New β-ketophosphonates for the Synthesis of Prostaglandin Analogues. 1. Phosphonates with a Bicyclo[3.3.0]octene Scaffold Spaced by a Methylene Group from the β-ketone †
| The new β-ketophosphonates are key intermediates for obtaining new prostaglandin analogues with a bicyclo[3.3.0]octene fragment in the ω-side chain I and II. | 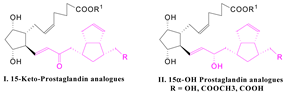 |
| The use of bis β-ketophosphonate 7 in the usual stereoselective Z-Horner-Emmons-Wadsworts selective olefination conditions should gave the pseudo-prostaglandin compound III: | 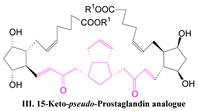 |
Acknowledgments
References
- Collins, P.W.; Djuric, S.W. Synthesis of therapeutically useful prostaglandin and prostacyclin analogs. Chem. Rev. 1993, 93, 1533–1564. [Google Scholar] [CrossRef]
- Das, S.; Chandrasekhar, S.; Yadav, J.S.; Gree, R. Recent developments in the synthesis of prostaglandins and analogues. Chem. Rev. 2007, 107, 3286–3337. [Google Scholar] [CrossRef] [PubMed]
- 1,2,3,3a,4,6a-hexahydro-1,3-pentalenedimethanol, mono and bis oh-protected derivatives, useful intermediates for fine organic synthesis. Rev. Roum. Chim. 2008, 53, 195–202.
- Tănase, C.; Cocu, F.; Drăghici, C.; Hanganu, A.; Pintilie, L.; Maganu, M.; Munteanu, C.V.A.; Shova, S. Secondary compounds in the catalytic hydrogenation of enone and allylic alcohol prostaglandin intermediates: Isolation, characterization, and X-ray crystallography. New J. Chem. 2019, 43, 7582–7599. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tănase, C.I.; Căproiu, M.T.; Drăghici, C.; Pintilie, L.; Paladache, L. New β-ketophosphonates for the Synthesis of Prostaglandin Analogues. 1. Phosphonates with a Bicyclo[3.3.0]octene Scaffold Spaced by a Methylene Group from the β-ketone. Proceedings 2020, 57, 43. https://doi.org/10.3390/proceedings2020057043
Tănase CI, Căproiu MT, Drăghici C, Pintilie L, Paladache L. New β-ketophosphonates for the Synthesis of Prostaglandin Analogues. 1. Phosphonates with a Bicyclo[3.3.0]octene Scaffold Spaced by a Methylene Group from the β-ketone. Proceedings. 2020; 57(1):43. https://doi.org/10.3390/proceedings2020057043
Chicago/Turabian StyleTănase, Constantin I., Miron Teodor Căproiu, Constantin Drăghici, Lucia Pintilie, and Laura Paladache. 2020. "New β-ketophosphonates for the Synthesis of Prostaglandin Analogues. 1. Phosphonates with a Bicyclo[3.3.0]octene Scaffold Spaced by a Methylene Group from the β-ketone" Proceedings 57, no. 1: 43. https://doi.org/10.3390/proceedings2020057043
APA StyleTănase, C. I., Căproiu, M. T., Drăghici, C., Pintilie, L., & Paladache, L. (2020). New β-ketophosphonates for the Synthesis of Prostaglandin Analogues. 1. Phosphonates with a Bicyclo[3.3.0]octene Scaffold Spaced by a Methylene Group from the β-ketone. Proceedings, 57(1), 43. https://doi.org/10.3390/proceedings2020057043





