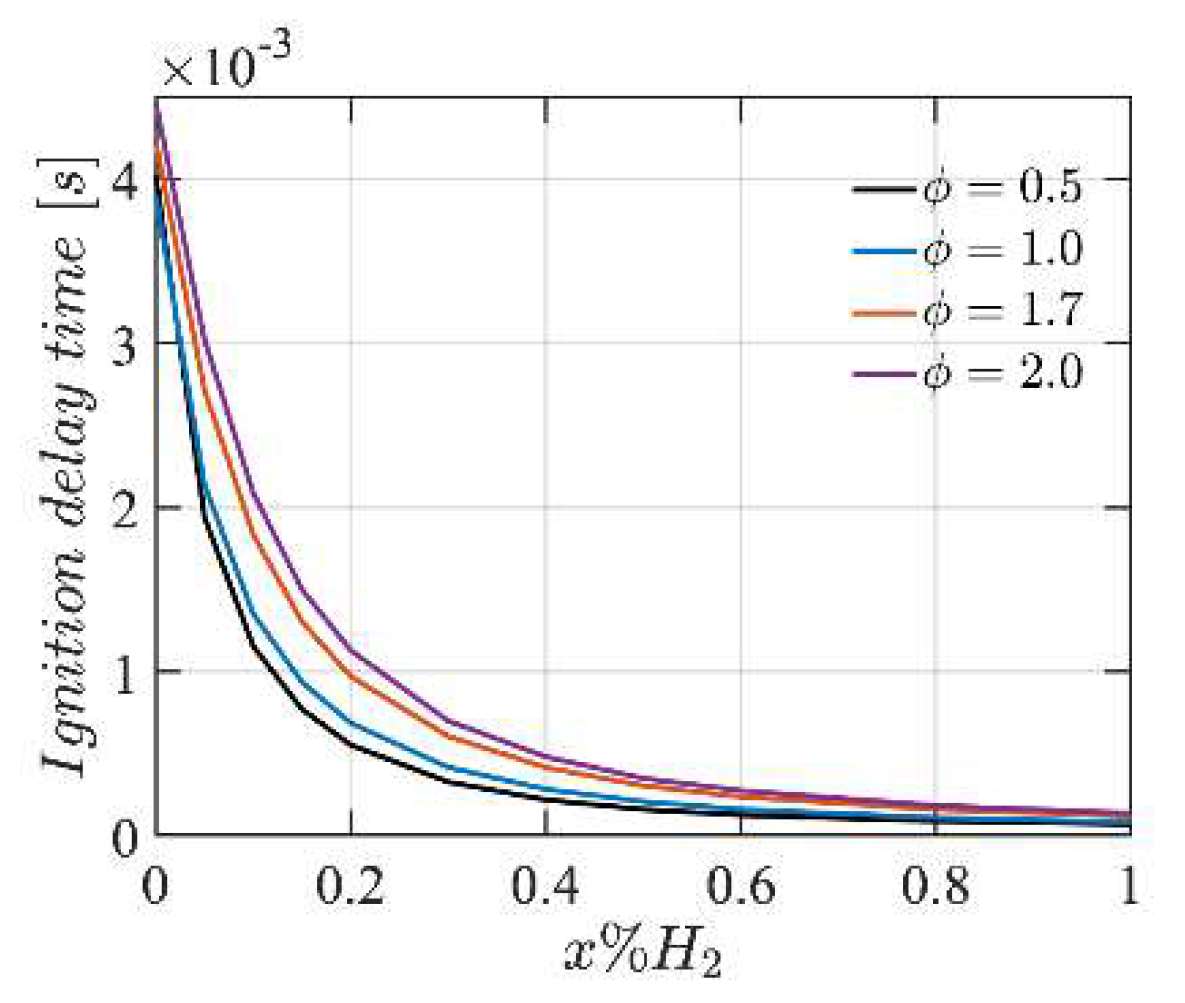
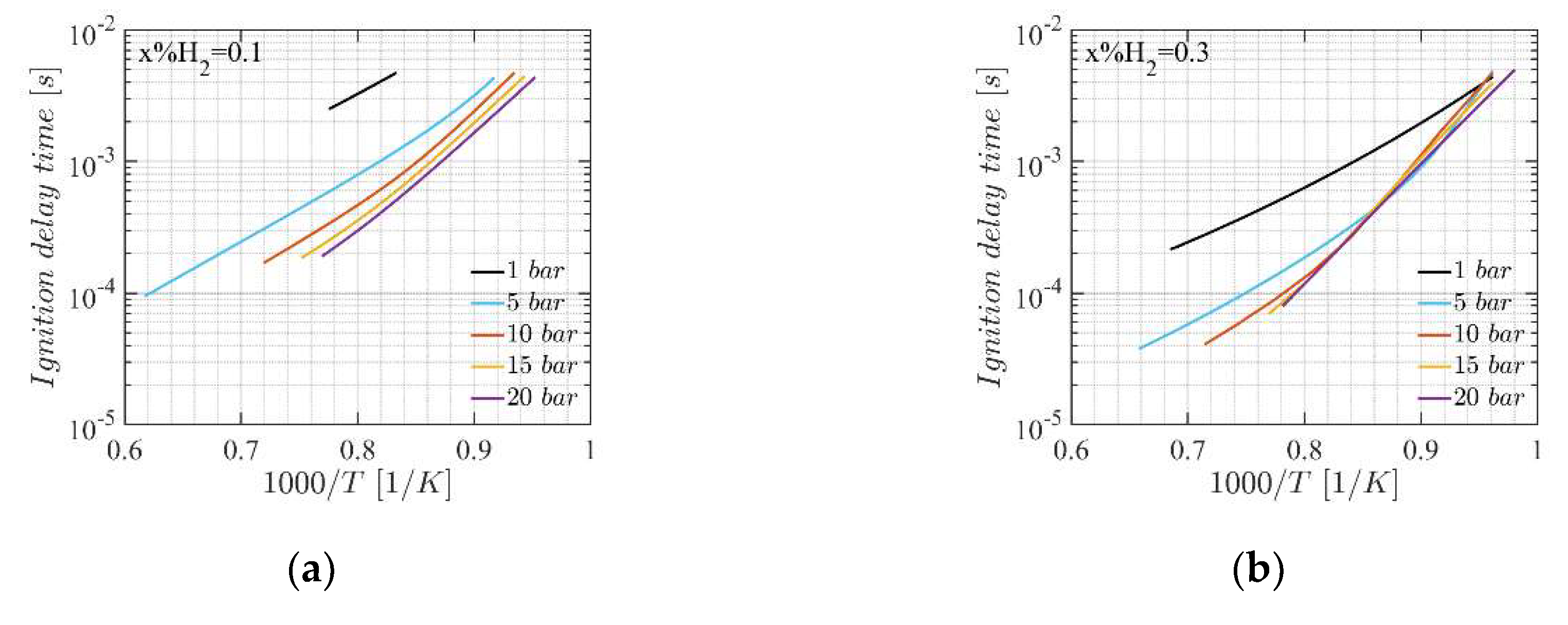
4.2. Ignition Delay Time
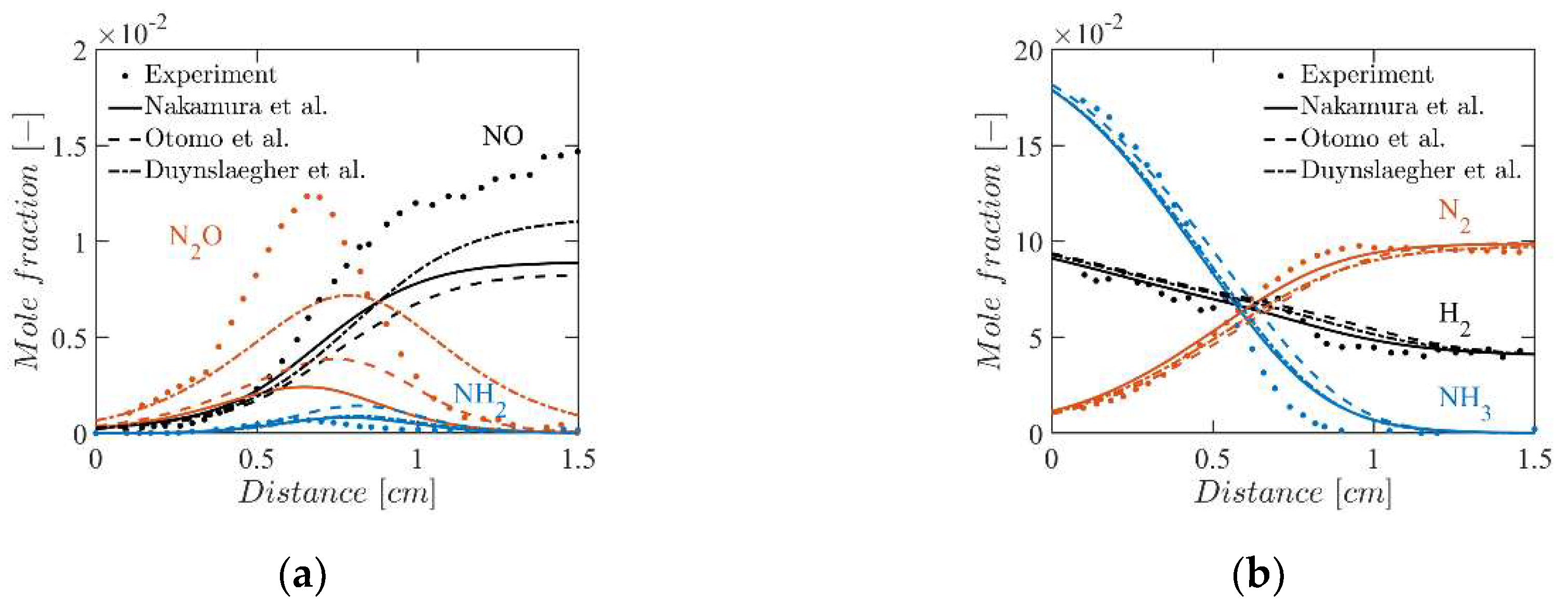
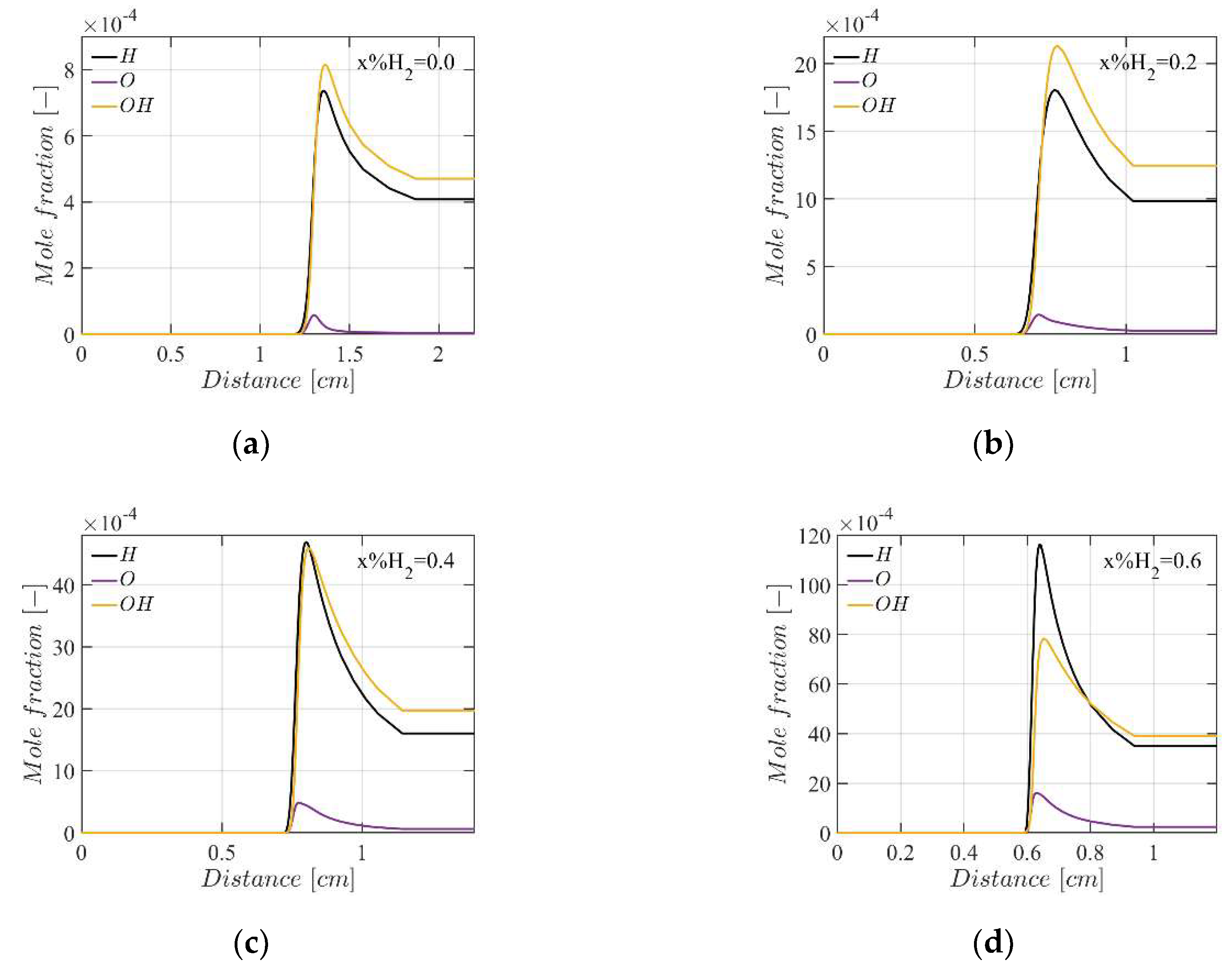
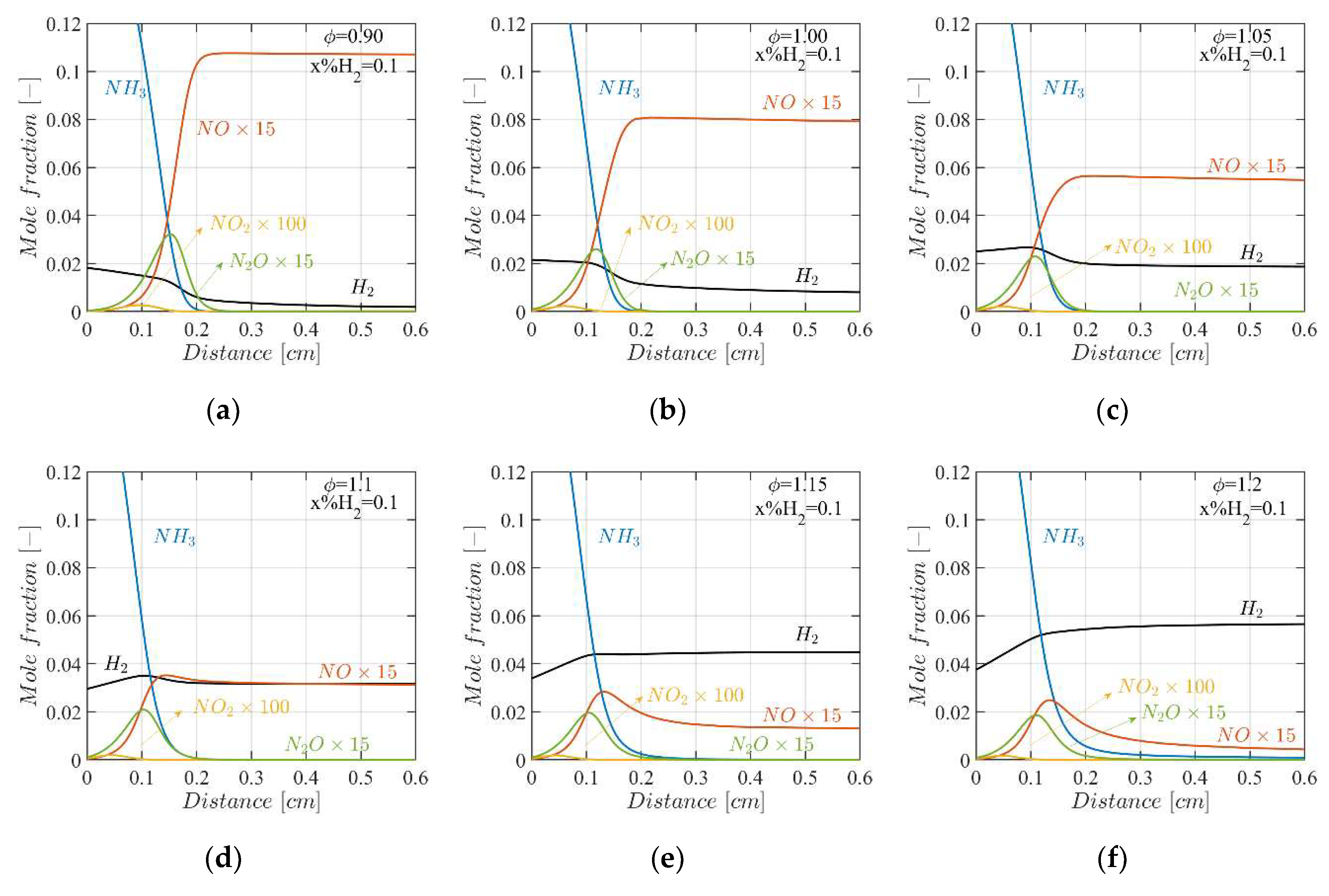
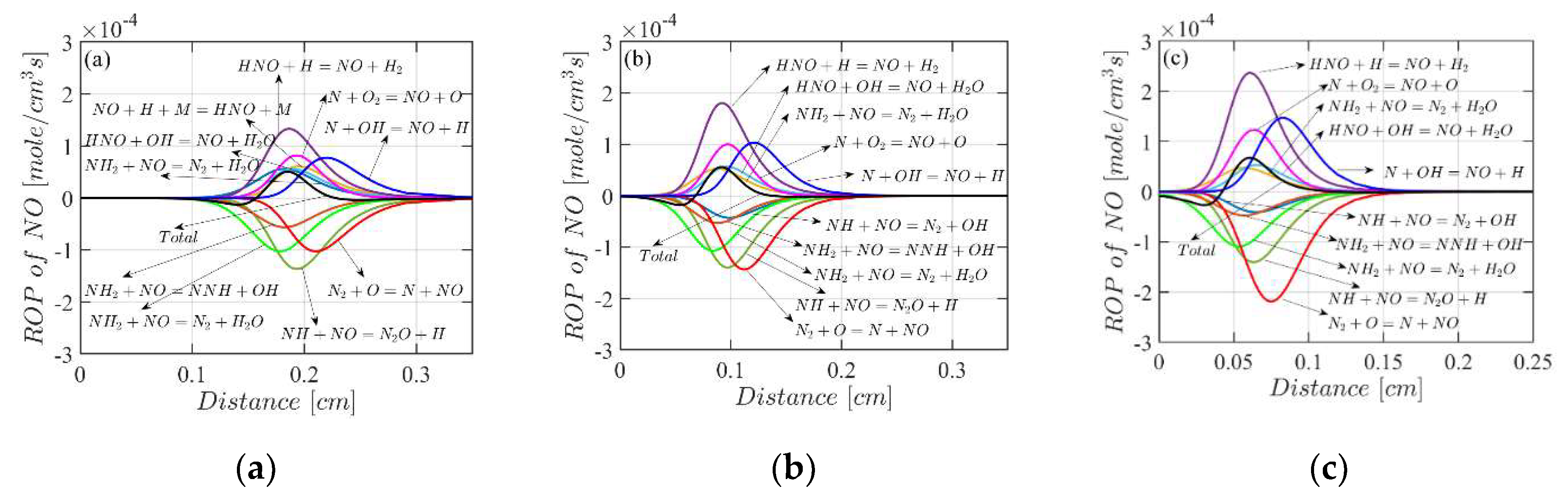
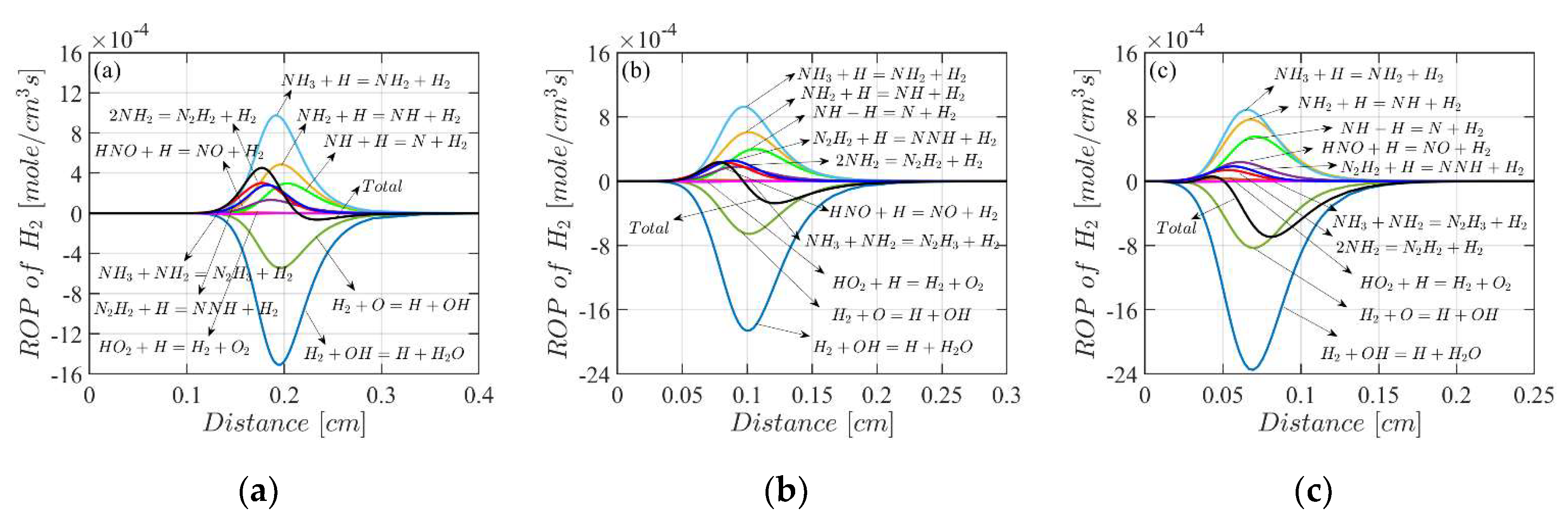
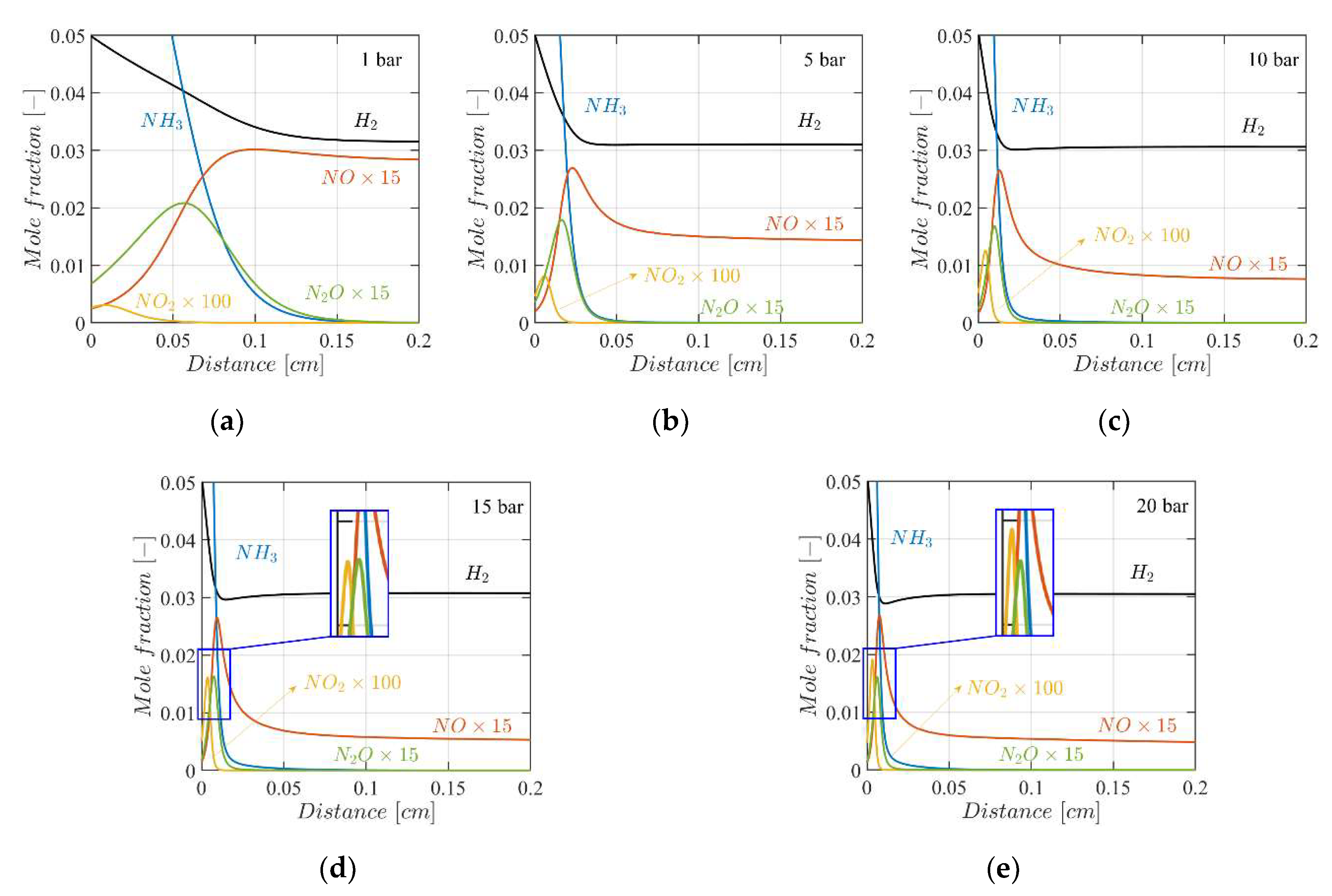
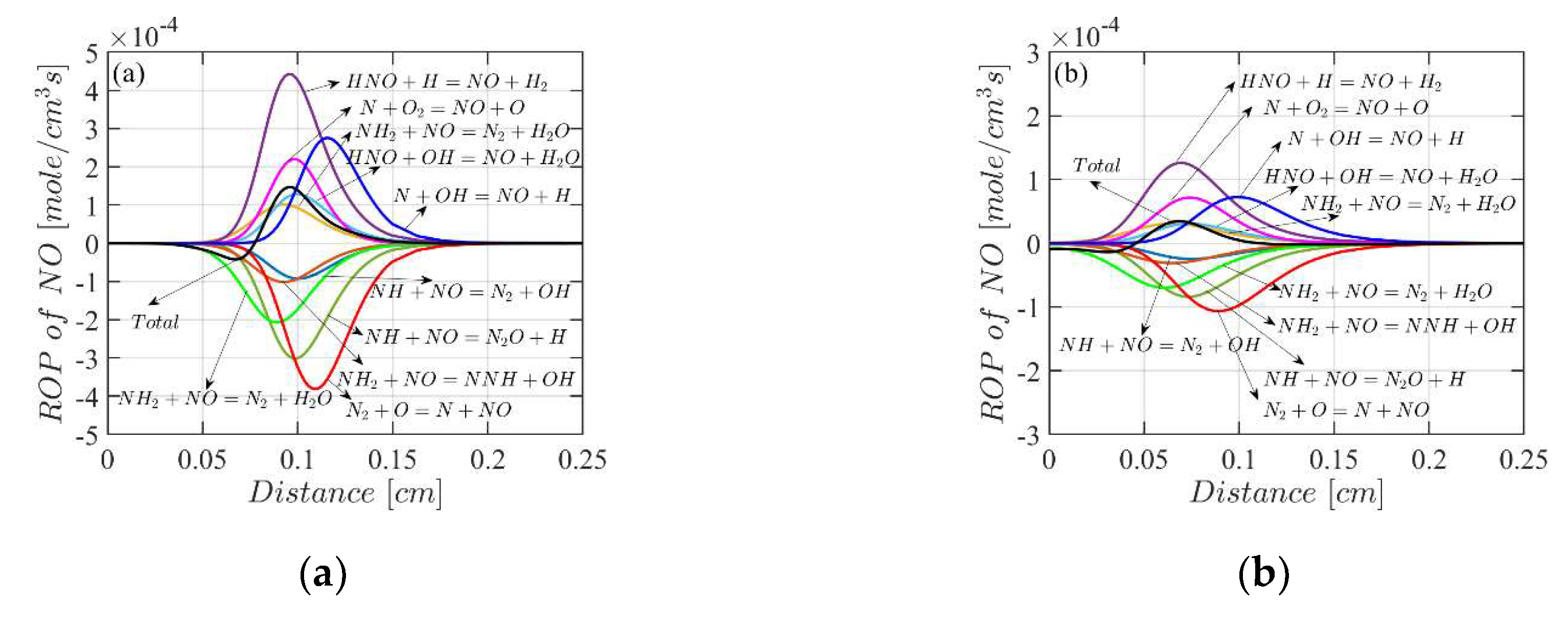
4.3. Mole Fractions of the Flame Species
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Mikalsen, R.; Roskilly, A.P. A review of free-piston engine history and applications. Appl. Therm. Eng. 2007, 27, 2339–2352. [Google Scholar] [CrossRef]
- Hung, N.B.; Lim, O. A review of free-piston linear engines. Appl. Energy 2016, 178, 78–97. [Google Scholar] [CrossRef]
- Schneider, S.; Chiodi, M.; Friedrich, H.; Bargende, M. Development and Experimental Investigation of a Two-Stroke Opposed-Piston Free-Piston Engine. SAE Tech. Pap. 2016, 1, 16. [Google Scholar] [CrossRef]
- Johnson, T.A.; Leick, M.T.; Moses, R.W. Experimental Evaluation of a Prototype Free Piston Engine—Linear Alternator (FPLA) System. SAE Tech. Pap. 2016, 1, 12. [Google Scholar] [CrossRef]
- Kosaka, H.; Akita, T.; Moriya, K.; Goto, S.; Hotta, Y.; Umeno, T.; Nakakita, K. Development of Free Piston Engine Linear Generator System Part 1—Investigation of Fundamental Characteristics. SAE Tech. Pap. 2014, 1, 8. [Google Scholar] [CrossRef]
- Goto, S.; Moriya, K.; Kosaka, H.; Akita, T.; Hotta, Y.; Umeno, T.; Nakakita, K. Development of Free Piston Engine Linear Generator System Part 2—Investigation of Control System for Generator. SAE Tech. Pap. 2014, 1, 8. [Google Scholar] [CrossRef]
- Mikalsen, R.; Roskilly, A.P. The free-piston reciprocating Joule Cycle engine: A new approach to efficient domestic CHP generation. In Proceeding of ICAE2012 Conference; 2012; Applied Energy: Suzhou, China, 2012. [Google Scholar]
- Jia, B.; Wu, D.; Smallbone, A.; Ngwaka, U.C.; Roskilly, A.P. Dynamic and thermodynamic characteristics of a linear Joule engine generator with different operating conditions. Energy Convers. Manag. 2018, 173, 375–382. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.D.K.A.; Okafor, E.C. Science and technology of ammonia combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- SIP energy carriers 2016. Available online: http://www.jst.go.jp/sip/pdf/SIP_energycarriers2016_en.pdf (accessed on 14 May 2020).
- Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B.D. Ammonia as Effective Hydrogen Storage: A Review on Production, Storage and Utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Crolius, S.H. NH3 Energy Implementation Conference: A Brief Report. Available online: https://www.ammoniaenergy.org/articles/nh3-energy-implementation-conference-a-brief-report/ (accessed on 8 July 2020).
- Kojima, Y.; Miyaoka, H.; Ichikawa, T. Ammonia-Based Hydrogen Storage Materials. In Advanced Materials for Clean Energy; CRC Press: Boca Raton, FL, USA, 2015; pp. 497–526. [Google Scholar] [CrossRef]
- Sanchez Garcia, A.; Martín, M. Optimal renewable production of ammonia from water and air. J. Clean. Prod. 2018, 178, 325–342. [Google Scholar] [CrossRef]
- Frigo, S.; Gentili, R. Analysis of the behaviour of a 4-stroke Si engine fuelled with ammonia and hydrogen. Int. J. Hydrog. Energy 2013, 38, 1607–1615. [Google Scholar] [CrossRef]
- Ryu, K.; Zacharakis-Jutz, G.E.; Kong, S.-C. Performance characteristics of compression-ignition engine using high concentration of ammonia mixed with dimethyl ether. Appl. Energy 2014, 113, 488–499. [Google Scholar] [CrossRef]
- Kurata, O.; Iki, N.; Matsunuma, T.; Inoue, T.; Tsujimura, T.; Furutani, H.; Kobayashi, H.; Hayakawa, A. Performances and emission characteristics of NH3–air and NH3CH4–air combustion gas-turbine power generations. Proc. Combust. Inst. 2017, 36, 3351–3359. [Google Scholar] [CrossRef]
- Xiao, H.; Valera-Medina, A.; Bowen, P.J. Modeling Combustion of Ammonia/Hydrogen Fuel Blends under Gas Turbine Conditions. Energy Fuels 2017, 31, 8631–8642. [Google Scholar] [CrossRef]
- Hayakawa, A.; Arakawa, Y.; Mimoto, R.; Somarathne, K.D.K.A.; Kudo, T.; Kobayashi, H. Experimental investigation of stabilization and emission characteristics of ammonia/air premixed flames in a swirl combustor. Int. J. Hydrog. Energy 2017, 42, 14010–14018. [Google Scholar] [CrossRef]
- Okafor, E.C.; Naito, Y.; Colson, S.; Ichikawa, A.; Kudo, T.; Hayakawa, A.; Kobayashi, H. Measurement and modelling of the laminar burning velocity of methane-ammonia-air flames at high pressures using a reduced reaction mechanism. Combust. Flame 2019, 204, 162–175. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Pugh, D.G.; Marsh, P.; Bulat, G.; Bowen, P. Preliminary study on lean premixed combustion of ammonia-hydrogen for swirling gas turbine combustors. Int. J. Hydrog. Energy 2017, 42, 24495–24503. [Google Scholar] [CrossRef]
- Hiroyuki, T.; Jun, H.; Shota, K.; Kimio, I.; Fumiteru, A. Characteristics of ammonia/N2/O2 laminar flame in oxygen-enriched air condition. Trans. JSME 2015, 81, 14–00423. [Google Scholar]
- Irvin, G.; Richard, A.Y.; Nick, G.G. Combustion, 5th ed.; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Kobayashi, N.; He, Z.; Nagai, Y. Study on using hydrogen and ammonia as fuels: Combustion characteristics and NOx formation. Int. J. Energy Res. 2014, 38, 1214–1223. [Google Scholar] [CrossRef]
- Ichikawa, A.; Hayakawa, A.; Kitagawa, Y.; Kunkuma Amila Somarathne, K.D.; Kudo, T.; Kobayashi, H. Laminar burning velocity and Markstein length of ammonia/hydrogen/air premixed flames at elevated pressures. Int. J. Hydrog. Energy 2015, 40, 9570–9578. [Google Scholar] [CrossRef]
- Nozari, H.; Karabeyoğlu, A. Numerical study of combustion characteristics of ammonia as a renewable fuel and establishment of reduced reaction mechanisms. Fuel 2015, 159, 223–233. [Google Scholar] [CrossRef]
- Vigueras-Zuniga, M.-O.; Tejeda-del-Cueto, M.-E.; Vasquez-Santacruz, J.-A.; Herrera-May, A.-L.; Valera-Medina, A. Numerical Predictions of a Swirl Combustor Using Complex Chemistry Fueled with Ammonia/Hydrogen Blends. Energies 2020, 13, 288. [Google Scholar] [CrossRef]
- Miller, J.A.; Bowman, C.T. Mechanism and modeling of nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 1989, 15, 287–338. [Google Scholar] [CrossRef]
- Klippenstein, S.J.; Harding, L.B.; Glarborg, P.; Miller, J.A. The role of NNH in NO formation and control. Combust. Flame 2011, 158, 774–789. [Google Scholar] [CrossRef]
- Song, Y.; Hashemi, H.; Christensen, J.M.; Zou, C.; Marshall, P.; Glarborg, P. Ammonia oxidation at high pressure and intermediate temperatures. Fuel 2016, 181, 358–365. [Google Scholar] [CrossRef]
- Otomo, J.; Koshi, M.; Mitsumori, T.; Iwasaki, H.; Yamada, K. Chemical kinetic modeling of ammonia oxidation with improved reaction mechanism for ammonia/air and ammonia/hydrogen/air combustion. Int. J. Hydrog. Energy 2018, 43, 3004–3014. [Google Scholar] [CrossRef]
- Konnov, A.A. Implementation of the NCN pathway of prompt-NO formation in the detailed reaction mechanism. Combust. Flame 2009, 156, 2093–2105. [Google Scholar] [CrossRef]
- Meyer, T.; Kumar, P.; Li, M.; Redfern, K.; Diaz, D. Ammonia Combustion with Near-zero Pollutant Emissions. In NH3 Congress: Iowa, USA; Iowa State University: Ames, IA, USA, 2011. [Google Scholar]
- Duynslaegher, C.; Contino, F.; Vandooren, J.; Jeanmart, H. Modeling of ammonia combustion at low pressure. Combust. Flame 2012, 159, 2799–2805. [Google Scholar] [CrossRef]
- Duynslaegher, C.; Jeanmart, H.; Vandooren, J. Flame structure studies of premixed ammonia/hydrogen/oxygen/argon flames: Experimental and numerical investigation. Proc. Combust. Inst. 2009, 32, 1277–1284. [Google Scholar] [CrossRef]
- Gregory, P.S.; David, M.G.; Michael, F.; Nigel, W.M.; Boris, E.; Mikhail, G.; Ronald, K.H.; Soonho, S.; William, C.G. GRI-Mech 3.0. Available online: http://www.me.berkeley.edu/gri_mech/ (accessed on 14 May 2020).
- Mechanical and Aerospace Engineering (Combustion Research), U.o.C.a.S.D. Chemical-Kinetic Mechanisms for Combustion Applications. Available online: http://combustion.ucsd.edu (accessed on 14 May 2020).
- Lindstedt, R.P.; Lockwood, F.C.; Selim, M.A. Detailed Kinetic Modelling of Chemistry and Temperature Effects on Ammonia Oxidation. Combust. Sci. Technol. 1994, 99, 253–276. [Google Scholar] [CrossRef]
- Jiang, B. Mécanisme de Formation et de Disparition des Oxydes D’azote dans les Flammes: Étude Expérimentale et Modélisation. Ph.D. Thesis, Université Catholique de Louvain, Ottignies-Louvain-la-Neuve, Belgium, 1990. [Google Scholar]
- Mathieu, O.; Petersen, E.L. Experimental and modeling study on the high-temperature oxidation of Ammonia and related NOx chemistry. Combust. Flame 2015, 162, 554–570. [Google Scholar] [CrossRef]
- Bugler, J.; Somers, K.P.; Simmie, J.M.; Güthe, F.; Curran, H.J. Modeling Nitrogen Species as Pollutants: Thermochemical Influences. J. Phys. Chem. A 2016, 120, 7192–7197. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Hasegawa, S.; Tezuka, T. Kinetic modeling of ammonia/air weak flames in a micro flow reactor with a controlled temperature profile. Combust. Flame 2017, 185, 16–27. [Google Scholar] [CrossRef]
- ANSYS CHEMKIN PRO Version 17.0. Available online: http://www.ansys.com/products/fluids/ansys-chemkin-proANSYS (accessed on 10 March 2016).
- Kumar, P.; Meyer, T.R. Experimental and modeling study of chemical-kinetics mechanisms for H2–NH3–air mixtures in laminar premixed jet flames. Fuel 2013, 108, 166–176. [Google Scholar] [CrossRef]
- Okafor, E.C.; Naito, Y.; Colson, S.; Ichikawa, A.; Kudo, T.; Hayakawa, A.; Kobayashi, H. Experimental and numerical study of the laminar burning velocity of CH4–NH3–air premixed flames. Combust. Flame 2018, 187, 185–198. [Google Scholar] [CrossRef]
- Xiao, H.; Valera-Medina, A.; Bowen, P.J. Study on premixed combustion characteristics of co-firing ammonia/methane fuels. Energy 2017, 140, 125–135. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Chen, G.; Wu, D.; Li, T.; Zhang, Z.; Wang, N. Characteristics of Ammonia/Hydrogen Premixed Combustion in a Novel Linear Engine Generator. Proceedings 2020, 58, 2. https://doi.org/10.3390/WEF-06925
Zhang F, Chen G, Wu D, Li T, Zhang Z, Wang N. Characteristics of Ammonia/Hydrogen Premixed Combustion in a Novel Linear Engine Generator. Proceedings. 2020; 58(1):2. https://doi.org/10.3390/WEF-06925
Chicago/Turabian StyleZhang, Fangyu, Gen Chen, Dawei Wu, Tie Li, Zhifei Zhang, and Ning Wang. 2020. "Characteristics of Ammonia/Hydrogen Premixed Combustion in a Novel Linear Engine Generator" Proceedings 58, no. 1: 2. https://doi.org/10.3390/WEF-06925
APA StyleZhang, F., Chen, G., Wu, D., Li, T., Zhang, Z., & Wang, N. (2020). Characteristics of Ammonia/Hydrogen Premixed Combustion in a Novel Linear Engine Generator. Proceedings, 58(1), 2. https://doi.org/10.3390/WEF-06925






