Abstract
The ubiquitin proteasomal system and autophagic pathway are two main protein degradation systems in eukaryotic cells. Inhibition of the proteasomal system with proteasome inhibitors for cancer treatment can cause neurotoxic side effects. In this study, we investigated neurotoxic side effects of bortezomib (BTZ) and carfilzomib (CFZ) in a human neuronal cell model. Inhibition of proteasome with BTZ upregulated autophagy receptor protein p62 level. BTZ caused reduced mitochondrial mass per cell in a greater extent than CFZ. BTZ caused more clustering of mitochondria than CFZ. In conclusion, mitochondrial toxicity and autophagic upregulation with BTZ may be the reason for more severe neurotoxic profile than CFZ.
1. Introduction
The ubiquitin proteasomal system and autophagic pathway are two main protein degradation systems in eukaryotic cells. The proteasomal system is major pathway for degradation of damaged, misfolded unnecessary and short-lived proteins whereas, autophagy is responsible for the degradation of long-lived or aggregated proteins and cellular organelles. Therefore, both pathways play critical role in the maintenance of cellular homeostasis []. The 26S proteasome is a multi-subunit protein degradation machinery, which consists of two 19S regulatory complexes and a 20S catalytic core. The three catalytic sites in the 20S proteasome are the β5, β1, and β2 subunits. Inhibition of catalytic sites of 20S proteasome has been identified as a powerful strategy for cancer therapy []. Bortezomib (BTZ) is a selective proteasome inhibitor, which approved by Food and Drug Administration (FDA) for treating multiple myeloma and mantle cell lymphoma. However, the development of BTZ induced painful peripheral neuropathy limits treatment. About 33–66% of the patients who receive BTZ will experience peripheral neurological complications that can cause abandonment of proper treatment. Second-generation proteasome inhibitor carfilzomib (CFZ) approved by FDA for patients whose myeloma has relapsed or became resistant to another treatment. CFZ appears to have better neurotoxic profile than BTZ with a lower incidence of peripheral neuropathy in patients. However, mechanisms underlying this difference is unclear []. In this study, we aimed to investigate the underlying molecular neurotoxicity mechanisms of BTZ and CFZ on human neuronal cells.
2. Materials and Methods
ReNcell CX human neuronal precursor cells (Millipore, Baltimore, MD, USA) were grown with DMEM:F12 medium supplemented with B27 neural cell supplement, 20 ng/mL EGF, 20 ng/mL β-FGF and 10 Units/mL heparin in laminin coated flasks. Neuronal differentiation was initiated by changing the medium to that without growth factors and cells were maintained for 10 days (All from Gibco, Waltham, MA, USA). Then, neuronal cells were treated with 100 nM BTZ or CFZ (ApexBio, Houston, TX, USA) along with controls for 3 hours (h) and 24 h. Cell viability was evaluated with WST-1 assay (Roche, Mannheim, Germany). Proteasome activity was assayed with flourometric proteasome activity assay. Mitochondrial mass was measured with Mitotracker Green FM (Invitrogen, Carlsbad, CA, USA) and autophagy receptor protein p62 (Millipore, Baltimore, MD, USA) level was evaluated by FACSCalibur flow cytometry system (BD, Franklin Lakes, NJ, USA). In addition, mitochondrial localisation within cells was visualised with Mitotracker Green FM by confocal microscopy (Zeiss, Jena, Germany). Statistical analysis was carried out using SPSS 21 (IBM, New York, NY, USA). Results were analysed by one-way ANOVA followed by Tukey’s post hoc test. A p value < 0.05 was considered statistically significant.
3. Results
According to WST-1 assay results, 100 nM BTZ and CFZ exposure for 24 h did not change cell viability in human neuronal cells (Figure 1).
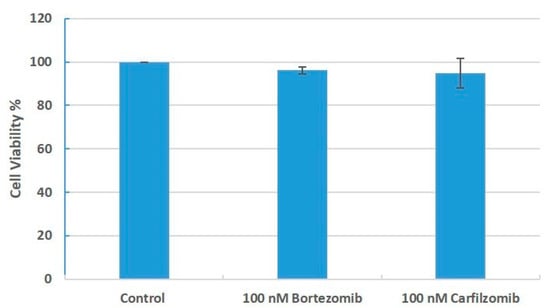
Figure 1.
Percentage viability of human neuronal cells after 24 h of BTZ and CFZ treatments with WST-1 assay. Values represent means ± SD, n = 3.
We have determined 26S and 20S proteasome activities. BTZ and CFZ treatments caused similar and significant inhibition of 26S and 20S proteasome activities for both endpoints (Figure 2).
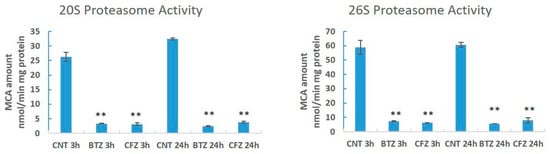
Figure 2.
Chymotrypsin like proteasome activities in neuronal cells after BTZ and CFZ treatments. Values represent means ± SD, n = 3. ** p < 0.01 (CNT: Control).
BTZ exposure for 24 h caused 10-fold increase in autophagy receptor protein p62 level and CFZ did not cause significant increase (Figure 3). Mitochondrial mass per cell was significantly reduced after 24 h BTZ and CFZ treatments (Figure 4). Confocal microscopy results showed that BTZ treatment for 24 h caused more perinuclear clustering of mitochondria than CFZ (Figure 5).
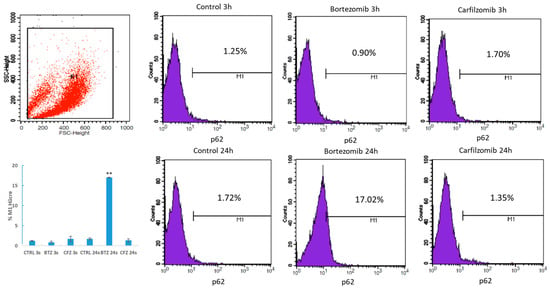
Figure 3.
Flow cytometry analysis of p62 protein level in neuronal cells after BTZ and CFZ treatments. Values represent means ± SD, n = 3. ** p < 0.01 (CTRL: Control).
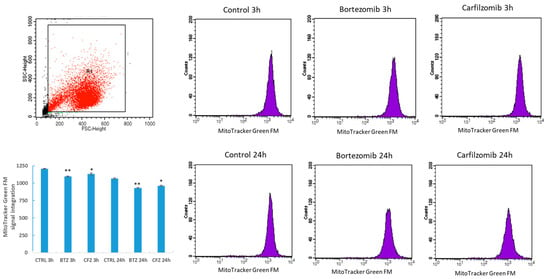
Figure 4.
Flow cytometry analysis of MitoTracker Green FM mitochondrial staining in neuronal cells after BTZ and CFZ treatments. Values represent means ± SD, n = 3. * p < 0.05, ** p < 0.01 (CNTRL: Control).
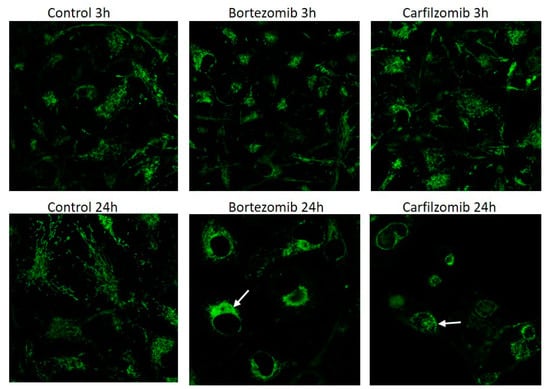
Figure 5.
Confocal images of MitoTracker Green FM mitochondrial staining in live neuronal cells after BTZ and CFZ treatments. The white arrows indicate clusters of mitochondria.
4. Discussion
Two main protein degradation systems, the ubiquitin proteasomal system and autophagy cooperate with each other to maintain proteostasis. This crosstalk is considered to help compensating the proteotoxic stress in eukaryotic cells []. Studies showed that proteasomal inhibition with BTZ activates autophagy in cancer cells []. In our study, proteasome inhibition with BTZ increased autophagy receptor protein p62 levels. However, proteasome inhibition with CFZ did not cause significant change in human neuronal cells.
Mitochondria are the cellular powerhouses and they are critical for cell function and survival. Mitochondrial impairment could be “off-target” effect of drugs and contribute organ toxicities []. Mitochondria constantly change shape size and move inside the cells. Disruption of these dynamics can affect the function of the mitochondria []. After BTZ treatment, a significant reduction in mitochondria mass was observed which may account for mitochondrial toxicity in neuronal cells. Okatsu et al. demonstrated that p62 is required for perinuclear clustering of mitochondria []. BTZ treatment for 24 h caused more perinuclear clustering of mitochondria than CFZ in neuronal cells. Mitochondrial clustering may be depended on p62 upregulation with BTZ treatment.
Thus, our data suggest that different mitochondrial toxic profile of BTZ and CFZ may contribute to the different neurotoxic effects of these drugs.
Acknowledgments
This study supported by The Scientific and Technological Research Council of Turkey—TUBITAK (216S838) and Istanbul University Research Fund (TDK-2017-24221 and TSA-2017-25380).
References
- Ji, C.H.; Kwon, Y.T. Crosstalk and interplay between the ubiquitin-proteasome system and autophagy. Mol. Cells 2017, 40, 441. [Google Scholar] [PubMed]
- Crawford, L.J.; Walker, B.; Irvine, A.E. Proteasome inhibitors in cancer therapy. J. Cell Commun. Signal. 2011, 5, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.S.; Torcun, C.C.; Grune, T.; Ozer, N.K.; Karademir, B. Proteasome inhibitors in cancer therapy: Treatment regimen and peripheral neuropathy as a side effect. Free Radic. Biol. Med. 2017, 103, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lilienbaum, A. Relationship between the proteasomal system and autophagy. Int. J. Biochem. Mol. Biol. 2013, 4, 1–26. [Google Scholar] [PubMed]
- Kao, C.; Chao, A.; Tsai, C.; Chuang, W.; Huang, W.; Chen, G.; Lin, C.; Wang, T.; Wang, H.; Lai, C.; et al. Bortezomib enhances cancer cell death by blocking the autophagic flux through stimulating ERK phosphorylation. Cell Death Dis. 2014, 5, e1510. [Google Scholar] [CrossRef] [PubMed]
- Dykens, J.A.; Will, Y. The significance of mitochondrial toxicity testing in drug development. Drug Discov. Today 2007, 12, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C. Mitochondrial fusion and fission in mammals. Annu. Rev. Cell Dev. Biol. 2006, 22, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Okatsu, K.; Saisho, K.; Shimanuki, M.; Nakada, K.; Shitara, H.; Sou, Y.S.; Kimura, M.; Sato, S.; Hattori, N.; Komatsu, M. p62/SQSTM1 cooperates with Parkin for perinuclear clustering of depolarized mitochondria. Genes Cells 2010, 15, 887–900. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).