Topological Machine Learning for Raman Spectroscopy: Perspectives for Pancreatic Diseases †
Abstract
1. Introduction
- Imaging (CT/MRI): Conventional imaging methods have limited sensitivity for early chronic pancreatitis and small pancreatic ductal adenocarcinoma, as current standards lack universal criteria to detect early parenchymal changes and rely heavily on ductal abnormalities captures by the Cambridge Classification, which misses subtle early-stage features [2];
- Biomarkers (CA 19-9): Despite its widespread clinical use, CA 19-9 exhibits stage-dependent sensitivity in dag, with pooled sensitivity of in all-stage analysis, but only 48% for localized T1 tumors, while specificity is compromised by false positives in 15–20% of benign biliary obstructions and undetectable in 5–10% of Lewis antigen-negative individuals [3];
- Histopathology (EUS-FNA): Despite being the gold standard, EUS-guided FNA histopathology is inherently invasive, carrying risks of pancreatitis (1–2%) and bleeding, while exhibiting a 10–20% non-diagnostic rate due to insufficient cellularity, sampling error, or obscuring blood—with accuracy further compromised in early-stage lesions (<1 cm) or fibrotic pancreatitis [4].
2. Materials and Methods
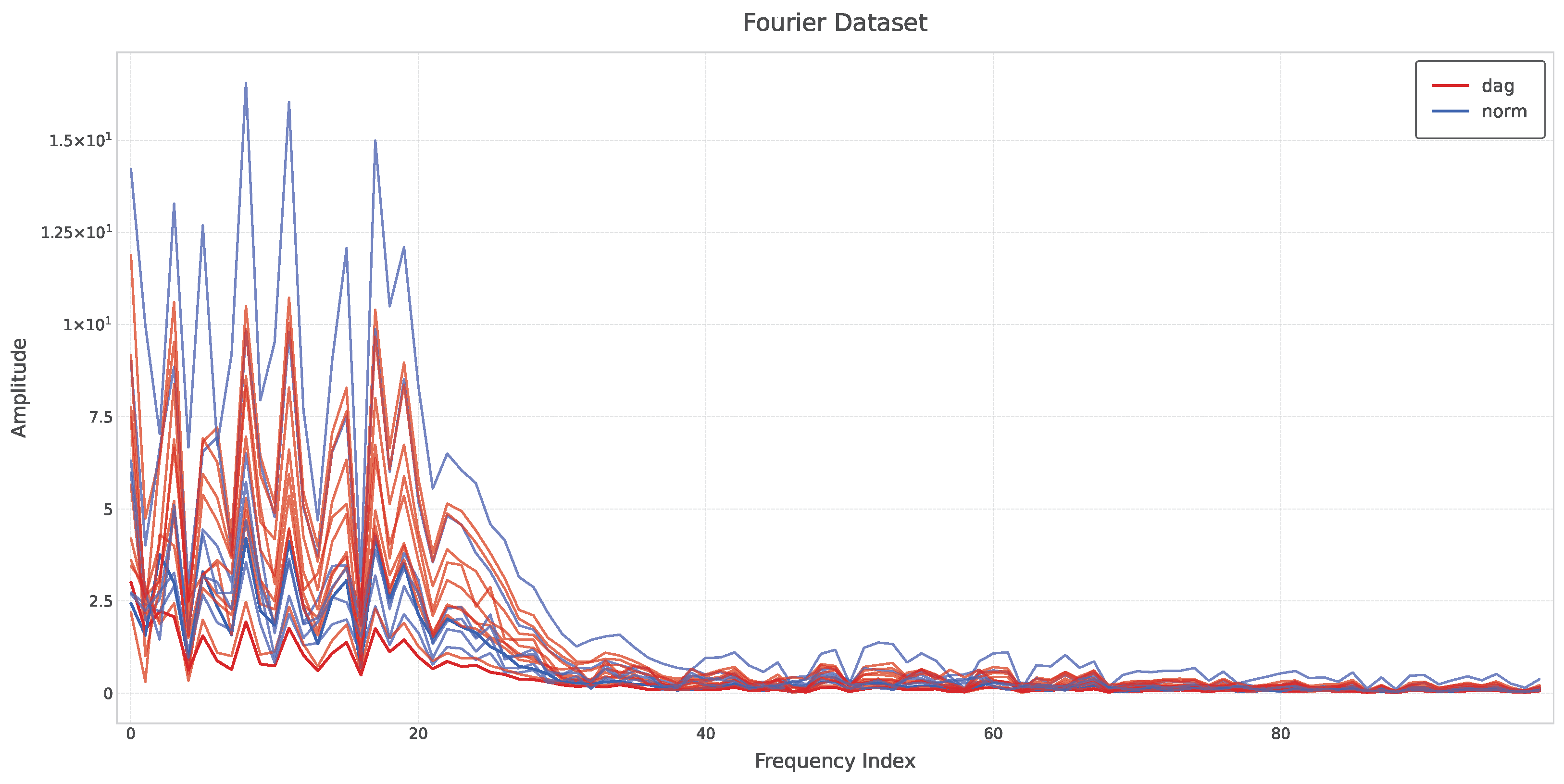
3. Results
CNN Implementation Details
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Tirkes, T.; Shah, Z.K.; Takahashi, N.; Grajo, J.R.; Chang, S.T.; Venkatesh, S.K.; Conwell, D.L.; Fogel, E.L.; Park, W.; Topazian, M.; et al. Reporting standards for chronic pancreatitis by using CT, MRI, and MR cholangiopancreatography: The consortium for the study of chronic pancreatitis, diabetes, and pancreatic cancer. Radiology 2019, 290, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Poruk, K.E.; Gay, D.Z.; Brown, K.; Mulvihill, J.D.; Boucher, K.M.; Scaife, C.L.; Firpo, M.A.; Mulvihill, S.J. The clinical utility of CA 19-9 in pancreatic adenocarcinoma: Diagnostic and prognostic updates. Curr. Mol. Med. 2013, 13, 340–351. [Google Scholar] [PubMed]
- Puli, S.R.; Bechtold, M.L.; Buxbaum, J.L.; Eloubeidi, M.A. How good is endoscopic ultrasound–guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass? A meta-analysis and systematic review. Pancreas 2013, 42, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, K.; Stiebing, C.; Matthäus, C.; Schmitt, M.; Popp, J. Advantages and limitations of Raman spectroscopy for molecular diagnostics: An update. Expert Rev. Mol. Diagn. 2015, 15, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Auner, G.W.; Koya, S.K.; Huang, C.; Broadbent, B.; Trexler, M.; Auner, Z.; Elias, A.; Mehne, K.C.; Brusatori, M.A. Applications of Raman spectroscopy in cancer diagnosis. Cancer Metastasis Rev. 2018, 37, 691–717. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Zhang, S.; Yue, S. Raman spectroscopy and imaging for cancer diagnosis. J. Healthc. Eng. 2018, 2018, 8619342. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; D’Acunto, M.; Caudai, C.; Colantonio, S.; Gaeta, R.; Moroni, D.; Pascali, M.A. Raman spectroscopy and topological machine learning for cancer grading. Sci. Rep. 2023, 13, 7282. [Google Scholar] [CrossRef] [PubMed]
- Lazzini, G.; Gaeta, R.; Pollina, L.E.; Comandatore, A.; Furbetta, N.; Morelli, L.; D’Acunto, M. Raman spectroscopy based diagnosis of pancreatic ductal adenocarcinoma. Sci. Rep. 2025, 15, 13240. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; Banchelli, M.; Bessi, V.; Cecchi, C.; Chiti, F.; Colantonio, S.; D’Andrea, C.; de Angelis, M.; Moroni, D.; Nacmias, B.; et al. Harnessing topological machine learning in Raman spectroscopy: Perspectives for Alzheimer’s disease detection via cerebrospinal fluid analysis. J. Frankl. Inst. 2024, 361, 107249. [Google Scholar] [CrossRef]
- Conti, F.; Moroni, D.; Pascali, M.A. A topological machine learning pipeline for classification. Mathematics 2022, 10, 3086. [Google Scholar] [CrossRef]
- Bubenik, P. Statistical topological data analysis using persistence landscapes. J. Mach. Learn. Res. 2015, 16, 77–102. [Google Scholar]
- Liu, J.; Osadchy, M.; Ashton, L.; Foster, M.; Solomon, C.J.; Gibson, S.J. Deep convolutional neural networks for Raman spectrum recognition: A unified solution. Analyst 2017, 142, 4067–4074. [Google Scholar] [CrossRef] [PubMed]
| Predicted: | DAG | No DAG |
|---|---|---|
| True DAG | 9 | 1 |
| True No DAG | 1 | 6 |
| Predicted: | DAG | PC | NORM |
|---|---|---|---|
| True DAG | 9 | 0 | 1 |
| True PC | 0 | 3 | 0 |
| True NORM | 1 | 1 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conti, F.; Lazzini, G.; Gaeta, R.; Pollina, L.E.; Comandatore, A.; Furbetta, N.; Morelli, L.; D’Acunto, M.; Moroni, D.; Pascali, M.A. Topological Machine Learning for Raman Spectroscopy: Perspectives for Pancreatic Diseases. Proceedings 2025, 129, 61. https://doi.org/10.3390/proceedings2025129061
Conti F, Lazzini G, Gaeta R, Pollina LE, Comandatore A, Furbetta N, Morelli L, D’Acunto M, Moroni D, Pascali MA. Topological Machine Learning for Raman Spectroscopy: Perspectives for Pancreatic Diseases. Proceedings. 2025; 129(1):61. https://doi.org/10.3390/proceedings2025129061
Chicago/Turabian StyleConti, Francesco, Gianmarco Lazzini, Raffaele Gaeta, Luca Emanuele Pollina, Annalisa Comandatore, Niccolò Furbetta, Luca Morelli, Mario D’Acunto, Davide Moroni, and Maria Antonietta Pascali. 2025. "Topological Machine Learning for Raman Spectroscopy: Perspectives for Pancreatic Diseases" Proceedings 129, no. 1: 61. https://doi.org/10.3390/proceedings2025129061
APA StyleConti, F., Lazzini, G., Gaeta, R., Pollina, L. E., Comandatore, A., Furbetta, N., Morelli, L., D’Acunto, M., Moroni, D., & Pascali, M. A. (2025). Topological Machine Learning for Raman Spectroscopy: Perspectives for Pancreatic Diseases. Proceedings, 129(1), 61. https://doi.org/10.3390/proceedings2025129061









