Fourier-Transform Infrared Microspectroscopy (FT-IR) Study on Caput and Cauda Mouse Spermatozoa †
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Sperm Samples from Caput and Cauda Epididymis
2.2. FT-IR Spectral Analysis
3. Results and Discussion
4. Conclusions
Acknowledgments
Conflicts of Interest
Compliance with Ethical Standards
References
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [PubMed]
- Shivanoor, S.M.; David, M. Fourier transform infrared (FT-IR) study on cyanide induced biochemical and structural changes in rat sperm. Toxicol. Rep. 2015, 2, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, V.; Redmann, K.; Wistuba, J.; Wübbeling, F.; Burger, M.; Oldenhof, H.; Wolkers, W.F.; Kliesch, S.; Schlatt, S.; Mallidis, C. Oxidative DNA damage in human sperm can be detected by Raman microspectroscopy. Fertil. Steril. 2012, 98, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Marciani, S.; Tamburrino, L.; Muratori, M.; Baldi, E. Epididymal sperm transport and fertilization. In Endocrinology of the Testis and Male Reproduction Endocrinology 1; Simoni, M., Huhtaniemi, I.T., Eds.; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2017; pp. 457–478. [Google Scholar]
- Cooper, T.G. Epididymis. In Enciclopedia of Reproduction; Knobil, E., Neill, J.D., Eds.; Academic Press: Cambridge, MA, USA, 2003; pp. 1–17. [Google Scholar]
- Portaccio, M.; d’Apuzzo, F.; Perillo, L.; Grassia, V.; Errico, S.; Lepore, M. Infrared microspectroscopy characterization of gingival crevicular fluid during orthodontic treatment. J. Mol. Struct. 2019, 1176, 847–854. [Google Scholar] [CrossRef]
- Liu, K.Z.; Schultz, C.P.; Mohammad, R.M.; Johnston, J.B.; Mantsch, H.H. Infrared spectroscopic study of bryostatin 1-induced membrane alterations in a B-CLLcell line. Leukemia 1999, 13, 1273–1280. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Severcan, F.; Gorgulu, G.; Gorgulu, S.T.; Guray, T. Rapid monitoring of diabetes-induced lipid peroxidation by Fourier transform infrared spectroscopy: Evidence from rat liver microsomal membranes. Anal. Biochem. 2005, 339, 36–40. [Google Scholar] [CrossRef] [PubMed]
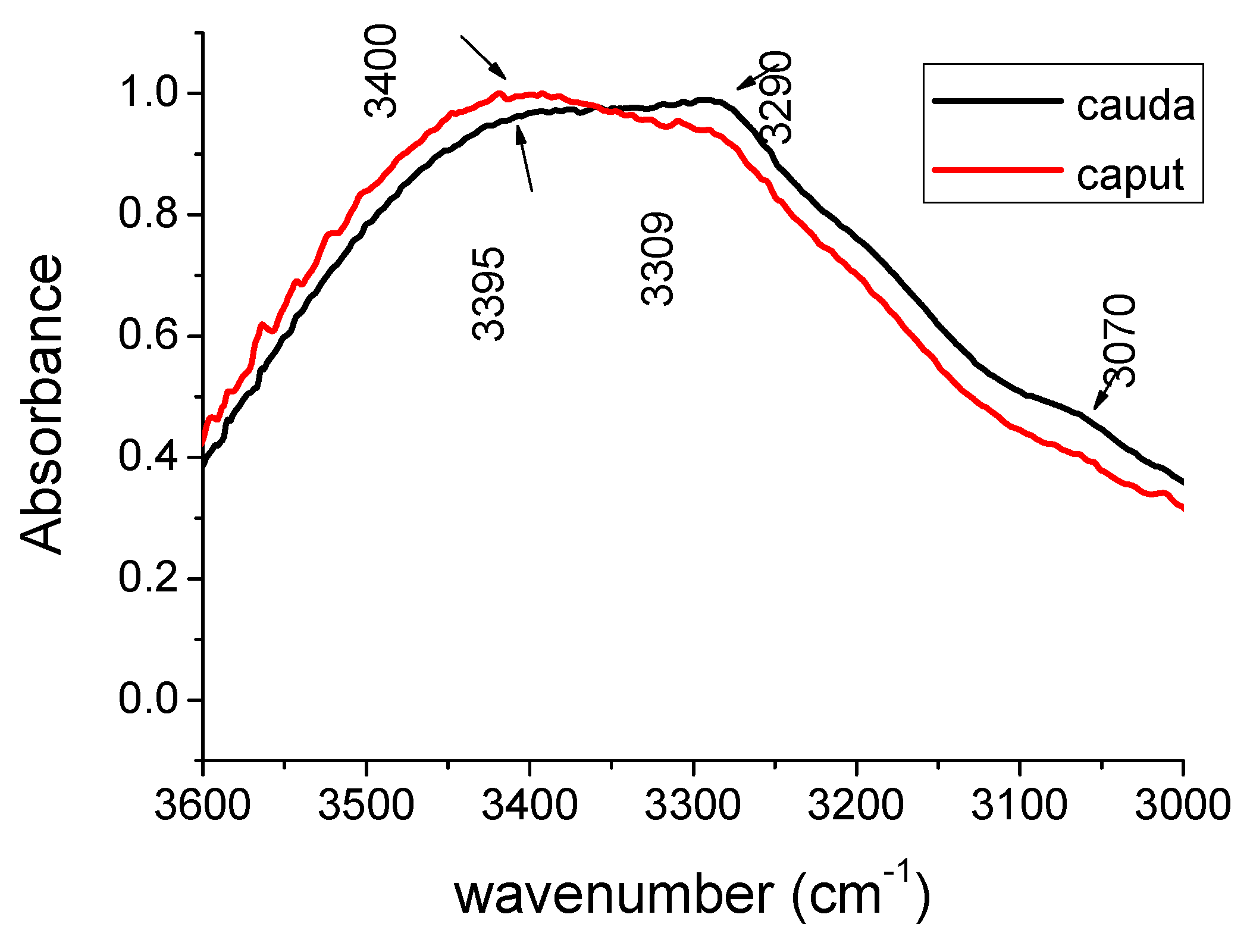
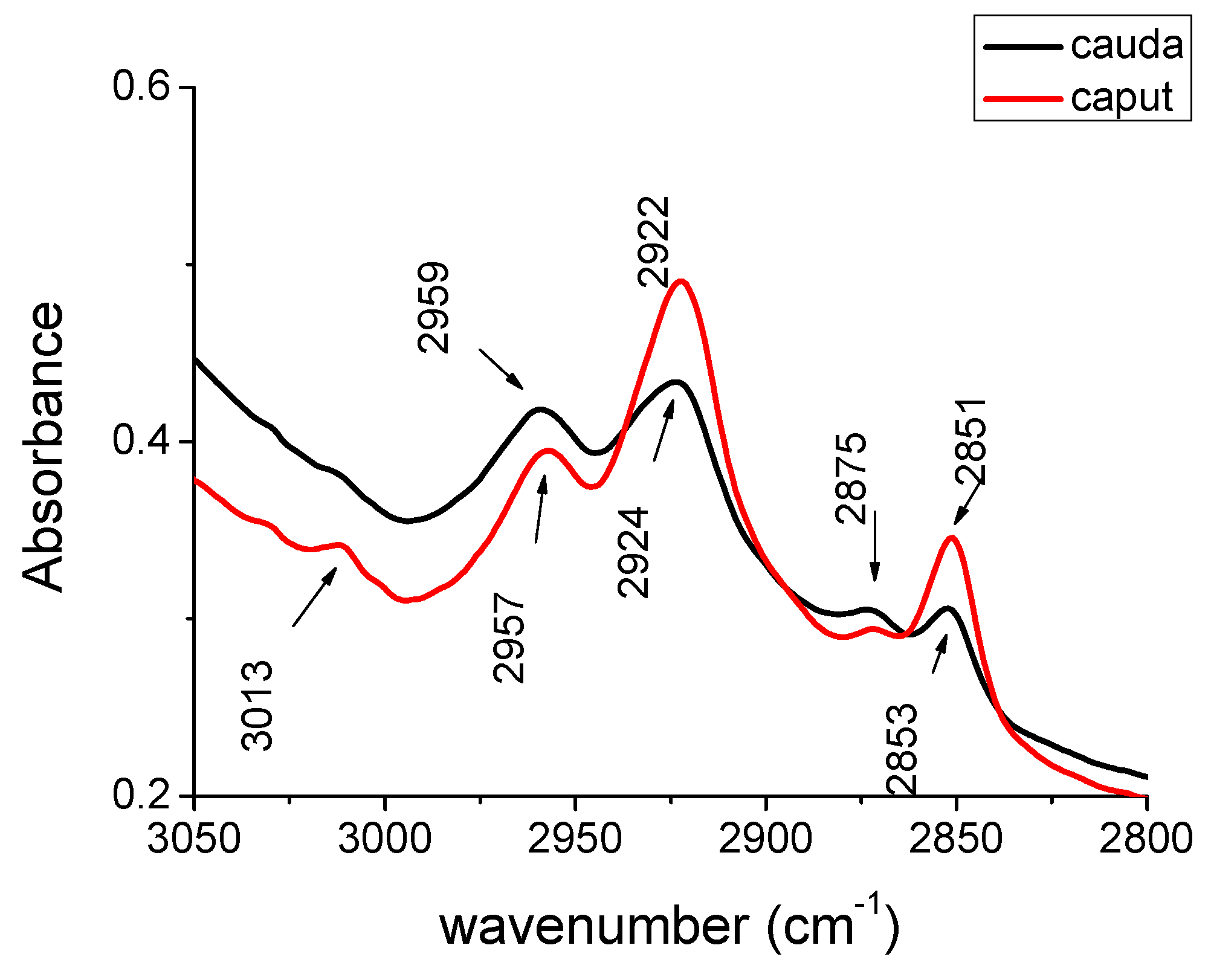
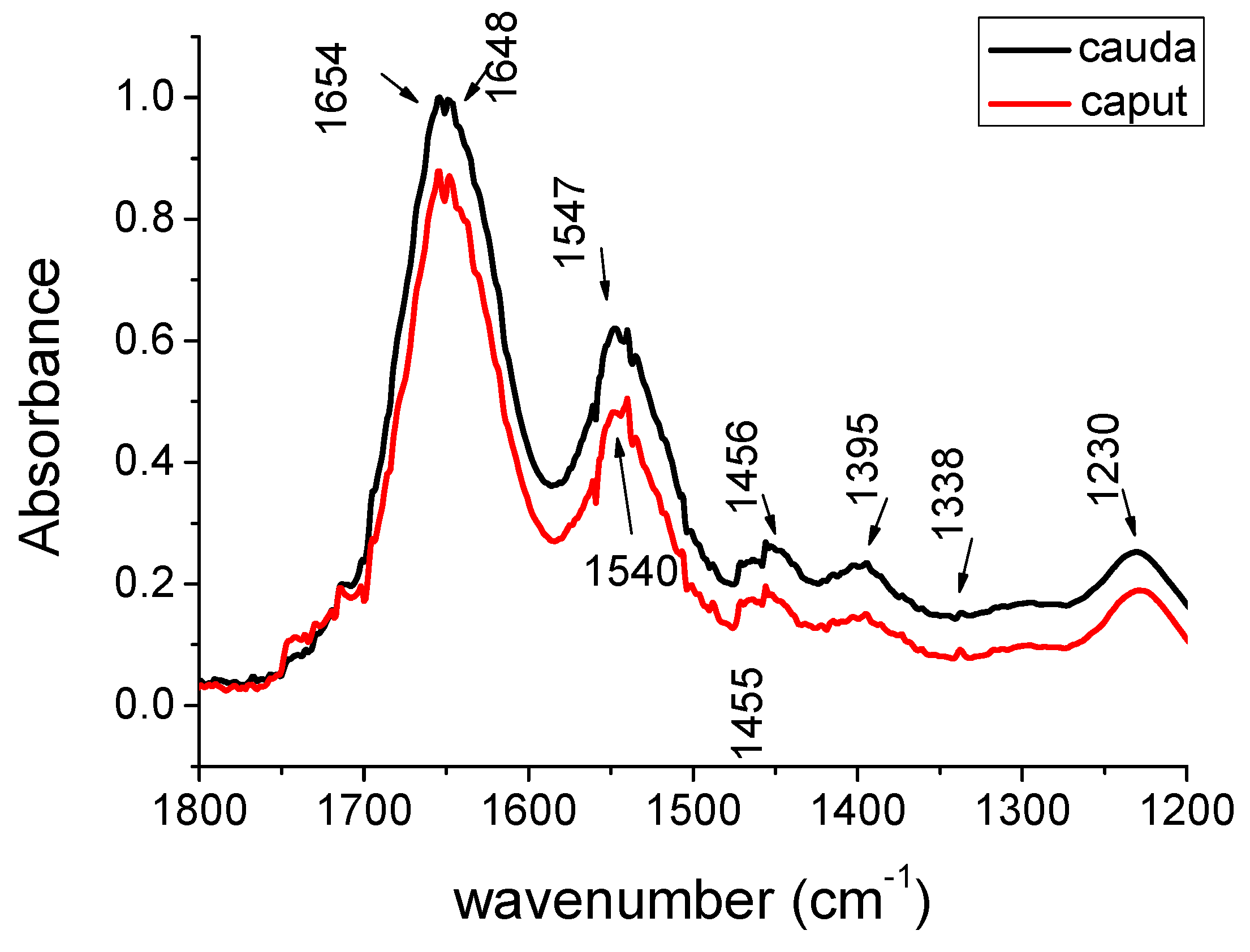
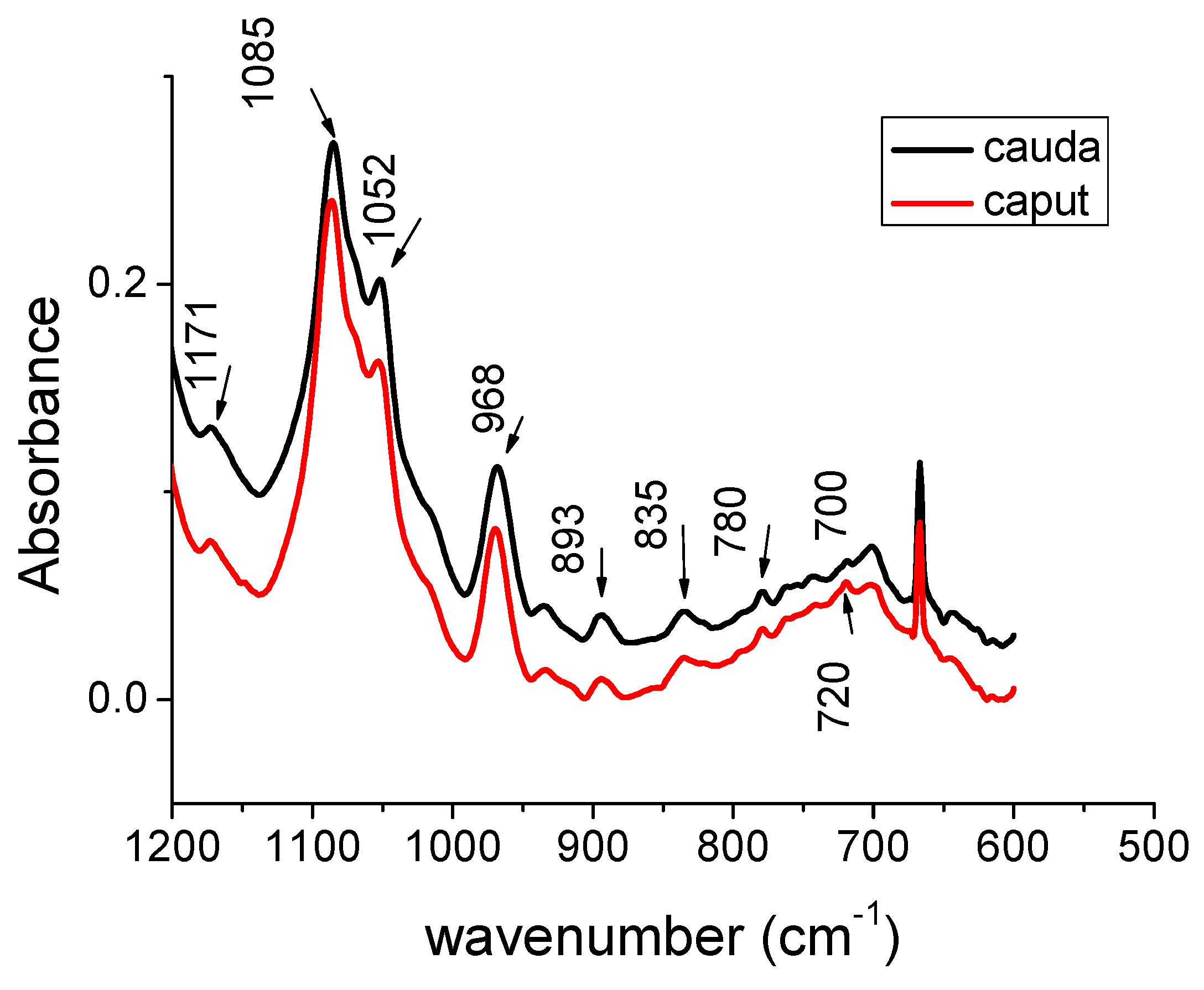
| Peak caput cm−1 | Peak cauda cm−1 | Peak Assignments | Biochemical Molecules |
|---|---|---|---|
| 3400 | 3395 | Hydrogen bonded O-H N-H | protein water |
| 3309 | 3290 | Amide A N-H | protein |
| 3013 | 3013 | olefinic = C-H | unsaturated lipid |
| 2957 | 2959 | CH3 as | lipid |
| 2922 | 2924 | CH2 as | lipid |
| 2875 | 2875 | CH3 s | lipid |
| 2851 | 2853 | CH2 s | lipid |
| 1654 | 1654 | Amide I-helix | protein |
| 1648 | 1648 | Amide I | protein |
| 1540 | 1547 | Amide II | protein |
| 1455 | 1456 | CH3 as bCH2 scis | lipid and protein |
| 1395 | 1395 | COO- s | fatty acid |
| 1300 | 1300 | Amide III | protein |
| 1230 | 1230 | PO2- as | nucleic acids and phospholipids |
| 1171 | 1171 | CO-O-C | glycogen |
| 1085 | 1085 | PO2- s | nucleic acids |
| 1052 | 1052 | C-O | saccharides and DNA deoxyribose |
| 968 | 968 | C-C | DNA backbone |
| 893 | 893 | Deoxyribose ring | DNA |
| 835 | 835 | DNA S-type sugar | DNA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portaccio, M.; Errico, S.; Chioccarelli, T.; Cobellis, G.; Lepore, M. Fourier-Transform Infrared Microspectroscopy (FT-IR) Study on Caput and Cauda Mouse Spermatozoa. Proceedings 2020, 42, 19. https://doi.org/10.3390/ecsa-6-06537
Portaccio M, Errico S, Chioccarelli T, Cobellis G, Lepore M. Fourier-Transform Infrared Microspectroscopy (FT-IR) Study on Caput and Cauda Mouse Spermatozoa. Proceedings. 2020; 42(1):19. https://doi.org/10.3390/ecsa-6-06537
Chicago/Turabian StylePortaccio, Marianna, Sonia Errico, Teresa Chioccarelli, Gilda Cobellis, and Maria Lepore. 2020. "Fourier-Transform Infrared Microspectroscopy (FT-IR) Study on Caput and Cauda Mouse Spermatozoa" Proceedings 42, no. 1: 19. https://doi.org/10.3390/ecsa-6-06537
APA StylePortaccio, M., Errico, S., Chioccarelli, T., Cobellis, G., & Lepore, M. (2020). Fourier-Transform Infrared Microspectroscopy (FT-IR) Study on Caput and Cauda Mouse Spermatozoa. Proceedings, 42(1), 19. https://doi.org/10.3390/ecsa-6-06537






