Possibilities for Recovering Indium from Electronic Devices †
1. Introduction
2. Materials and Methods
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Indium Tin Oxide Market. Available online: https://www.prnewswire.com/news-releases/indium-tin-oxide-market-size-to-increase-by-usd-213-87-million-between-2022-to-2027--amalgamated-metal-corp-plc-american-elements-diamond-coatings-inc-and-more-among-key-companies-technavio-302089762.html (accessed on 25 June 2024).
- European Commission. Study on the EU’s List of Critical Raw Materials—Final Report; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Global Indium Market—Industry Trends and Forecast to 2030. Available online: https://www.databridgemarketresearch.com/reports/global-indium-market (accessed on 25 June 2024).
- Willner, J.; Fornalczyk, A.; Jablonska-Czapla, M.; Grygoyc, K.; Rachwal, M. Studies on the content of selected technology critical elements (Germanium, Tellurium and Thallium) in electronic waste. Materials 2021, 14, 3722. [Google Scholar] [CrossRef] [PubMed]
- Willner, J.; Fornalczyk, A.; Saternus, M.; Sedlakova-Kadukova, J.; Gajda, B. LCD panels bioleaching with pure and mixed culture of Acidithiobacillus. Physicochem. Probl. Miner. Process. 2022, 58, 15–23. [Google Scholar] [CrossRef]
- Xu, L.; Chen, G.; Zhang, X.; Yang, Y.; Leng, C.; Yang, C.; Tian, Y.; Zhao, Z. Waste ITO target recycling for efficient indium recovery through a closed-loop process. J. Environ. Chem. Eng. 2024, 12, 112136. [Google Scholar] [CrossRef]
- Sverdrup, H.U.; Allen, O.; Haraldsson, H.V. Modeling indium extraction, supply, price, use and recycling 1930–2200 using the WORLD7 Model: Implication for the imaginaries of sustainable Europe 2050. Nat. Resour. Res. 2024, 33, 539–570. [Google Scholar] [CrossRef]
- Krzymińska, N. Possibilities of Indium Recovery from Waste Liquid Crystal Display Materials. Master’s Thesis, Silesian University of Technology, Faculty of Materials Engineering, Katowice, Poland, 2019. [Google Scholar]
- Wang, J.; Faraji, F.; Ghahreman, A. Evaluation of ozone as an efficient and sustainable reagent for chalcopyrite leaching: Process optimization and oxidative mechanism. J. Ind. Eng. Chem. 2021, 104, 333–334. [Google Scholar] [CrossRef]
- Fornalczyk, A.; Willner, J.; Gajda, B.; Sedlakova-Kadukova, J. Influence of H2O2 and O3 on PGM extraction from used car catalysts. Arch. Metall. Mater. 2018, 63, 963–968. [Google Scholar] [CrossRef] [PubMed]
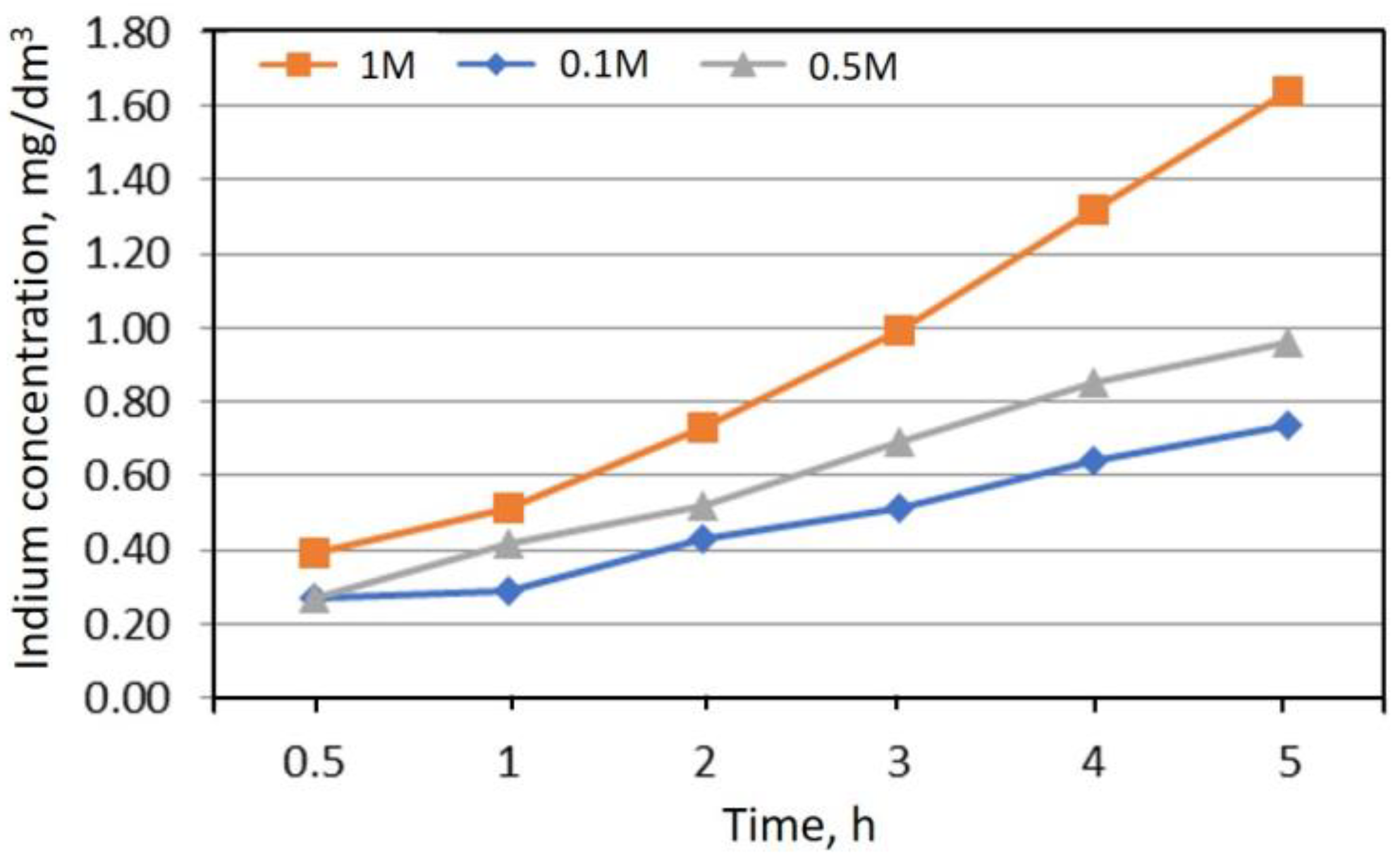
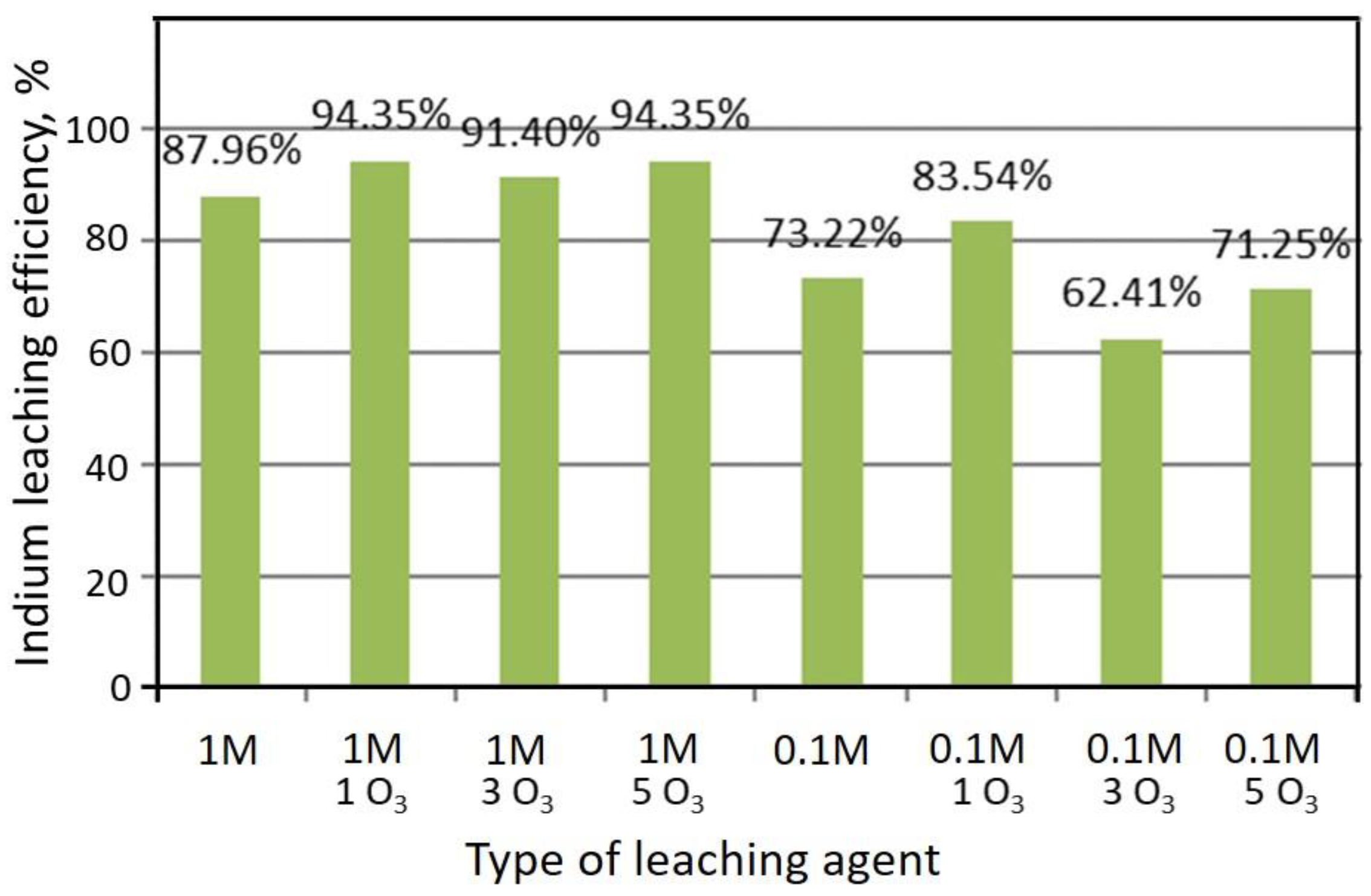
| Indium g/unit | Computer | Screen | Cellphone | LED TV | CdTe | CuInGaSe |
| 0.040 | 0.082 | 0.010 | 0.003 | 0.015 | 0.120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willner, J.; Krzymińska, N. Possibilities for Recovering Indium from Electronic Devices. Proceedings 2024, 108, 11. https://doi.org/10.3390/proceedings2024108011
Willner J, Krzymińska N. Possibilities for Recovering Indium from Electronic Devices. Proceedings. 2024; 108(1):11. https://doi.org/10.3390/proceedings2024108011
Chicago/Turabian StyleWillner, Joanna, and Natalia Krzymińska. 2024. "Possibilities for Recovering Indium from Electronic Devices" Proceedings 108, no. 1: 11. https://doi.org/10.3390/proceedings2024108011
APA StyleWillner, J., & Krzymińska, N. (2024). Possibilities for Recovering Indium from Electronic Devices. Proceedings, 108(1), 11. https://doi.org/10.3390/proceedings2024108011






