Improving Nitrogen Fertilization Recommendations in Temperate Agricultural Systems: A Study on Walloon Soils Using Anaerobic Incubation and POxC
Abstract
1. Introduction
2. Material and Methods
2.1. Study Site and Experimental Design
2.2. Soil Analyses
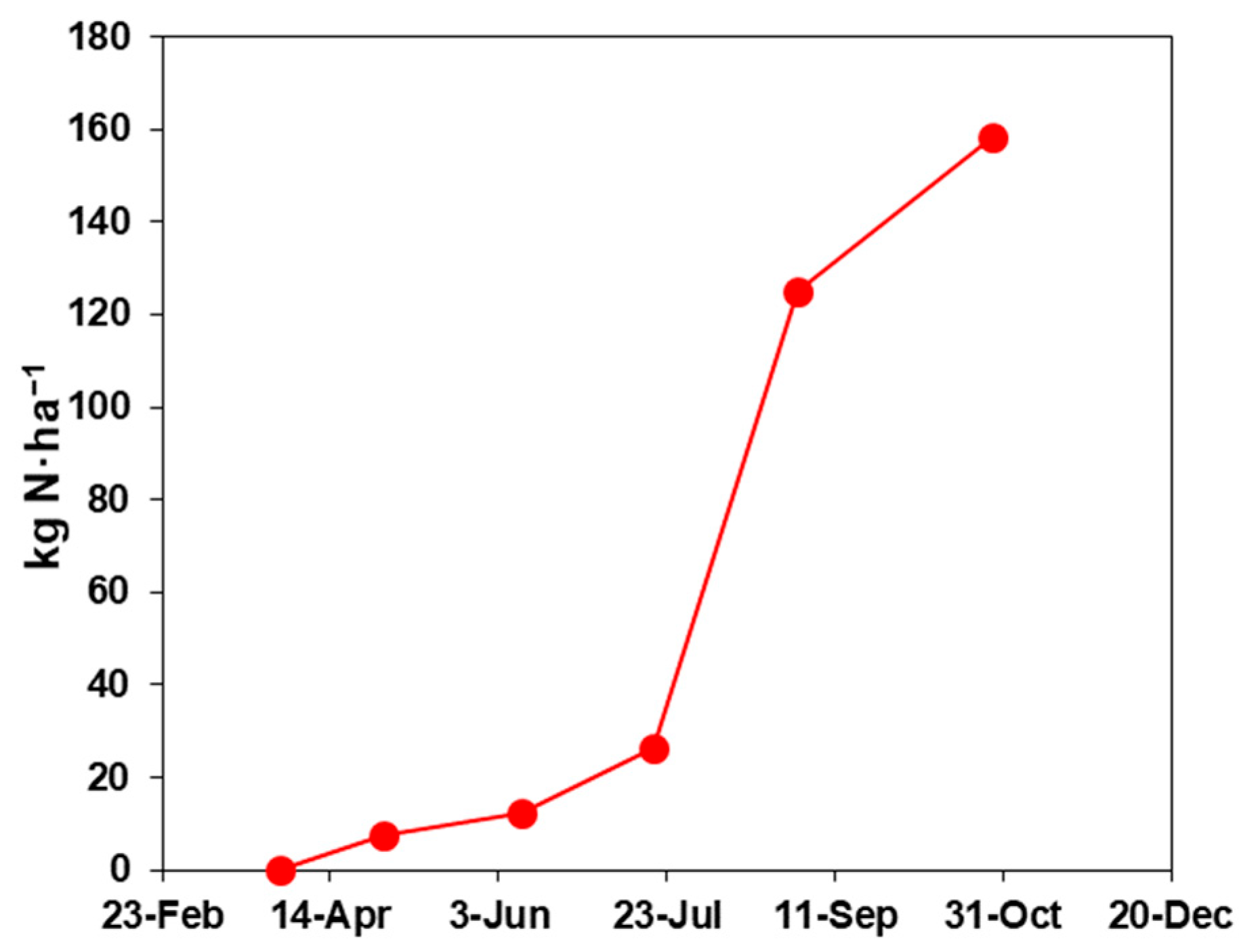
2.3. In Situ Nitrogen Mineralization
2.4. Statistical Analyses and Modeling Approach
3. Results
3.1. Soil Diversity
3.2. Factors Influencing Soil Nitrogen Mineralization
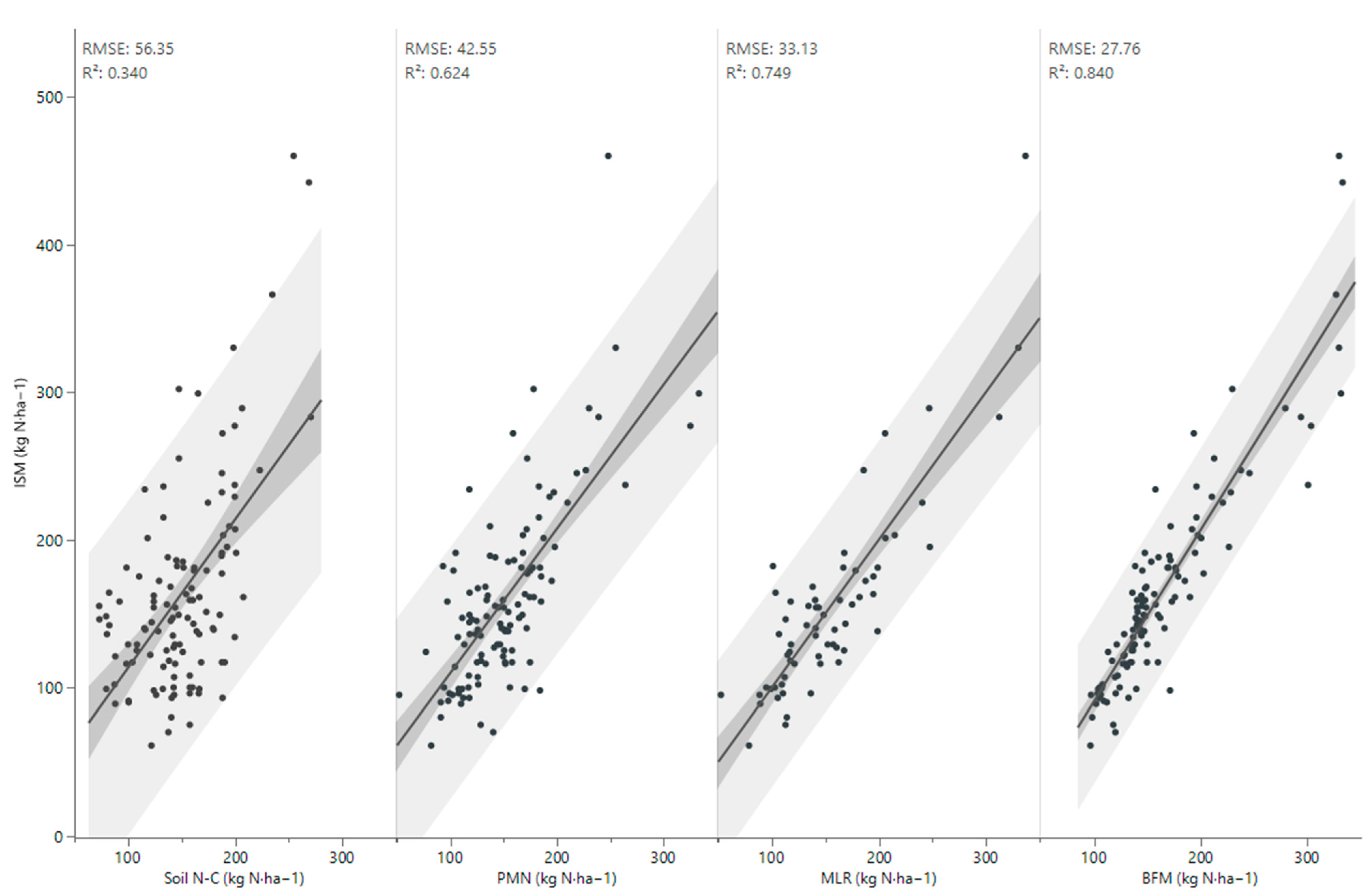
3.3. Improving the Prediction of Soil Nitrogen Mineralization
4. Discussion
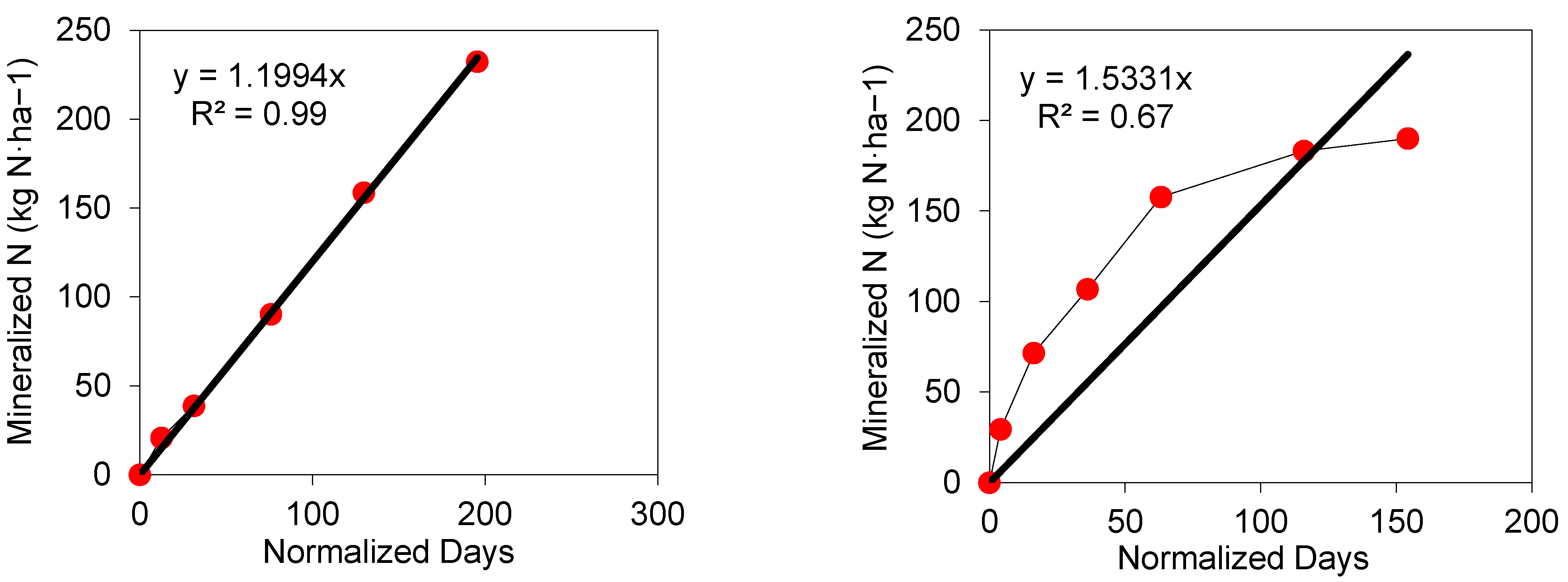
4.1. Time Normalization
4.2. Major Influencing Factors
4.3. Current and Further Improvement
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Recous, S.; Mary, B.; Faurie, G. Microbial immobilization of ammonium and nitrate in cultivated soils. Soil Biol. Biochem. 1995, 27, 365–373. [Google Scholar] [CrossRef]
- Cabrera, M.L.; Kissel, D.E.; Vigil, M.F. Nitrogen mineralization from organic residues: Research opportunities. J. Environ. Qual. 2005, 34, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Carreón, G.; Flores-Márgez, J.P.; Osuna-Avila, P.; Sanogo, S. Importance and inconsistencies of the influence of soil properties on nitrogen mineralization: A systematic review. Soil Health 2023, 1, 2. [Google Scholar] [CrossRef]
- Li, X.; Wang, A.; Huang, D.; Qian, H.; Luo, X.; Che, W.; Huang, Q. Patterns and drivers of soil net nitrogen mineralization and its temperature sensitivity across eastern China. Plant Soil 2023, 485, 475–488. [Google Scholar] [CrossRef]
- Keeney, D.R.; Bremner, J.M. Comparison and Evaluation of Laboratory Methods of Obtaining an Index of Soil Nitrogen Availability. Agron. J. 1966, 58, 498–503. [Google Scholar] [CrossRef]
- Stanford, G.; Smith, S.J. Nitrogen mineralization potentials of soils. Soil Sci. Soc. Am. J. 1972, 36, 465–472. [Google Scholar] [CrossRef]
- Curtin, D.; Campbell, C.A. Mineralizable nitrogen. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 599–606. [Google Scholar]
- Brisson, N.; Gary, C.; Justes, E.; Roche, R.; Mary, B.; Ripoche, D.; Zimmer, D.; Sierra, J.; Bertuzzi, P.; Burger, P.; et al. An overview of the crop model STICS. Eur. J. Agron. 2003, 18, 309–332. [Google Scholar] [CrossRef]
- Keating, B.A.; Carberry, P.C.; Hammer, G.L.; Probert, M.E.; Robertson, M.J.; Holzworth, D.; Huth, N.I.; Hargreaves, J.N.G.; Meinke, H.; Hochman, Z.; et al. An overview of APSIM, a model designed for farming systems simulation. Eur. J. Agron. 2003, 18, 267–288. [Google Scholar] [CrossRef]
- Ros, G.H.; Temminghoff, E.J.M.; Hoffland, E. Nitrogen mineralization: A review and meta-analysis of the predictive value of soil tests. Eur. J. Soil Sci. 2011, 62, 162–173. [Google Scholar] [CrossRef]
- Silva, L.; Conceição, L.A.; Lidon, F.C.; Patanita, M.; D’Antonio, P.; Fiorentino, C. Digitization of crop nitrogen modelling: A review. Agronomy 2023, 13, 1964. [Google Scholar] [CrossRef]
- Thompson, L.J.; Archontoulis, S.V.; Puntel, L.A. Simulating within-field spatial and temporal corn yield response to nitrogen with APSIM model. Precis. Agric. 2024, 25, 2421–2446. [Google Scholar] [CrossRef]
- Ruma, F.Y.; Munnaf, M.A.; De Neve, S.; Mouazen, A.M. Management zone-specific N mineralization rate estimation in unamended soil. Precis. Agric. 2023, 24, 1906–1931. [Google Scholar] [CrossRef]
- Santiago, S.T.; Clark, N.; Leinfelder-Miles, M.; Light, S.; Mathesius, K.; Wilson, R.; Parikh, S.; Savidge, M.; Geisseler, D. Nitrogen mineralization from soil organic matter under field conditions in California soils. Soil Sci. Soc. Am. J. 2025, 89, e70055. [Google Scholar] [CrossRef]
- Ruma, F.Y.; Munnaf, M.A.; De Neve, S.; Mouazen, A.M. Visible-to-near-infrared spectroscopy for prediction of soil nitrogen mineralization after sample stratification by textural homogeneity criteria. Soil Tillage Res. 2024, 244, 106250. [Google Scholar] [CrossRef]
- Yin, X.; Kersebaum, K.-C.; Beaudoin, N.; Constantin, J.; Chen, F.; Louarn, G.; Manevski, K.; Hoffmann, M.; Kollas, C.; Armas-Herrera, C.M.; et al. Uncertainties in simulating N uptake, net N mineralization, soil mineral N and N leaching in European crop rotations using process-based models. Field Crops Res. 2020, 255, 107863. [Google Scholar] [CrossRef]
- Abras, M.; Goffart, J.-P.; Detain, J.-P. Prospects for improving the provisional nitrogen fertilization recommendation at field scale in Wallonia using the AzoFert® software. Biotechnol. Agron. Soc. Environ. 2012, 16, 215–220. [Google Scholar]
- Cugnon, T.; Vilret, A.; Blondiau, L.M.; Colli, C.; Genot, V.; Lizin, P.; Renneson, M.; Lambert, R. Établissement du Conseil de Fumure Azotée en Cultures (Internal Belgian Laboratory Reference); REQUASUD: Gembloux, Belgium, 2021; 24p. [Google Scholar]
- Mariano, E.; Trivelin, P.C.O.; Leite, J.M.; Vieira-Megda, M.X.; Otto, R.; Franco, H.C.J. Incubation methods for assessing mineralizable nitrogen in soils under sugarcane. Rev. Bras. Cienc. Solo 2013, 37, 450–461. [Google Scholar] [CrossRef]
- Urakawa, R.; Ohte, N.; Shibata, H.; Tateno, R.; Inagaki, Y.; Oda, T.; Toda, H.; Fukuzawa, K.; Watanabe, T.; Hishi, T.; et al. Estimation of field soil nitrogen mineralization and nitrification rates using soil N transformation parameters obtained through laboratory incubation. Ecol. Res. 2017, 32, 279–285. [Google Scholar] [CrossRef]
- Cugnon, T.; Mahillon, J.; Lambert, R. In vitro anaerobic incubation: A reliable method to predict the potential of nitrogen mineralization after grassland ploughing. Biotechnol. Agron. Soc. Environ. 2024, 28, 17–27. [Google Scholar] [CrossRef]
- Mary, B.; Beaudoin, N.; Justes, E.; Machet, J. Calculation of nitrogen mineralization and leaching in fallow soil using a simple dynamic model. Eur. J. Soil Sci. 1999, 50, 549–566. [Google Scholar] [CrossRef]
- Vale, M. Quantification et Prédiction de la Minéralisation Nette de L’azote du Sol In Situ, Sous Divers Pédoclimats et Systèmes de Culture Français. Ph.D. Thesis, Institut National Polytechnique de Toulouse, Toulouse, France, 2006. [Google Scholar]
- ISO 11465:1993; Soil Quality—Determination of Dry Matter and Water Content on a Mass Basis—Gravimetric Method. International Organization for Standardization: Geneva, Switzerland, 1993.
- ISO 14256-2:2005; Soil Quality—Determination of Nitrogen Mineralization and Nitrification in Soils and the Influence of Chemicals—Part 2: Laboratory Incubation Method. International Organization for Standardization: Geneva, Switzerland, 2005.
- Walkley, A.; Black, I.A. Determination of organic matter in the soil by chromic acid digestion. Soil Sci. 1947, 63, 251–264. [Google Scholar] [CrossRef]
- ISO 11261:1995; Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 1995.
- ISO 10390:2005; Soil Quality—Determination of pH. International Organization for Standardization: Geneva, Switzerland, 2005.
- Série de Données de Référence en Matière de Textures et de Fractions Granulométriques des Sols de Wallonie; Ephesia Consult/SPW-ARNE: Namur, Belgium, 2020. Available online: https://geoportail.wallonie.be/catalogue/e90eb7cf-8f7d-40ab-9df9-5c34ddf387ea.html (accessed on 21 September 2025).
- Martínez, J.M.; Galantini, J. A rapid chemical method for estimating potentially mineralizable and particulate organic nitrogen in Mollisols. Commun. Soil Sci. Plant Anal. 2017, 48, 113–123. [Google Scholar] [CrossRef]
- Weil, R.R.; Islam, K.R.; Stine, M.A.; Gruver, J.B.; Samson-Liebig, S.E. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agric. 2003, 18, 3–17. [Google Scholar] [CrossRef]
- Culman, S.W.; Snapp, S.S.; Freeman, M.A.; Schipanski, M.E.; Beniston, J.; Lal, R.; Drinkwater, L.E.; Franzluebbers, A.J.; Glover, J.D.; Grandy, A.S.; et al. Permanganate oxidizable carbon reflects a processed soil fraction that is sensitive to management. Soil Sci. Soc. Am. J. 2012, 76, 494–504. [Google Scholar] [CrossRef]
- Fine, A.K.; van Es, H.M.; Schindelbeck, R.R. Statistics, scoring functions, and regional analysis of a comprehensive soil health database. Soil Sci. Soc. Am. J. 2017, 81, 589–601. [Google Scholar] [CrossRef]
- Culman, S.W.; Hurisso, T.T.; Wade, J. Permanganate Oxidizable Carbon. In Soil Health Series; Karlen, D.L., Stott, D.E., Mikha, M.M., Eds.; ASA-CSSA-SSSA: Madison, WI, USA, 2021; Chapter 9. [Google Scholar]
- Christy, I.; Moore, A.; Myrold, D.; Kleber, M. A mechanistic inquiry into the applicability of permanganate oxidizable carbon as a soil health indicator. Soil Sci. Soc. Am. J. 2023, 87, 1083–1095. [Google Scholar] [CrossRef]
- Morvan, T.; Beff, L.; Lambert, Y.; Mary, B.; Germain, P.; Louis, B.; Beaudoin, N. An original experimental design to quantify and model net mineralization of organic nitrogen in the field. Nitrogen 2022, 3, 197–212. [Google Scholar] [CrossRef]
- Jones, D.L.; Kielland, K. Amino acid, peptide and protein mineralization dynamics in a taiga forest soil. Soil Biol. Biochem. 2012, 55, 60–69. [Google Scholar] [CrossRef]
- Romillac, N. Ammonification. In Encyclopedia of Ecology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 256–263. [Google Scholar]
- Geisseler, D.; Horwath, W.R.; Joergensen, R.G.; Ludwig, B. Pathways of nitrogen utilization by soil microorganisms—A review. Soil Biol. Biochem. 2010, 42, 2058–2067. [Google Scholar] [CrossRef]
- Clivot, H.; Mary, B.; Vale, M.; Cohan, J.-P.; Champolivier, L.; Piraux, F.; Laurent, F.; Justes, E. Quantifying in situ and modeling net nitrogen mineralization from soil organic matter in arable cropping systems. Soil Biol. Biochem. 2017, 111, 44–59. [Google Scholar] [CrossRef]
- Hurisso, T.T.; Culman, S.W.; Horwath, W.R.; Wade, J.; Cass, D.; Beniston, J.W.; Bowles, T.M.; Grandy, A.S.; Franzluebbers, A.J.; Schipanski, M.E.; et al. Comparison of Permanganate-Oxidizable Carbon and Mineralizable Carbon for Assessment of Organic Matter Stabilization and Mineralization. Soil Sci. Soc. Am. J. 2016, 80, 1352–1364. [Google Scholar] [CrossRef]
- De Toffoli, M.; Bontemps, P.-Y.; Lambert, R. Synthèse de résultats d’essais de cultures intermédiaires pièges à nitrate à l’Université catholique de Louvain. Biotechnol. Agron. Soc. Environ. 2010, 14 (Suppl. 1), 79–89. [Google Scholar]
- Dabney, S.M.; Delgado, J.A.; Meisinger, J.J.; Schomberg, H.H.; Liebig, M.A.; Kaspar, T.; Mitchell, J.; Reeves, W. Using cover crops and cropping systems for nitrogen management. In Advances in Nitrogen Management for Water Quality; Delgado, J.A., Follett, R.F., Eds.; Soil and Water Conservation Society: Ankeny, IA, USA, 2010; pp. 231–282. [Google Scholar]
- Kühling, I.; Mikuszies, P.; Helfrich, M.; Flessa, H.; Schlathölter, M.; Sieling, K.; Kage, H. Effects of winter cover crops from different functional groups on soil–plant nitrogen dynamics and silage maize yield. Eur. J. Agron. 2023, 148, 126878. [Google Scholar] [CrossRef]
- Redin, M.; Recous, S.; Aita, C.; Dietrich, G.; Skolaude, A.C.; Ludke, W.H.; Schmatz, R.; Giacomini, S.J. How the chemical composition and heterogeneity of crop residue mixtures decomposing at the soil surface affects C and N mineralization. Soil Biol. Biochem. 2014, 78, 65–75. [Google Scholar] [CrossRef]
- Weiler, D.A.; Giacomini, S.J.; Aita, C.; Schmatz, R.; Pilecco, G.E.; Chaves, B.; Bastos, L.M. Summer cover crops shoot decomposition and nitrogen release in a no-tilled sandy soil. Rev. Bras. Cienc. Solo 2019, 43, e0190027. [Google Scholar] [CrossRef]
- Oliveira, M.; Rebac, D.; Coutinho, J.; Ferreira, L.; Trindade, H. Nitrogen mineralization of legume residues: Interactions between species, temperature and placement in soil. Span. J. Agric. Res. 2020, 18, e1101. [Google Scholar] [CrossRef]
- Chaves, B.; Redin, M.; Giacomini, S.J.; Schmatz, R.; Léonard, J.; Ferchaud, F.; Recous, S. The combination of residue quality, residue placement and soil mineral N content drives C and N dynamics by modifying N availability to microbial decomposers. Soil Biol. Biochem. 2021, 163, 108434. [Google Scholar] [CrossRef]
- Constantin, J.; Minette, S.; Vericel, G.; Jordan-Meille, L.; Justes, E. MERCI: A simple method and decision support tool to estimate availability of nitrogen from a wide range of cover crops to the next cash crop. Plant Soil 2024, 494, 333–351. [Google Scholar] [CrossRef]
- Castellano, M.; Kaye, J.P.; Lin, H.; Schmidt, J.P. Reactive nitrogen retention and flushing along a soil texture gradient. In Abstracts of the 94th Ecological Society of America Annual Meeting; Ecological Society of America: Albuquerque, NM, USA, 2009; pp. 67–68. [Google Scholar]
- Batten, G.D. An appreciation of the contribution of NIR to agriculture. J. Near Infrared Spectrosc. 1998, 6, 105–114. [Google Scholar] [CrossRef]
- Fystro, G. The prediction of C and N content and their potential mineralisation in heterogeneous soil samples using Vis–NIR spectroscopy and comparative methods. Plant Soil 2002, 246, 139–149. [Google Scholar] [CrossRef]
- Russell, C.; Angus, J.; Batten, G.; Dunn, B.W.; Williams, R.L. The potential of NIR spectroscopy to predict nitrogen mineralization in rice soils. Plant Soil 2002, 247, 243–252. [Google Scholar] [CrossRef]
- Calderón, F.J.; Culman, S.; Six, J.; Franzluebbers, A.J.; Schipanski, M.; Beniston, J.; Grandy, S.; Kong, A.Y.Y. Quantification of soil permanganate oxidizable C (POXC) using infrared spectroscopy. Soil Sci. Soc. Am. J. 2017, 81, 277–288. [Google Scholar] [CrossRef]


| Location | Agricultural Region | Xp Plots | T °C | Rain (mm/y) | Years |
|---|---|---|---|---|---|
| Avernas | Loamy Region | 30 | 11.0 ± 0.5 | 632.6 ± 25.2 | 2016–2018 |
| Buzet | Loamy Region | 8 | 11.7 | 668.4 | 2018 |
| Ciney | Condroz | 5 | 10.0 | 709.0 | 2017 |
| Enghien | Loamy Region | 2 | 11.0 | 660.8 | 2017 |
| Gembloux | Loamy Region | 4 | 10.8 | 603.7 | 2017 |
| Givry | Loamy Region | 4 | 11.7 | 641.0 | 2020 |
| Louvain | Sandy Loamy Region | 9 | 10.7 ± 0.4 | 713.8 ± 153.2 | 2017; 2021 |
| Marbisoux | Loamy Region | 15 | 10.4 ± 0.6 | 751.9 ± 142.5 | 2019; 2021 |
| Michamps | Ardenne | 34 | 9.0 ± 0.4 | 750.9 ± 41.0 | 2015–2018 |
| Tinlot | Condroz | 10 | 11.3 | 615.0 | 2017 |
| Location | Xp Plots | Nt (‰) | TOC (%) | Clay (G·KG−1) | NL (MG·KG−1) | POXC (MG·KG−1) | PH |
|---|---|---|---|---|---|---|---|
| Avernas | 30 | 1.37 ± 0.15 cd * | 1.24 ± 0.15 c | 114.0 ± 0.0 e | 218 ± 24 de | 380 ± 33 bc | 7.11 ± 0.33 a |
| Buzet | 8 | 1.30 ±0.05 cd | 0.98 ± 0.11 c | 138.0 ± 0.0 bc | 209 ± 31 e | 293 ± 31 c | 6.35 ± 0.05 b |
| Ciney | 5 | 1.63 ± 0.17 bcd | 1.60 ± 0.10 bc | 144.0 ± 0.0 b | 430 ± 43 ab | 319 ± 18 bc | 5.81 ± 0.26 bc |
| Enghien | 2 | 1.35 ± 0.07 bcd | 1.55 ± 0.07 bc | 114.0 ± 0.0 cde | 209 ± 6 de | 241 ± 0 bc | 6.35 ± 0.07 b |
| Gembloux | 4 | 1.91 ± 0.37 bc | 2.03 ± 0.44 ab | 114.0 ± 0.0 de | 365 ± 31 bc | 632 ± 118 a | 7.14 ± 0.63 a |
| Givry | 4 | 2.00 ± 0.08 b | 1.61 ± 0.16 bc | 138.0 ± 0.0 bcd | 248 ± 10 de | 518 ± 30 ab | 6.11 ± 0.24 b |
| Louvain | 9 | 1.21 ± 0.10 d | 1.01 ± 0.10 c | 146.0 ± 38.1 b | 221 ± 24 de | 260 ± 37 c | 7.13 ± 0.52 a |
| Marbisoux | 15 | 1.34 ± 0.14 cd | 1.25 ± 0.17 c | 151.1 ± 9.8 b | 194 ± 16 e | 304 ± 10 bc | 5.74 ± 0.14 bc |
| Michamps | 34 | 2.88 ± 0.57 a | 2.51 ± 0.66 a | 165.8 ± 4.3 a | 496 ± 63 a | 435 ± 148 bc | 5.49 ± 0.42 c |
| Tinlot | 10 | 1.56 ± 0.38 bcd | 1.58 ± 0.37 bc | 138.0 ± 0.0 bc | 303 ± 45 cd | 368 ± 115 bc | 6.13 ± 0.38 b |
| Location | ISM | PMN | Soil N-C | Soil N-N |
|---|---|---|---|---|
| Avernas | 151 ± 27 a * | 142 ± 22 a | 163 ± 23 a | 174 ± 27 a |
| Buzet | 139 ± 62 ab | 116 ± 21 b | 126 ± 11 ab | 163 ± 14 a |
| Ciney | 151 ± 9 a | 134 ± 16 a | 78 ± 5 b | 80 ± 8 b |
| Enghien | 187 ± 1 a | 151 ± 12 b | 141 ± 6 b | 134 ± 6 b |
| Gembloux | 341 ± 82 a | 243 ± 11 ab | 233 ± 36 b | 236 ± 32 b |
| Givry | 102 ± 28 c | 154 ± 12 ab | 140 ± 14 b | 178 ± 7 a |
| Louvain | 138 ± 21 ab | 149 ± 29 a | 110 ± 16 ab | 125 ± 17 b |
| Marbisoux | 211 ± 61 a | 183 ± 65 a | 175 ± 35 a | 187 ± 35 a |
| Michamps | 159 ± 81 a | 158 ± 79 a | 149 ± 41 a | 173 ± 41 a |
| Tinlot | 131 ± 44 a | 151 ± 31 a | 127 ± 27 a | 130 ± 28 a |
| Practice | n | Mean Effect (kg N·ha−1) | SD (kg N·ha−1) | Min (kg N·ha−1) | Max (kg N·ha−1) |
|---|---|---|---|---|---|
| Grassland effect (GE) | 29 | 42.7 | 32.2 | 10 | 140 |
| Cover cropping (CC) | 53 | 27.0 | 6.1 | 15 | 45 |
| Manure effect (ME) | 30 | 65.6 | 27.9 | 27.4 | 131.6 |
| ISM | aLIX | Vp | PMN | PMN/ND | TOC (%) | NT (‰) | TOC/N | Clay | TOC/Clay | Soil N-C | Soil N-N | Nl | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aLIX | 0.73 * | ||||||||||||
| Vp | 0.77 * | 0.94 * | |||||||||||
| PMN | 0.79 * | 0.49 * | 0.55 * | ||||||||||
| PMN/ND | 0.36 * | 0.59 * | 0.66 * | 0.61 * | |||||||||
| TOC (%) | 0.03 | 0.11 | 0.27 * | 0.06 | 0.29 * | ||||||||
| NT (‰) | −0.06 | 0.06 | 0.21 * | 0.00 | 0.27 * | 0.95 * | |||||||
| TOC/N | 0.26 * | 0.16 | 0.19 * | 0.20 * | 0.06 | 0.19 * | −0.13 | ||||||
| Clay | −0.01 | −0.30 * | −0.17 | 0.12 | −0.02 | 0.53 * | 0.59 * | −0.12 | |||||
| TOC/Clay | 0.08 | 0.33 * | 0.44 * | 0.06 | 0.38 * | 0.92 * | 0.82 * | 0.32 * | 0.18 * | ||||
| Soil N-C | 0.58 * | 0.47 * | 0.45 * | 0.51 * | 0.26 * | 0.14 | 0.04 | 0.30 * | −0.11 | 0.26 * | |||
| Soil N-N | 0.49 * | 0.45 * | 0.41 * | 0.43 * | 0.27 * | 0.08 | 0.13 | −0.19 * | −0.01 | 0.13 | 0.86 * | ||
| Nl | 0.12 | −0.01 | 0.10 | 0.09 | 0.00 | 0.82 * | 0.82 * | 0.26 * | 0.80 * | 0.70 * | 0.07 | −0.05 | |
| POxC | 0.53 * | 0.46 * | 0.41 * | 0.46 * | 0.25 * | 0.26 * | 0.24 * | 0.16 | 0.18 | 0.35 * | 0.57 * | 0.50 * | 0.32 * |
| Manure Effect (ME) | Cover Crop Effect (CC) | Grassland Effect (GE) | ||||
|---|---|---|---|---|---|---|
| Yes (n = 30) | NO (n = 91) | Yes (n = 53) | No (n = 68) | Yes (n = 29) | No (n = 92) | |
| ISM-Vp | 0.44 * | 0.81 * | 0.70 * | 0.84 * | 0.87 * | 0.60 * |
| ISM-PMN | 0.79 * | 0.78 * | 0.55 * | 0.85 * | 0.87 * | 0.62 * |
| ISM-POxC | −0.19 | 0.68 * | 0.46 | 0.63 * | 0.96 * | 0.40 * |
| PMN-Vp | 0.22 | 0.61 * | 0.18 | 0.67 * | 0.55 * | 0.32 * |
| PMN-POxC | 0.37 | 0.65 * | 0.75 * | 0.56 * | 0.82 * | 0.38 * |
| POxC-Vp | −0.06 | 0.44 * | 0.18 | 0.46 * | 0.89 * | 0.11 |
| Term | BFM | MLR | |||
|---|---|---|---|---|---|
| Main Effect | Total Effect | Weights | Logworth | p-Value | |
| PMN | 0.440 | 0.641 | +++++++ | 7.180 | 0.0000 |
| POxC | 0.215 | 0.215 | ++ | 5.066 | 0.0001 |
| TOC/N | 0.126 | 0.126 | + | 0.175 | 0.6686 |
| pHKCl | 0.120 | 0.120 | + | 2.437 | 0.0037 |
| TOC/Clay | 0.098 | 0.098 | + | 0.692 | 0.2032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cugnon, T.; De Toffoli, M.; Mahillon, J.; Lambert, R. Improving Nitrogen Fertilization Recommendations in Temperate Agricultural Systems: A Study on Walloon Soils Using Anaerobic Incubation and POxC. Nitrogen 2025, 6, 91. https://doi.org/10.3390/nitrogen6040091
Cugnon T, De Toffoli M, Mahillon J, Lambert R. Improving Nitrogen Fertilization Recommendations in Temperate Agricultural Systems: A Study on Walloon Soils Using Anaerobic Incubation and POxC. Nitrogen. 2025; 6(4):91. https://doi.org/10.3390/nitrogen6040091
Chicago/Turabian StyleCugnon, Thibaut, Marc De Toffoli, Jacques Mahillon, and Richard Lambert. 2025. "Improving Nitrogen Fertilization Recommendations in Temperate Agricultural Systems: A Study on Walloon Soils Using Anaerobic Incubation and POxC" Nitrogen 6, no. 4: 91. https://doi.org/10.3390/nitrogen6040091
APA StyleCugnon, T., De Toffoli, M., Mahillon, J., & Lambert, R. (2025). Improving Nitrogen Fertilization Recommendations in Temperate Agricultural Systems: A Study on Walloon Soils Using Anaerobic Incubation and POxC. Nitrogen, 6(4), 91. https://doi.org/10.3390/nitrogen6040091






