Rapid, Wide-Range, and Low-Cost Determination of Formaldehyde Based on Porous Silica Gel Plate by Digital Image Colorimetry †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Sensing Elements
2.3. Colorimetric Test of Formaldehyde
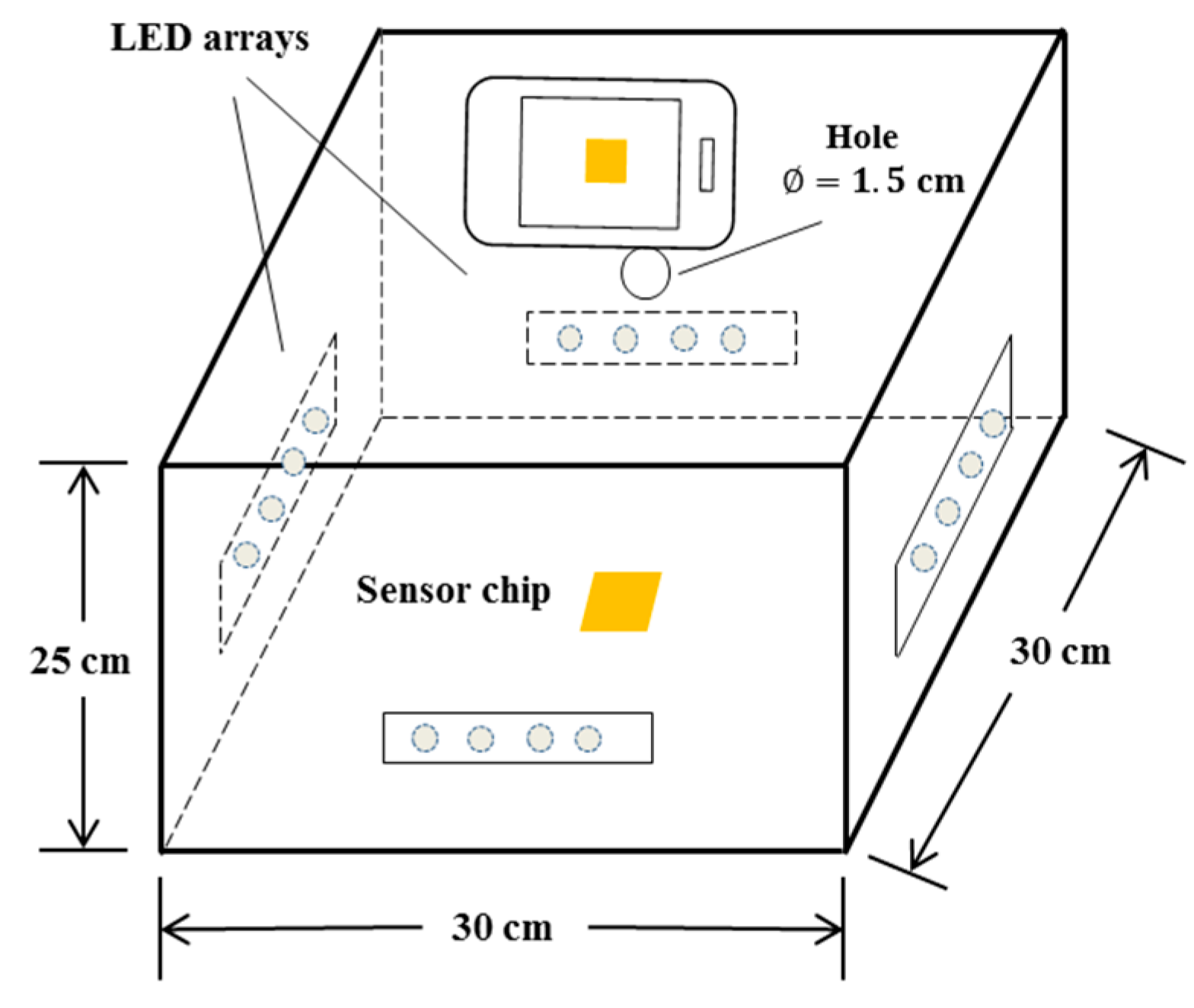
2.4. Photographic System and Procedure
2.5. Analytical Performance and Method Validation
3. Results and Discussion
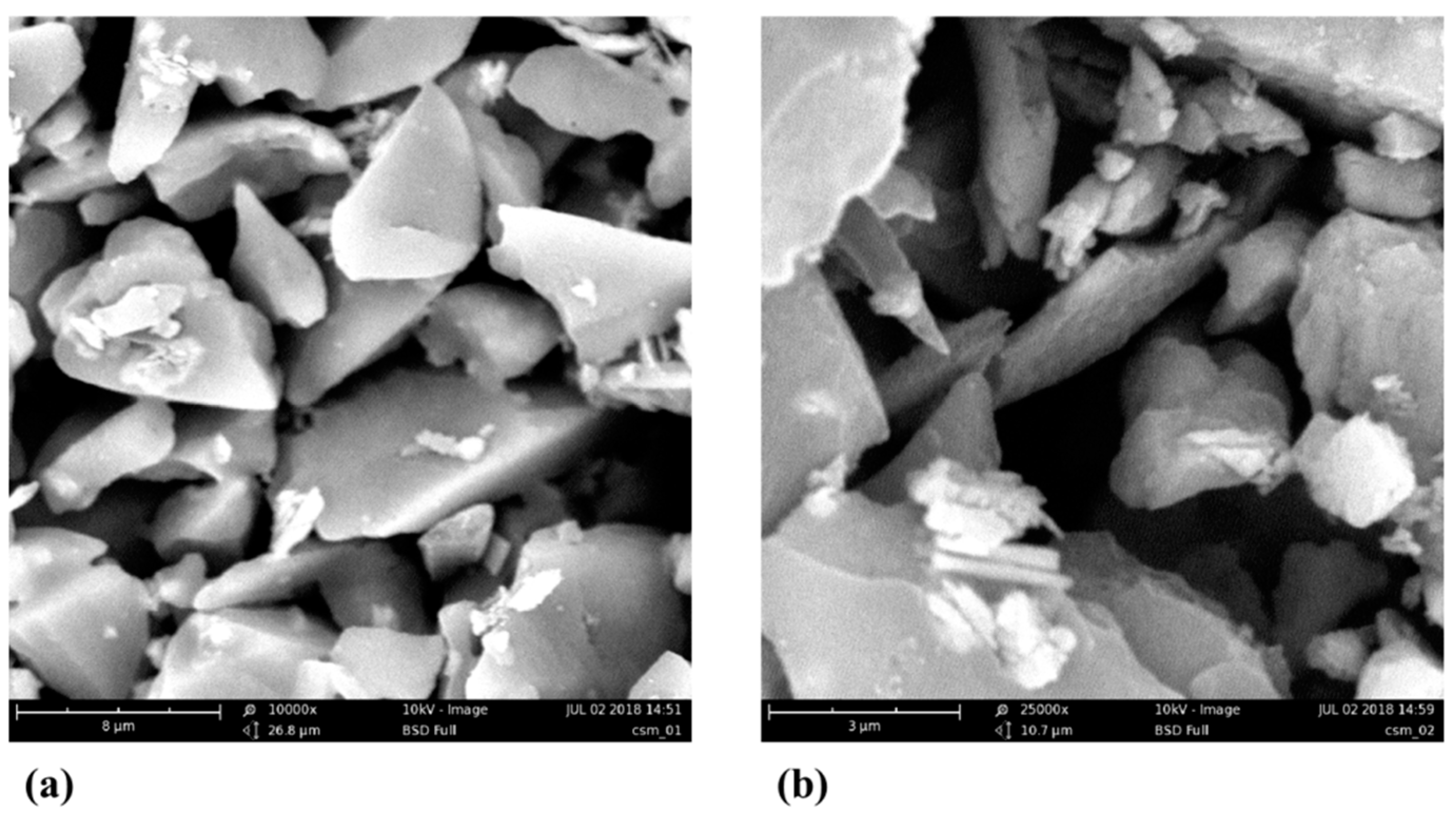
3.1. Characterization of Silica Gel Plates and Preparation of the Sensing Elements
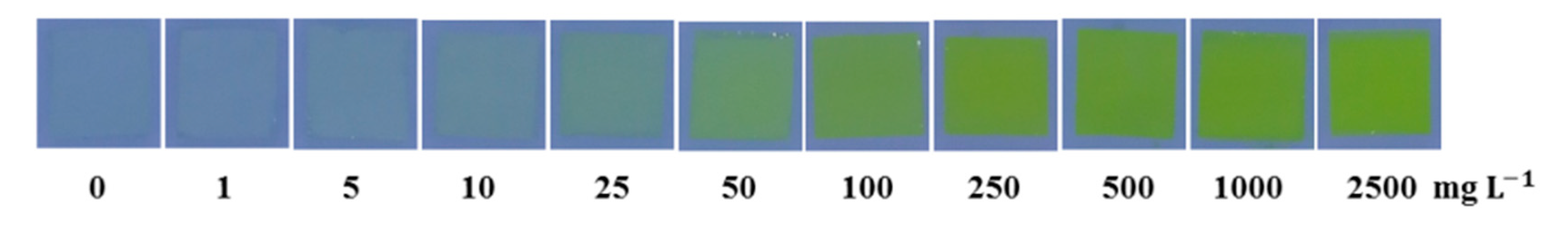
3.2. Colorimetric Test of the Sensing Elements
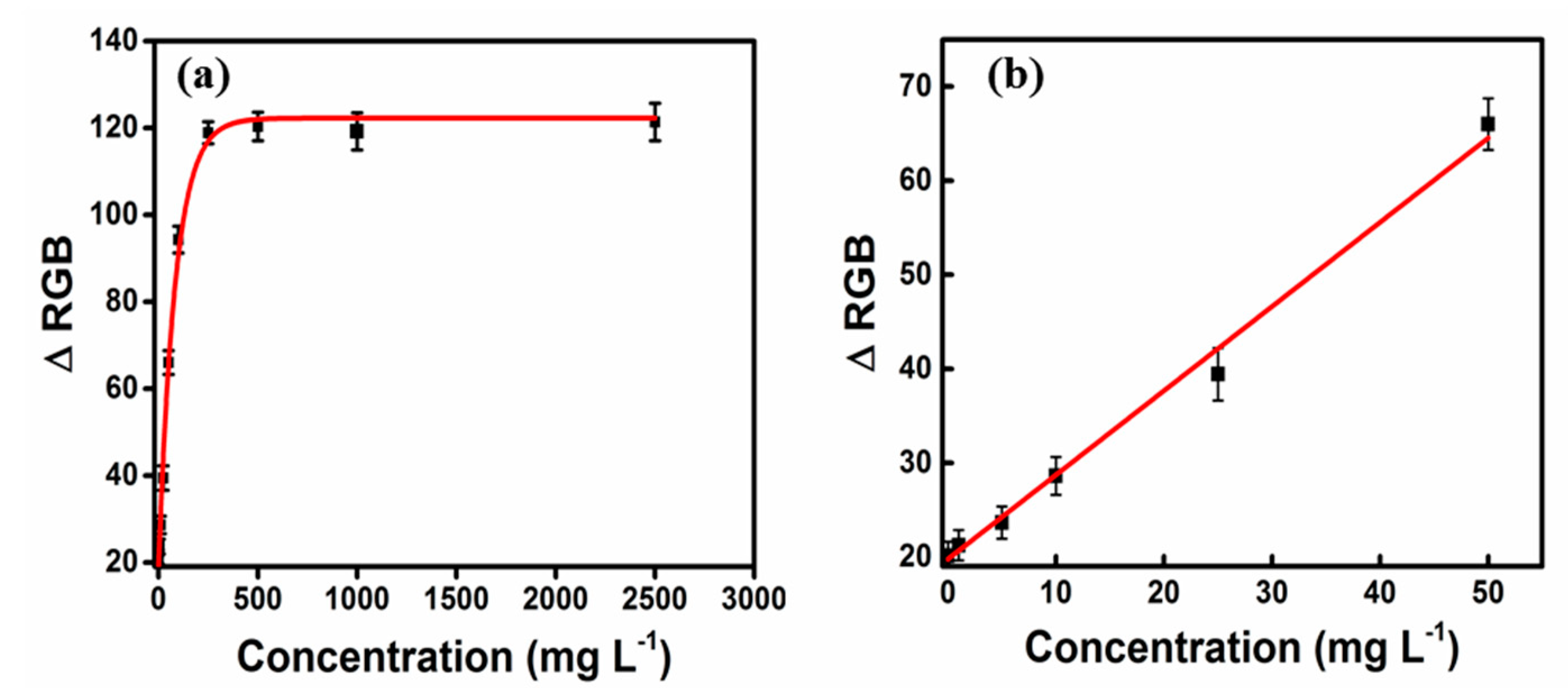
3.3. Digital Image Analysis for Quantification of Formaldehyde
3.4. Analytical Performance and Method Validation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chung, P.R.; Tzeng, C.T.; Ke, M.T.; Lee, C.Y. Formaldehyde Gas Sensors: A Review. Sensors 2013, 13, 4468–4484. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Formaldehyde, 2-butoxyethanol and 1-tert-butoxypropan-2-ol. IARC Monogr Eval. Carcinog. Risks Hum. 2006, 88, 1–478. [Google Scholar]
- World Health Organization. Environmental Health Criteria 89 Formaldehyde; WHO: Geneva, Switzerland, 1989. [Google Scholar]
- Hopkins, J.R.; Still, T.; Al-Haider, S.; Fisher, I.R.; Lewis, A.C.; Seakins, P.W. A simplified apparatus for ambient formaldehyde detection via GC-pHID. Atmos. Environ. 2003, 37, 2557–2565. [Google Scholar] [CrossRef]
- Wahed, P.; Razzaq, M.A.; Dharmapuri, S.; Corrales, M. Determination of formaldehyde in food and feed by an in-house validated HPLC method. Food Chem. 2016, 202, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Maruo, Y.Y.; Nakamura, J. Portable formaldehyde monitoring device using porous glass sensor and its applications in indoor air quality studies. Anal. Chim. Acta 2011, 702, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Mariano, S.; Wang, W.; Brunelle, G.; Bigay, Y.; Tran-Thi, T.H. Colorimetric Detection of Formaldehyde: A Sensor for Air Quality Measurements and a Pollution-Warning Kit for Homes. Procedia Eng. 2010, 5, 1184–1187. [Google Scholar] [CrossRef][Green Version]
- Lin, C.; Zhu, Y.; Yu, J.; Qin, X.; Xian, X.; Tsow, F.; Forzani, E.S.; Wang, D.; Tao, N. Gradient-Based Colorimetric Sensors for Continuous Gas Monitoring. Anal. Chem. 2018, 90, 5375–5380. [Google Scholar] [CrossRef] [PubMed]
- Rungroadsri, J.; Limsakul, W.; Wongniramaikul, W.; Choodum, A. Rapid Semi-Quantitative Analysis of Formaldehyde in Food by Digital Image Colorimetry. Int. J. Chem. Eng. Appl. 2017, 8, 294–298. [Google Scholar] [CrossRef][Green Version]
- Choodum, A.; Kanatharana, P.; Wongniramaikul, W.; Daeid, N.N. Using the iPhone as a device for a rapid quantitative analysis of trinitrotoluene in soil. Talanta 2013, 115, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Choodum, A.; Daeid, N.N. Rapid and semi-quantitative presumptive tests for opiate drugs. Talanta 2011, 86, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Wongniramaikul, W.; Limsakul, W.; Choodum, A. A biodegradable colorimetric film for rapid low-cost field determination of formaldehyde contamination by digital image colorimetry. Food Chem. 2018, 249, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Choodum, A.; Kanatharana, P.; Wongniramaikul, W.; Nicdaeid, N. A sol–gel colorimetric sensor for methamphetamine detection. Sens. Actuators B Chem. 2015, 215, 553–560. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Drug Discov. Ther. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Pinheiro, H.L.; de Andrade, M.V.; de Paula Pereira, P.A.; de Andrade, J.B. Spectrofluorimetric determination of formaldehyde in air after collection onto silica cartridges coated with Fluoral P. Microchem. J. 2004, 78, 15–20. [Google Scholar] [CrossRef]
- Wang, S.E. Spectrophotometric Method with Silica-Gel Beads for Determination of Trace Formaldehyde in Air. Spectrosc. Lett. 2005, 38, 121–130. [Google Scholar]
- Choodum, A.; Kanatharana, P.; Wongniramaikul, W.; Nicdaeid, N. Poly vinyl alcohol cryogel as a selective test kit for pre and post blast trinitrotoluene. Sens. Actuators B Chem. 2016, 222, 654–662. [Google Scholar] [CrossRef]
- Hoang, T.; Cho, Y.B.; Yong, S.K. A strip array of colorimetric sensors for visualizing a concentration level of gaseous analytes with basicity. Sens. Actuators B Chem. 2017, 251, 1089–1095. [Google Scholar] [CrossRef]

| Relationship | Calibration Equation | Linearity | Linear Range | LOD | LQD |
|---|---|---|---|---|---|
| 0.9892 | 0-50 | 0.1 | 0.3 | ||
| 0.9952 | 0-50 | 0.1 | 0.2 | ||
| 0.9926 | 0-50 | 0.1 | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, S.; Liu, Y.; Zhao, L.; Cao, X.; Wang, X.; Zheng, M.; Pan, H.; Chen, J.; Xu, J. Rapid, Wide-Range, and Low-Cost Determination of Formaldehyde Based on Porous Silica Gel Plate by Digital Image Colorimetry. Proceedings 2020, 42, 13. https://doi.org/10.3390/ecsa-6-06542
Cao S, Liu Y, Zhao L, Cao X, Wang X, Zheng M, Pan H, Chen J, Xu J. Rapid, Wide-Range, and Low-Cost Determination of Formaldehyde Based on Porous Silica Gel Plate by Digital Image Colorimetry. Proceedings. 2020; 42(1):13. https://doi.org/10.3390/ecsa-6-06542
Chicago/Turabian StyleCao, Simin, Yangyi Liu, Litao Zhao, Xiaodan Cao, Xueli Wang, Ming Zheng, Haifeng Pan, Jinquan Chen, and Jianhua Xu. 2020. "Rapid, Wide-Range, and Low-Cost Determination of Formaldehyde Based on Porous Silica Gel Plate by Digital Image Colorimetry" Proceedings 42, no. 1: 13. https://doi.org/10.3390/ecsa-6-06542
APA StyleCao, S., Liu, Y., Zhao, L., Cao, X., Wang, X., Zheng, M., Pan, H., Chen, J., & Xu, J. (2020). Rapid, Wide-Range, and Low-Cost Determination of Formaldehyde Based on Porous Silica Gel Plate by Digital Image Colorimetry. Proceedings, 42(1), 13. https://doi.org/10.3390/ecsa-6-06542




