Abstract
Our contribution focuses on a correlative study of polyaniline (PANI) electropolymerisation and UV/VIS spectroscopy. PANI was prepared via electro-oxidation using a potentiodynamic method on commercial gold screen-printed electrodes (Au-SPE). By using an in-situ spectroelectrochemical method, the development of the polymer was observed from monomer, monomer oxidation to final polymer formation and its transformations between the oxidation forms. The results confirm the spontaneous doping of the polymer during the polymerisation, the instability of leucoemeraldine form in air and its two-stage oxidation to emeraldine form. The final conductive PANI deposited on Au-SPE will be used as sensor element for the detection of toxic organic compounds.
1. Introduction
The conducting polymer polyaniline (PANI) is one of the most investigated polymers, due to its interesting properties, such as tunable morphology, environmental stability, conductivity, reversible redox chemistry, easy synthesis, and low cost [1]. Potential PANI applications include as sensor elements for ammonia sensing [2], humidity sensing [3], pH sensing [4], etc. PANI can be processed via cyclic voltammetry (CV) which presents an easy path for the deposition of nanostructured PANI in doped oxidation form. Basic representation of PANI is a combination of two repetition units, oxidized quinoid rings and reduced benzene rings. Depending on their ratio it has three the most characteristic forms, regarding oxidation state: leucoemeraldine (fully reduced form), emeraldine (half oxidized form) and pernigraniline (fully oxidized form) [5]. Emeraldine salt form is the most conductive form of PANI. Additionally, PANI is also an electrochromic material, meaning it undergoes visible absorbance changes due to the reversible redox processes. These changes are closely related to the energies of the PANI oxidation forms as a consequence of the formation of sub-bands by charge carriers (polarons—cation-radical and bipolarons—double charge) [5]. The most straightforward technique to observe color changes and redox responses of PANI is CV in combination with UV/VIS spectroscopy. Therefore, the in-situ spectroelectrochemical measurement paves the way to understand the polymerization steps and mechanism of PANI formation.
2. Materials and Methods
The experiments were performed using a new type of spectroelectrochemistry device, that enables the in-situ electrochemical and spectroscopic measurements. Commercial Au-SPE were used for the polymerization of PANI via CV. The 80 µL droplet of aniline polymerization suspension was added into the cell and cycled between −0.3 and 1 V vs. Ag at scan rate 50 mV/s were performed. Spectroscopic measurements from 200 to 900 nm were performed in-situ during the polymerization.
3. Results and Discussion
Figure 1 shows cyclic voltammogram (a) and UV/VIS spectra (b) for PANI-Au-SPE during separate stages of polymerisation. At −0.2 V vs. Ag leucoemeraldine is formed (yellow), at 0.2 V vs. Ag, conductive emeraldine salt (dark green) is obtained, which is further oxidized to pernigraniline (0.5 V). Polymerisation ends at −0.3 V vs. Ag, where again the leucoemeraldine form is obtained (Figure 1a). This form is known to be unstable and immediately oxidizes in air to the light green form—protoemeraldine—which is intermediate between leucoemeraldine and emeraldine. The appearance of shoulder in absorbance (Figure 1b) at 420 nm of PANI film obtained at 0.15 V vs. Ag, indicates the presence of state between leucoemeraldine and emeraldine, which later becomes the maximum for emeraldine salt obtained at 0.2 V vs. Ag. PANI obtained after polymerisation shows higher absorbance at 420 nm than leucomemeraldine, which confirms that exposing to air after polymerisation caused partial oxidation. Absorbance increases for emeraldine salt and decreases for pernigraniline. Emeraldine salt shows characteristic absorbance peaks located between 320–360 nm for π–π* transition, around 400 nm for polaron–π* transition and between 700–750 nm for polaron–π transition. One can see that at all mentioned potentials, green emeraldine salt at a potential 0.2 V vs. Ag shows the highest absorbance (Figure 1b). According to theory, the polarons present charge carries in conductive polymers [6]. Therefore, emelardine salt is known to be the most conductive form of PANI; hence, it will be further applied as sensor element for the detection of ammonia and acrylamide.
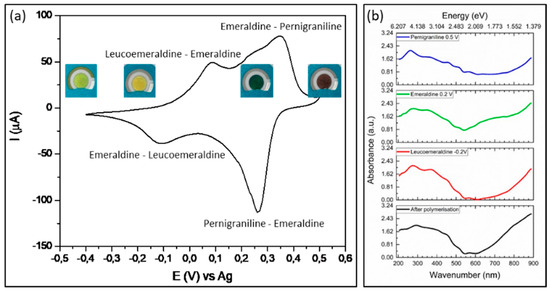
Figure 1.
(a) Cyclic voltammetry (CV) with color changes (insets) of polyaniline (PANI) forms and (b) UV/VIS spectra of the PANI oxidation forms obtained at certain potentials (−0.2 V, 0.2 V, 0.5 V and −0.3 V vs. Ag) in air.
Funding
The project (High performance nanostructured acrylamide sensors, ID J2-1739) was financially supported by the Slovenian Research Agency.
References
- Song, E.; Choi, J.-W. Conducting polyaniline nanowire and its applications in chemiresistive sensing. Nanomaterials 2013, 3, 498–523. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Debarnot, D.; Poncin-Epaillard, F. Polyaniline as a new sensitive layer for gas sensors. Anal. Chim. Acta 2003, 475, 1–15. [Google Scholar] [CrossRef]
- Kumar, R.; Yadav, B.C. Humidity sensing investigation on nanostructured polyaniline synthesized via chemical polymerization method. Mater. Lett. 2016, 167, 300–302. [Google Scholar] [CrossRef]
- Jin, Z.; Su, Y.; Duan, Y. An improved optical pH sensor based on polyaniline. Sens. Actuators B Chem. 2000, 71, 118–122. [Google Scholar] [CrossRef]
- Gvozdenovic, M.; Jugovic, B.; Stevanovic, J.; Grgur, B. Electrochemical synthesis of electroconducting polymers. Hem. Ind. 2014, 68, 673–684. [Google Scholar] [CrossRef]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers (Basel) 2017, 9, 150. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).