Advanced Electrochemical Molecularly Imprinted Polymer as Sensor Interfaces †
Abstract
:1. Introduction
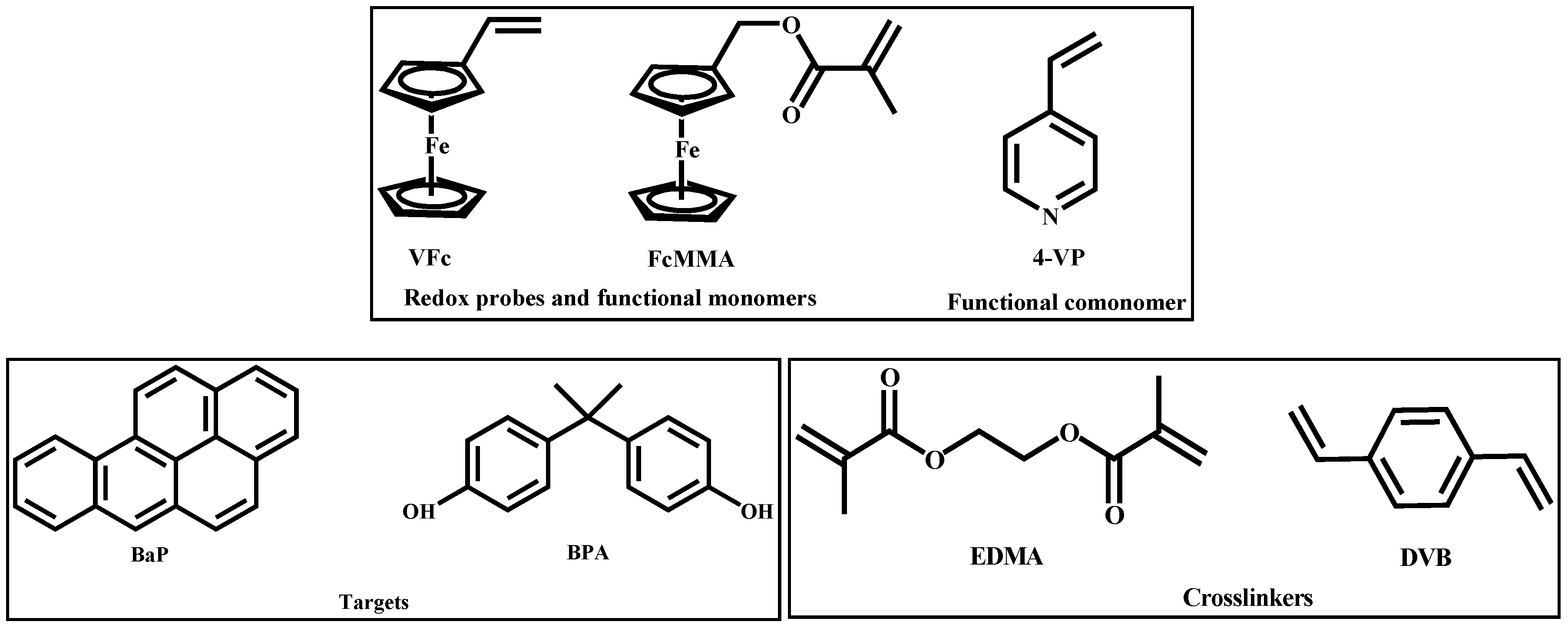
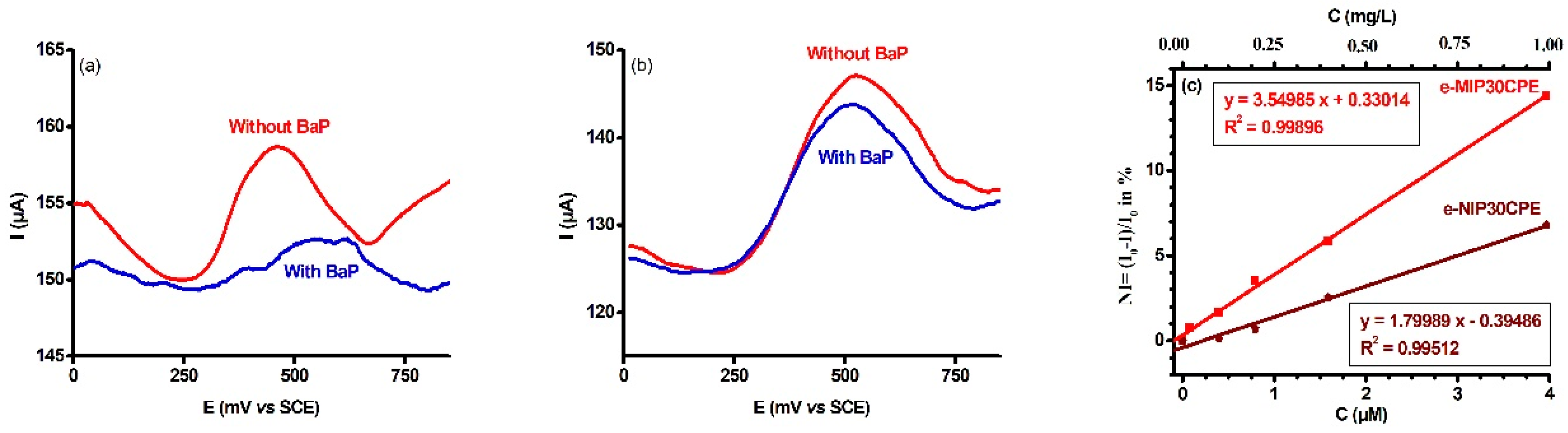
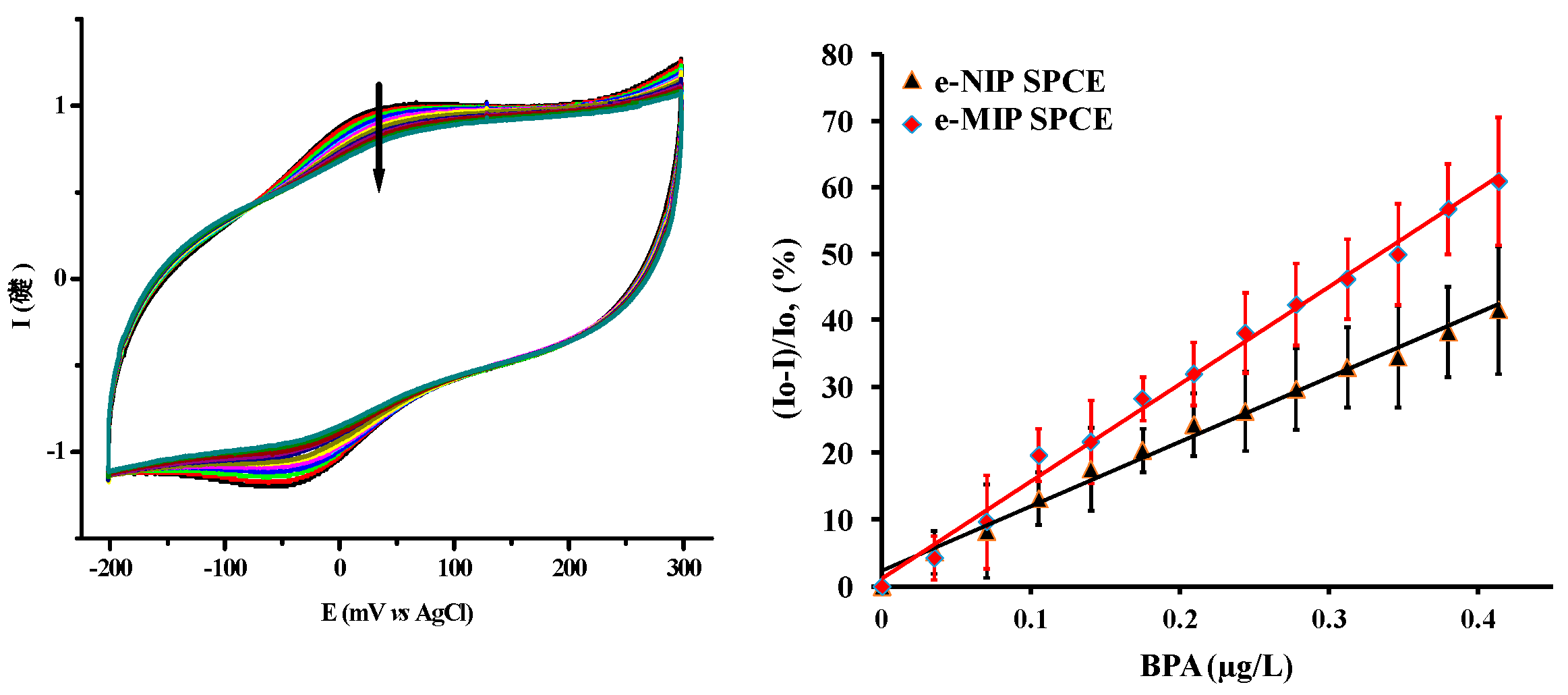
2. Results
3. Conclusions
References
- Haupt, K.; Mosbach, K. Molecularly Imprinted Polymers and Their Use in Biomimetic Sensors. Chem. Rev. 2000, 100, 100–2495. [Google Scholar] [CrossRef] [PubMed]
- Branger, C.; Brisset, H.; Udomsap, D. French patent FR 1262617; PCT/IB2013/ 061196. Available online: https://bases-brevets.inpi.fr/fr/document/FR3000076.html?p=6&s=1562916171252&cHash=16bb2dfb8f273a8af29456c2a11c412e; https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2014097248 (accessed on 26 June 2014).
- Udomsap, D.; Branger, C.; Culioli, G.; Dollet, P.; Brisset, H. A versatile electrochemical sensing receptor based on a molecularly imprinted polymer. Chem. Comm. 2014, 50, 50–7488. [Google Scholar] [CrossRef] [PubMed]
- Udomsap, D.; Brisset, H.; Culioli, G.; Dollet, P.; Laatikainen, K.; Siren, H.; Branger, C. Electrochemical molecularly imprinted polymers as material for pollutant detection. Mat. Today Comm. 2018, 17, 458–465. [Google Scholar] [CrossRef]
- Mba Ekomo, V.; Branger, C.; Bikanga, R.; Florea, A.-M.; Istamboulie, G.; Calas-Blanchard, C.; Noguer, T.; Sarbu, A.; Brisset, H. Detection of Bisphenol A in aqueous medium by screen printed carbon electrodes incorporating electrochemical molecularly imprinted polymers. Biosens. Bioelectron. 2018, 112, 158–161. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branger, C.; Brisset, H. Advanced Electrochemical Molecularly Imprinted Polymer as Sensor Interfaces. Proceedings 2019, 15, 22. https://doi.org/10.3390/proceedings2019015022
Branger C, Brisset H. Advanced Electrochemical Molecularly Imprinted Polymer as Sensor Interfaces. Proceedings. 2019; 15(1):22. https://doi.org/10.3390/proceedings2019015022
Chicago/Turabian StyleBranger, Catherine, and Hugues Brisset. 2019. "Advanced Electrochemical Molecularly Imprinted Polymer as Sensor Interfaces" Proceedings 15, no. 1: 22. https://doi.org/10.3390/proceedings2019015022
APA StyleBranger, C., & Brisset, H. (2019). Advanced Electrochemical Molecularly Imprinted Polymer as Sensor Interfaces. Proceedings, 15(1), 22. https://doi.org/10.3390/proceedings2019015022




