OGG1 Does not Interact with NKX3.1 and AR to Repair 8-OHdG Base Damage in LNCaP Cells †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Immunoprecipitation
2.3. GST-Pull-Down
3. Results
3.1. Interaction of OGG1 with NKX3.1 and AR Has Been Confirmed by Native Immunoprecipitation
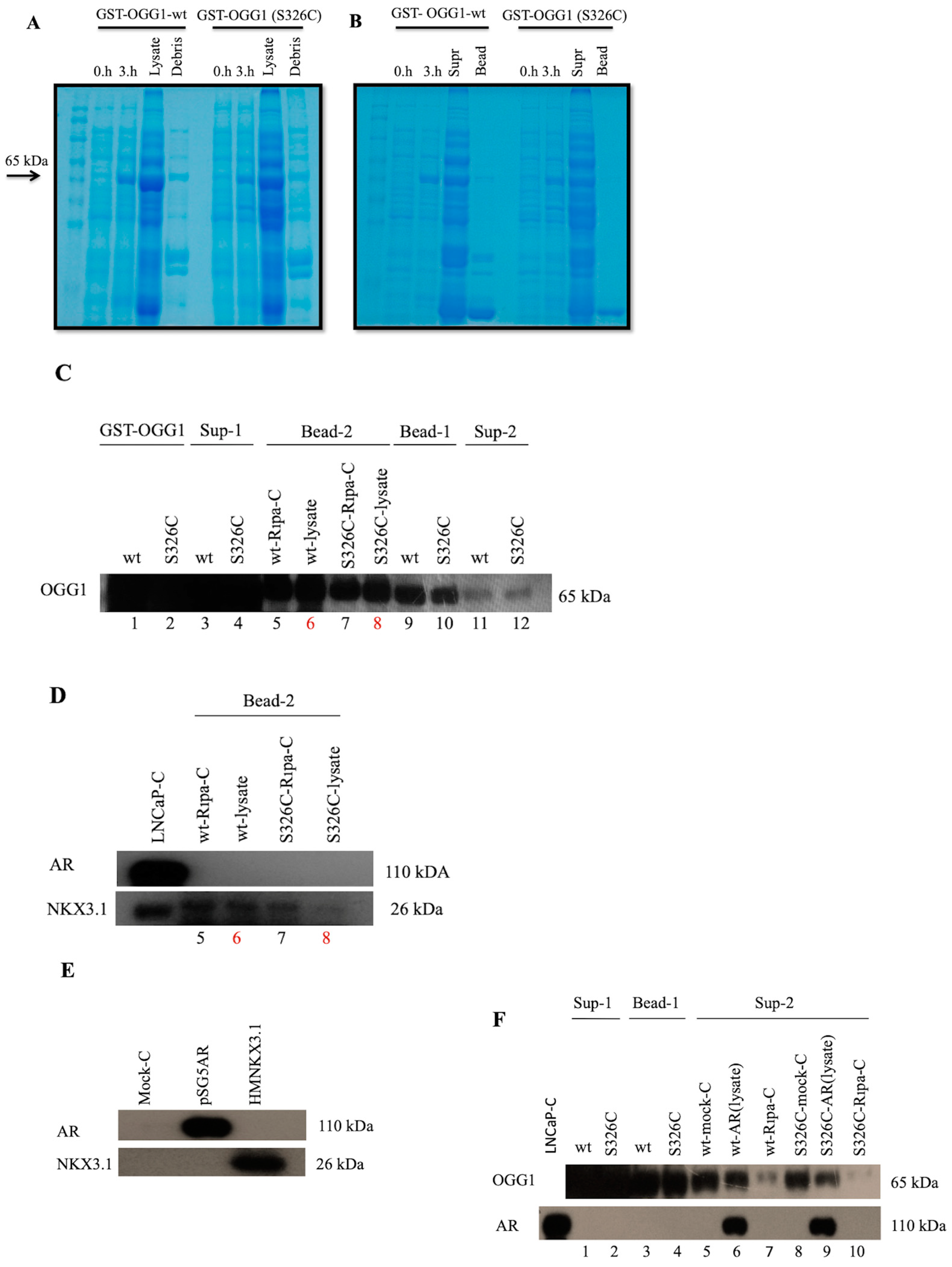
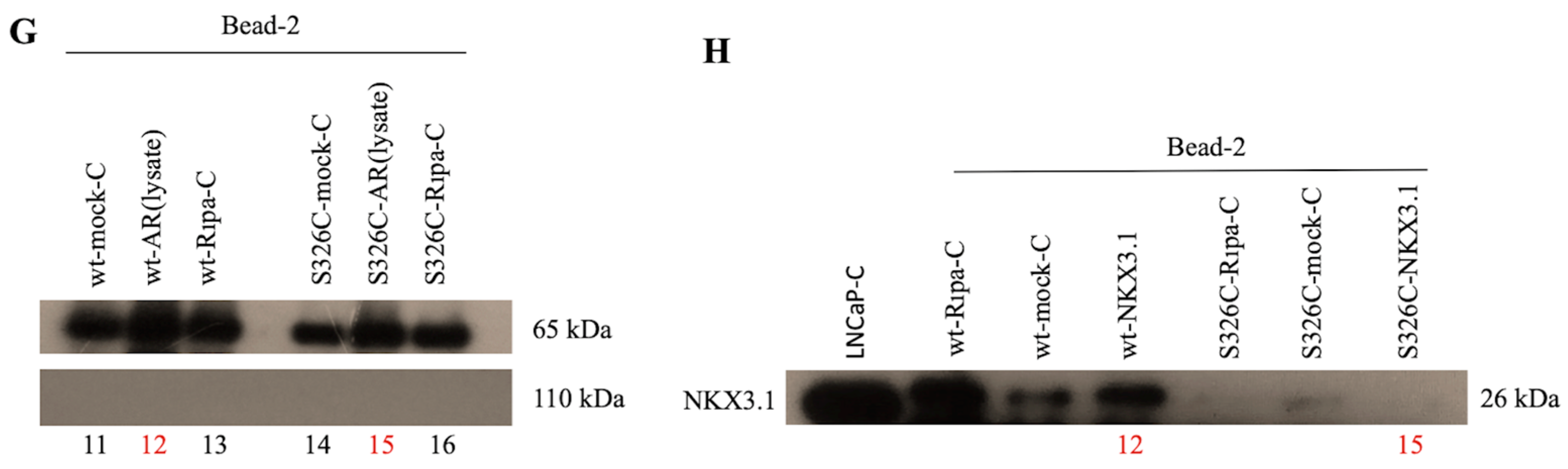
3.2. Interaction of OGG1 and S326C Its Polymorphic Variant with NKX3.1 and AR Has not Been Observed by GST-Pull Down Assay
4. Discussion
Acknowledgments
References
- Boiteux, S.; Radicella, J.P. The Human OGG1 Gene: Structure, Functions, and Its Implication in the Process of Carcinogenesis. Arch. Biochem. Biophys. 2000, 377, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Luna, L.; Rolseth, V.; Hilgrestrand, G.A.; Otteriei, M.; Dantzer, F.; Bjøra, M.; Seeberg, E. Dynamic relocalization of hOGG1 during the cell cycle is disrupted in cells harbouring the hOGG1-Cys326 polymorphic variant. Nucleic Acids Res. 2005, 33, 1813–1824. [Google Scholar] [CrossRef] [PubMed]
- Bowen, C.; Zheng, T.; Gelmann, E.P. NKX3.1 Suppresses TMPRSS2–ERG Gene Rearrangement and Mediates Repair of Androgen Receptor Induced DNA Damage. Can. Res. 2015, 75, 2686–2698. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.M.; Goode, E.L.; Ladiges, W.C.; Ulrich, C.M. Polymorphic Variation in hOGG1 and Risk of Cancer: A Review of the Functional and Epidemiologic Literature. Mol. Carcinog. 2005, 42, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Debelec-Butuner, B.; Alapinar, C.; Varişli, L.; Erbaykent-Tepedelen, B.; Muhammad Hamid, S.; Gonen-Korkmaz, C.; Korkmaz, K. Inflammation-mediated abrogation of androgen signaling: An in vitro model of prostate cell inflammation. Mol. Carcinog. 2014, 53, 85–97. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
İşel, E.; Bütüner, B.D.; Korkmaz, K.S. OGG1 Does not Interact with NKX3.1 and AR to Repair 8-OHdG Base Damage in LNCaP Cells. Proceedings 2018, 2, 1578. https://doi.org/10.3390/proceedings2251578
İşel E, Bütüner BD, Korkmaz KS. OGG1 Does not Interact with NKX3.1 and AR to Repair 8-OHdG Base Damage in LNCaP Cells. Proceedings. 2018; 2(25):1578. https://doi.org/10.3390/proceedings2251578
Chicago/Turabian Styleİşel, Elif, Bilge Debeleç Bütüner, and Kemal Sami Korkmaz. 2018. "OGG1 Does not Interact with NKX3.1 and AR to Repair 8-OHdG Base Damage in LNCaP Cells" Proceedings 2, no. 25: 1578. https://doi.org/10.3390/proceedings2251578
APA Styleİşel, E., Bütüner, B. D., & Korkmaz, K. S. (2018). OGG1 Does not Interact with NKX3.1 and AR to Repair 8-OHdG Base Damage in LNCaP Cells. Proceedings, 2(25), 1578. https://doi.org/10.3390/proceedings2251578



