Triclosan Detection in Aqueous Environmental Matrices by Thin-Films Sensors †
Abstract
:1. Introduction
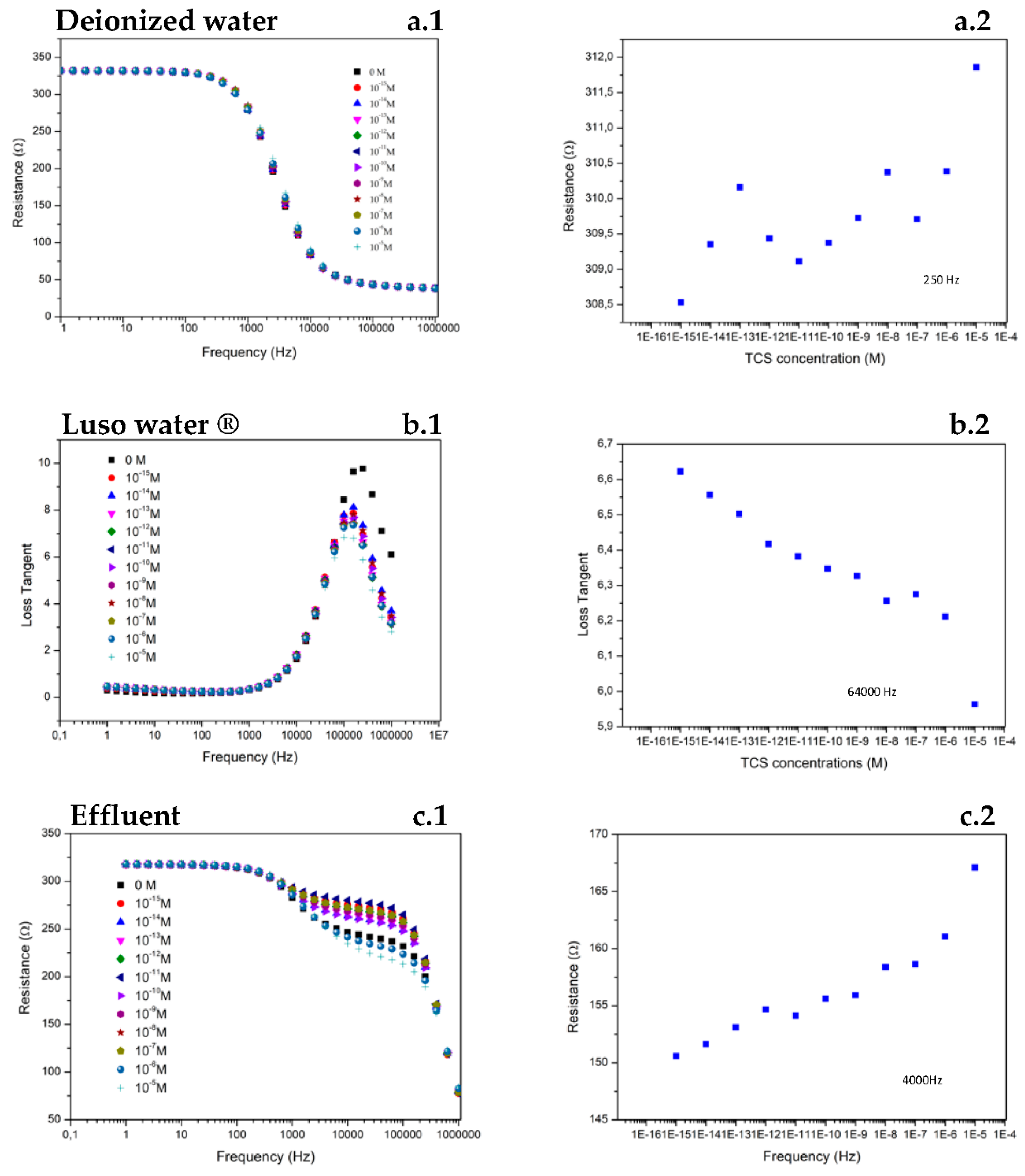
2. Results
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef] [PubMed]
- Chalew, E.A.; Halden, R.U. Environmental exposure of aquatic and terrestrial biota to triclosan and triclocarban. J. Am. Water Resour. Assoc. 2009, 45, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Stingley, R.L.; Beland, F.A.; Harrouk, W.; Lumpkins, D.L.; Howard, P. Occurrence, Efficacy, Metabolism, and Toxicity of Triclosan. J. Environ. Sci. Health Part C 2010, 28, 147–171. [Google Scholar] [CrossRef] [PubMed]
- Sabaliunas, D.; Webb, S.F.; Hauk, A.; Jacob, M.; Eckhoff, W.S. Environmental fate of Triclosan in the River Aire Basin, UK. Water Res. 2003, 37, 3145–3154. [Google Scholar] [CrossRef] [PubMed]
- Skarha, J.; Mínguez-alarcón, L.; Williams, P.L.; Korevaar, T.I.M.; de Poortere, R.A.; Broeren, M.A.C.; Ford, J.B.; Eliot, M.; Hauser, R.; Braun, J.M. Cross-sectional associations between urinary triclosan and serum thyroid function biomarker concentrations in women. Environ. Int. 2019, 122, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wan, Y.; Wu, M.; Xu, Y.; Xu, Q.; He, Z.; Xia, W. Occurrence of benzophenones, parabens and triclosan in the Yangtze River of China, and the implications for human exposure. Chemosphere 2018, 213, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Juric, A.; Singh, K.; Hu, X.F.; Chan, H.M. Exposure to triclosan among the Canadian population: Results of the Canadian Health Measures Survey (2009–2013). Environ. Int. 2019, 123, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Lehutso, R.F.; Daso, A.P.; Okonkwo, J.O. Occurrence and environmental levels of triclosan and triclocarban in selected wastewater treatment plants in Gauteng Province, South Africa. Emerg. Contam. 2017, 3, 107–114. [Google Scholar] [CrossRef]
- Wu, T.; Li, T.; Liu, Z.; Guo, Y.; Dong, C. Talanta Electrochemical sensor for sensitive detection of triclosan based on graphene / palladium nanoparticles hybrids. Talanta 2017, 164, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, Q.J.; Wang, L. Development and characterization of an amperometric sensor for triclosan detection based on electropolymerized molecularly imprinted polymer. Microchem. J. 2009, 91, 222–226. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, M.; Ling, Y.; Xu, J.; Hu, S.; Hayat, T.; Alharbi, N.S.; Yang, F. Fabrication of one dimensional CNTs/Fe3O4@PPy/Pd magnetic composites for the accumulation and electrochemical detection of triclosan. J. Electroanal. Chem. 2018, 818, 97–105. [Google Scholar] [CrossRef]
- Magro, C.; Mateus, E.; Raposo, M.; Ribeiro, A.B. Overview of electronic tongue sensing in environmental aqueous matrices: Potential for monitoring emerging organic contaminants. Environ. Rev. 2018, 27, 202–214. [Google Scholar] [CrossRef]
- Marques, I.; Magalhâes-Mota, G.; Pires, F.; Sério, S.; Ribeiro, P.A.; Raposo, M. Detection of traces of triclosan in water. Appl. Surf. Sci. 2017, 421, 142–147. [Google Scholar] [CrossRef]
- Abegão, L.; Ribeiro, J.; Ribeiro, P.; Raposo, M. Nano-Molar Deltamethrin Sensor Based on Electrical Impedance of PAH/PAZO Layer-by-Layer Sensing Films. Sensors 2013, 13, 10167–10176. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.P.; Zagalo, P.M.; Magalhões-Mota, G.; Ribeiro, P.A.; Raposo, M. Adsorption of Triclosan on sensors based on PAH/PAZO thin-films: The effect of pH. In Proceedings of 15th International Conference on Computational Intelligence methods for Bioinformatics and Biostatistics, Caparica, Portugal, 6–8 September 2018; pp. 1–48. [Google Scholar]
- Ferreira, Q.; Gomes, P.J.; Ribeiro, P.A.; Jones, N.C.; Hoffmann, S.V.; Mason, N.J.; Oliveira, O.N., Jr.; Raposo, M. Determination of Degree of Ionization of Poly(allylamine hydrochloride) (PAH) and Poly[1-[4-(3-carboxy-4 hydroxyphenylazo)benzene sulfonamido]-1,2-ethanediyl, sodium salt] (PAZO) in Layer-by-Layer Films using Vacuum Photoabsorption Spectroscopy. Langmuir 2013, 29, 448–455. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magro, C.C.; Zagalo, P.M.; Pereira-da-Silva, J.; Mateus, E.P.; Ribeiro, A.B.; Ribeiro, P.A.; Raposo, M.F. Triclosan Detection in Aqueous Environmental Matrices by Thin-Films Sensors. Proceedings 2019, 15, 24. https://doi.org/10.3390/proceedings2019015024
Magro CC, Zagalo PM, Pereira-da-Silva J, Mateus EP, Ribeiro AB, Ribeiro PA, Raposo MF. Triclosan Detection in Aqueous Environmental Matrices by Thin-Films Sensors. Proceedings. 2019; 15(1):24. https://doi.org/10.3390/proceedings2019015024
Chicago/Turabian StyleMagro, Cátia Costa, Paulo Morgado Zagalo, João Pereira-da-Silva, Eduardo Pires Mateus, Alexandra Branco Ribeiro, Paulo António Ribeiro, and Maria Fátima Raposo. 2019. "Triclosan Detection in Aqueous Environmental Matrices by Thin-Films Sensors" Proceedings 15, no. 1: 24. https://doi.org/10.3390/proceedings2019015024
APA StyleMagro, C. C., Zagalo, P. M., Pereira-da-Silva, J., Mateus, E. P., Ribeiro, A. B., Ribeiro, P. A., & Raposo, M. F. (2019). Triclosan Detection in Aqueous Environmental Matrices by Thin-Films Sensors. Proceedings, 15(1), 24. https://doi.org/10.3390/proceedings2019015024








