Enhancing Hybrid Maize Performance and Yield Through Potassium Sulfate Fertilization: A Field-Based Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Experiment Area and Design
2.2. Measured Characters
2.2.1. Growth Parameters
- Plant height (PH, cm): the length of the main stem from the soil surface to the plant apex has been measured using a ruler.
- Leaf number (LN, leaves plants−1): the total number of fully expanded leaves per plant.
- Flag leaf area (FLA, cm2): calculated using the method [17], it involved measuring the length of the leaf blade from its base to the tip of the leaf (leaf L) and the width of the leaf at its widest point (leaf W) and multiplying them by a correction factor (0.75), derived to account for the natural curvature and shape of maize leaves, which are not perfect rectangles as illustrated in the equation:Flag leaf area (FLA) = Leaf L × Leaf W × 0.75
- Stem diameter (SD, cm): measured at the base of the stem using a digital caliper.
- Ear height (EH, cm): the height from the soil surface to the top-most node bearing an ear.
2.2.2. Chlorophyll Content (Chl.)
2.2.3. Grain Yield and Its Attributes
- Ear diameter (ED, cm): measured at the midpoint of the ear using a digital caliper.
- Ear length (EL, cm): measured from the base to the tip of the ear.
- Grains yield per plant (GYP, g plants−1): the weight of grains per sampled plant.
- Shelling percentage (SP, %):
- 1000-grain weight (SI, g): the weight of 1000 randomly sampled grains.
- Grain yield (GY, ton ha−1): the weight of grain yield of each plot adjusted to 15.5% moisture content was recorded.
2.2.4. Grain Quality
- Protein content (PP, %): determined from grain nitrogen (N) concentration measured using the micro Kjeldahl method and expressed as N × 6.25 [19].
- Oil content (OP, %): extracted by Soxhlet apparatus using petroleum ether (boiling range of 60–80 °C) according to [19].
- Protein yield (kg ha−1):
- Oil yield (kg ha−1):
2.3. Statistical Analysis
3. Results
3.1. Analysis of Variance
3.2. Means of Characteristics over Two Seasons
3.3. Path Coefficient Analysis
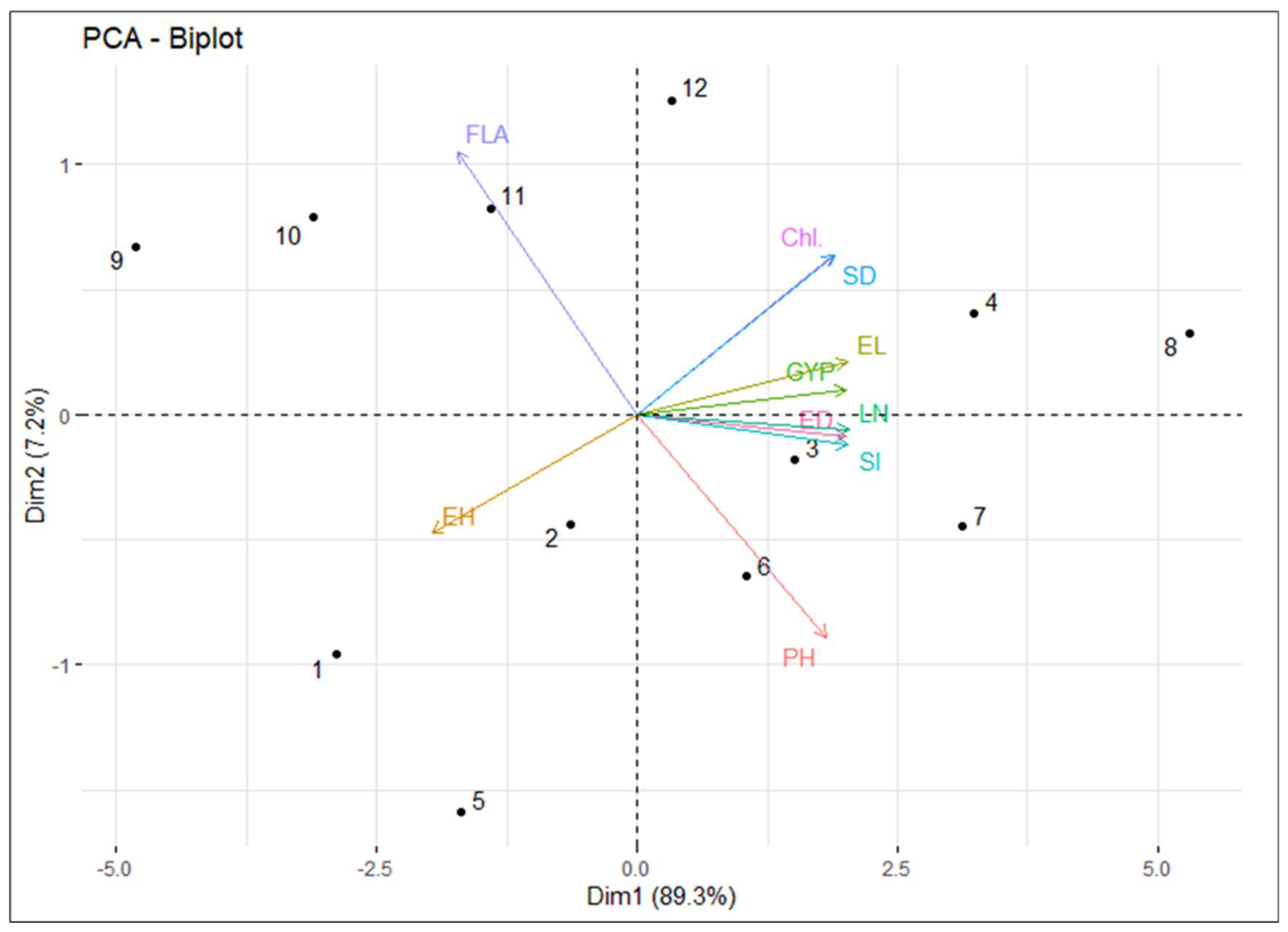
3.4. PCA Analysis
4. Discussion
5. Conclusions
6. Practical Recommendations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kyenpia, E.O.; Namo, O.A.; Gikyu, S.W.; Ifenkwe, O.P. A comparative study of the biochemical composition of some varieties of maize (Zea mays) grown in Nigeria. Niger. J. Bot. 2009, 22, 291–296. [Google Scholar]
- FAO Food Price Index. 2023. Available online: https://www.fao.org/worldfoodsituation/FoodPricesIndex/en/ (accessed on 23 July 2024).
- Sardans, J.; Peñuelas, J. Potassium Control of Plant Functions: Ecological and Agricultural Implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fan, Y.-F.; Sun, J.-Y.; Gao, J.-L.; Wang, Z.-G.; Yu, X.-F. Effects of Straw Return with Potassium Fertilizer on the Stem Lodging Resistance, Grain Quality and Yield of Spring Maize (Zea mays L.). Sci. Rep. 2023, 13, 20307. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Z.; Liang, L.; Zhao, Y.; Yang, B.; Ding, R.; Wang, J.; Nie, J. Changes in Soil Characteristics and Maize Yield under Straw Returning System in Dryland Farming. Field Crops Res. 2018, 218, 11–17. [Google Scholar] [CrossRef]
- Anandham, P.; Arul, J.; Senthilkumar, M.; Yang, Y.; Long, Y.; Li, S.; Liu, X. Straw Return Decomposition Characteristics and Effects on Soil Nutrients and Maize Yield. Agriculture 2023, 13, 1570. [Google Scholar] [CrossRef]
- Liu, R.Z.; Borjigin, Q.; Gao, J.; Yu, X.; Hu, S.; Li, R.P. Effects of Different Straw Return Methods on Soil Properties and Yield Potential of Maize. Sci. Rep. 2024, 14, 28682. [Google Scholar] [CrossRef]
- Król-Badziak, A.; Kozyra, J.; Rozakis, S. Evaluation of Climate Suitability for Maize Production in Poland under Climate Change. Sustainability 2024, 16, 6896. [Google Scholar] [CrossRef]
- Sun, L.; Lai, M.; Ghouri, F.; Nawaz, M.A.; Ali, F.; Baloch, F.S.; Nadeem, M.A.; Aasim, M.; Shahid, M.Q. Modern plant breeding techniques in crop improvement and genetic diversity: From molecular markers and gene editing to artificial intelligence—A critical review. Plants 2024, 13, 2676. [Google Scholar] [CrossRef]
- Wright, S. Correlation and causation. J. Agric. Res. 1921, 20, 557. [Google Scholar]
- Fernández, J.A.; Messina, C.D.; Salinas, A.; Prasad, P.V.V.; Nippert, J.B.; Ciampitti, I.A. Kernel Weight Contribution to Yield Genetic Gain of Maize: A Global Review and US Case Studies. J. Exp. Bot. 2022, 73, 3597–3609. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.A. Estimation of Some Breeding Parameters for Improvement of Grain Yield in Yellow Maize under Water Stress. J. Plant Prod. 2016, 7, 1509–1521. [Google Scholar] [CrossRef]
- Zhu, X.M.; Shao, X.Y.; Pei, Y.H.; Guo, X.M.; Li, J.; Song, X.Y.; Zhao, M.A. Genetic Diversity and Genome-Wide Association Study of Major Ear Quantitative Traits Using High-Density SNPs in Maize. Front. Plant Sci. 2018, 9, 966. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Singh, M.K. Correlation and Path Coefficient Analysis, for Yield and Yield-Related Traits in Maize (Zea mays L.). Int. J. Res. Agron. 2025, 8, 668–676. [Google Scholar] [CrossRef]
- Estefan, G.; Sommer, R.; Ryan, J. Methods of Soil, Plant, and Water Analysis. Man. West Asia N. Afr. Reg. 2013, 3, 65–119. [Google Scholar]
- McKee, G.W. A Coefficient for Computing Leaf Area in Hybrid Corn1. Agron. J. 1964, 56, 240–241. [Google Scholar] [CrossRef]
- Dash, J.; Curran, P.J.; Tallis, M.J.; Llewellyn, G.M.; Taylor, G.; Snoeij, P. The relationship between the MERIS terrestrial chlorophyll index and chlorophyll content. In Proceedings of the Second ENVISAT Symposium, ESA, SP-636, Montreux, Switzerland, 23–27 April 2007; European Space Agency: Noordwijk, The Netherlands, 2007. [Google Scholar]
- AOAC. Official Method of Analysis, 18th ed.; Association of Officiating Analytical Chemists: Washington, DC, USA, 2005; Method 935.14 and 992.24. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Bartlett, M.S. Some Examples of Statistical Methods of Research in Agriculture and Applied Biology. J. R. Stat. Soc. Ser. B Stat. Methodol. 1937, 4, 137–170. [Google Scholar] [CrossRef]
- Shaphiro, S.; Wilk, M.B.J.B. An analysis of variance test for normality. Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Waller, R.A.; Duncan, D.B. A Bayes Rule for the Symmetric Multiple Comparisons Problem. J. Am. Stat. Assoc. 1969, 64, 1484–1503. [Google Scholar] [CrossRef]
- Dewey, D.R.; Lu, K.H. A Correlation and Path-Coefficient Analysis of Components of Crested Wheatgrass Seed Production1. Agron. J. 1959, 51, 515–518. [Google Scholar] [CrossRef]
- Price, A.L.; Patterson, N.J.; Plenge, R.M.; Weinblatt, M.E.; Shadick, N.A.; Reich, D. Principal Components Analysis Corrects for Stratification in Genome-Wide Association Studies. Nat. Genet. 2006, 38, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Jeffers, J.N.R. Two Case Studies in the Application of Principal Component Analysis. J. R. Stat. Soc. Ser. C Appl. Stat. 1967, 16, 225–236. [Google Scholar] [CrossRef]
- Cakmak, I. The Role of Potassium in Alleviating Detrimental Effects of Abiotic Stresses in Plants. J. Plant Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Rengel, Z.; Damon, P.M. Crops and Genotypes Differ in Efficiency of Potassium Uptake and Use. Physiol. Plant 2008, 133, 624–636. [Google Scholar] [CrossRef]
- Mengel, K.; Kirkby, E. Principles of Plant Nutrition; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Pettigrew, W.T. Potassium influences yield and quality production for maize, wheat, soybean and cotton. Physiol. Plant 2008, 133, 670–681. [Google Scholar] [CrossRef]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in Agriculture—Status and Perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Yaseen Ashraf, M.; Saifullah, S.; Ahmad, M. Improving Agricultural Water Use Efficiency by Nutrient Management in Crop Plants. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2011, 61, 291–304. [Google Scholar] [CrossRef]
- Zhao, D.; Oosterhuis, D.M.; Bednarz, C.W. Influence of Potassium Deficiency on Photosynthesis, Chlorophyll Content, and Chloroplast Ultrastructure of Cotton Plants. Photosynthetica 2001, 39, 103–109. [Google Scholar] [CrossRef]
- Csathó, P.; Szabó, A.; Pokovai, K.; Árendás, T. Effect of Potassium Supply and Plant Density on Maize (Zea mays L.) Yields and Nutrient Contents: A Case Study in a Hungarian Long-Term Field Trial Set up on Calcareous Chernozem Soil. Cereal Res. Commun. 2025, 53, 1091–1103. [Google Scholar] [CrossRef]
- Nisa, Z.; Saifullah, N.; Ullah, M.; Zia, Z.; Ali, B. Comparison of Different Potassium Application Methods for Maize (Zea mays L.) Growth under Salt-Affected Soils. J. Pure Appl. Agric. 2021, 6, 44–53. [Google Scholar]
- Milad, R.A. Effect of Source and Rate of Potassium Fertilizer on Plant Growth Under Soil Water Stress. Sirte Univ. Sci. J. 2019, 9, 1–26. [Google Scholar]
- Song, J.; Wang, S.X.; Li, L.; Huang, J.L.; Zhao, B.; Zhang, J.W.; Ren, B.Z.; Liu, P. Effects of Potassium Application Rate on NPK Uptake and Utilization and Grain Yield in Summer Maize (Zea mays L.). Acta Agron. Sin. 2023, 49, 539–551. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Kronzucker, H.J. The Nitrogen–Potassium Intersection: Membranes, Metabolism, and Mechanism. Plant Cell Environ. 2017, 40, 2029–2041. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Bukhsh, H.A.; Ahmad, R.; Ali, A.; Ishaque, M.; Rehman, A.; Khan, G. Potassium use efficiency of maize hybrids. J. Anim. Plant Sci. 2012, 22, 728–732. [Google Scholar]
- Laidig, F.; Feike, T.; Klocke, B.; Macholdt, J.; Miedaner, T.; Rentel, D.; Piepho, H.P. Long-Term Breeding Progress of Yield, Yield-Related, and Disease Resistance Traits in Five Cereal Crops of German Variety Trials. Theor. Appl. Genet. 2021, 134, 3805–3827. [Google Scholar] [CrossRef]
- Liu, L.; Lindsay, P.L.; Jackson, D. Next Generation Cereal Crop Yield Enhancement: From Knowledge of Inflorescence Development to Practical Engineering by Genome Editing. Int. J. Mol. Sci. 2021, 22, 5167. [Google Scholar] [CrossRef] [PubMed]
- Yahaya, M.S.; Bello, I.; Unguwanrimi, A.Y. Correlation and path-coefficient analysis for grain yield and agronomic traits of maize (Zea mays L.). Sci. World J. 2021, 16, 10–13. [Google Scholar]
- Mendes-Moreira, P.M.R.; Mendes-Moreira, J.; Fernandes, A.; Andrade, E.; Hallauer, A.R.; Pêgo, S.E.; Vaz Patto, M.C. Is Ear Value an Effective Indicator for Maize Yield Evaluation? Field Crops Res. 2014, 161, 75–86. [Google Scholar] [CrossRef]
- Ma, J.; Cao, Y. Genetic Dissection of Grain Yield of Maize and Yield-Related Traits Through Association Mapping and Genomic Prediction. Front. Plant Sci. 2021, 12, 690059. [Google Scholar] [CrossRef]
- Ren, H.; Liu, M.; Zhang, J.; Liu, P.; Liu, C. Effects of Agronomic Traits and Climatic Factors on Yield and Yield Stability of Summer Maize (Zea mays L.) in the Huang-Huai-Hai Plain in China. Front. Plant Sci. 2022, 13, 1050064. [Google Scholar] [CrossRef]
- Khodarahmpour, Z. Morphological Classification of Maize (Zea mays L.) Genotypes in Heat Stress Condition. J. Agric. Sci. 2012, 4, 31–40. [Google Scholar] [CrossRef]
- Meng, S.; Huang, Y.; Lian, Y.; Chen, H.; Cao, X.; Ding, D.; Chen, X.; Tang, J. Transcriptomic Analysis Reveals the Regulation of Early Ear-Length Development in Maize. Plant Growth Regul. 2023, 100, 97–105. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Akinwale, R.O.; Ajala, S.O.; Menkir, A.; Fakorede, M.A.B.; Oyekunle, M. Relationships among Traits of Tropical Early Maize Cultivars in Contrasting Environments. Agron. J. 2011, 103, 717–729. [Google Scholar] [CrossRef]
- Hamid, M. Heritability and Trait Association Studies in Maize F1 Hybrids. Int. J. Biosci. 2018, 12, 18–26. [Google Scholar] [CrossRef]
- Mustafa, B.S.; Ismael, N.B.; Mustafa, N.R.; Kakarash, S.A.; Abdulazeez, S.D. Chlorophyll Content and Leaf Area Correlated with Corn (Zea mays) Yield Components in F1 Hybrids. Indian J. Agric. Sci. 2024, 94, 352–357. [Google Scholar] [CrossRef]
- Széles, A.; Horváth, É.; Simon, K.; Zagyi, P.; Huzsvai, L. Maize Production under Drought Stress: Nutrient Supply, Yield Prediction. Plants 2023, 12, 3301. [Google Scholar] [CrossRef]
- Fei, J.; Lu, J.; Jiang, Q.; Liu, Z.; Yao, D.; Qu, J.; Liu, S.; Guan, S.; Ma, Y. Maize Plant Architecture Trait QTL Mapping and Candidate Gene Identification Based on Multiple Environments and Double Populations. BMC Plant Biol. 2022, 22, 110. [Google Scholar] [CrossRef]
- Ul-Allah, S.; Ijaz, M.; Nawaz, A.; Sattar, A.; Sher, A.; Naeem, M.; Shahzad, U.; Farooq, U.; Nawaz, F.; Mahmood, K. Potassium Application Improves Grain Yield and Alleviates Drought Susceptibility in Diverse Maize Hybrids. Plants 2020, 9, 75. [Google Scholar] [CrossRef]

| Property (Unit) | Year 2023 | Year 2024 | Property | Year 2023 | Year 2024 |
|---|---|---|---|---|---|
| Particle size distribution | Soluble cations | ||||
| Sand (%) | 26.40 | 26.80 | Ca2+ (meq 100 g−1) | 9.5 | 9.4 |
| Silt (%) | 25.40 | 25.50 | Mg2+ (meq 100 g−1) | 3.0 | 3.0 |
| Clay (%) | 48.20 | 47.70 | Na+ (meq 100 g−1) | 6.1 | 6.0 |
| Soil texture class | Clay | Clay | K+ (meq 100 g−1) | 2.0 | 2.0 |
| Bulk density (g cm−3) | 1.21 | 1.20 | Soluble anions | ||
| Field capacity (%) | 40.27 | 40.20 | Cl− (meq 100 g−1) | 4.0 | 4.0 |
| Wilting point (%) | 21.00 | 21.00 | HCO3− + CO32− (meq 100 g−1) | 7.0 | 6.9 |
| Infiltration rate (cm h−1) | 0.13 | 0.12 | SO42− (meq 100 g−1) | 10.5 | 10.4 |
| CaCO3 (%) | 1.22 | 1.18 | Total nitrogen (%) | 0.08 | 0.09 |
| pH (1:2.5) | 7.77 | 7.78 | Available phosphorous (mg kg−1) | 11.2 | 11.2 |
| Electrical conductivity (dS m−1) | 2.03 | 2.03 | Available potassium (mg kg−1) | 240.0 | 235.0 |
| Organic matter (%) | 1.70 | 1.72 | |||
| Month | Temperature (°C) | Relative Humidity (%) | Precipitation (m3) | |
|---|---|---|---|---|
| Min | Max | |||
| 2023 | ||||
| May | 21.0 | 35.5 | 29 | 0 |
| June | 25.0 | 38.0 | 30 | 0 |
| July | 25.0 | 39.0 | 33 | 0 |
| August | 24.0 | 38.0 | 38 | 0 |
| September | 23.0 | 37.2 | 36 | 0 |
| 2024 | ||||
| May | 20.8 | 35.5 | 26 | 0 |
| June | 25.1 | 40.4 | 27 | 0 |
| July | 25.0 | 39.9 | 28 | 0 |
| August | 26.5 | 39.4 | 30 | 0 |
| September | 24.0 | 36.4 | 40 | 0 |
| S.O.V | D.F | Ear Diameter (cm) | Ear Length (cm) | Grain Yield (g plant−1) | 1000-Grain Weight (g) | Shelling Percentage (%) | Grain Yield (t ha−1) | Protein Content (%) | Oil Content (%) | Protein Yield (kg ha−1) | Oil Yield (kg ha−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S | 1 | 3.34 ** | 12.17 ** | 1063.4 ** | 11.9 * | 1.36 | 2.80 ** | 1.04 ** | 0.07 | 72,014.0 ** | 10,336.6 ** |
| Error a | 4 | 0.03 | 0.42 | 21.2 | 0.94 | 0.4 | 0.08 | 0.02 | 0.01 | 1989.8 | 542.1 |
| PS | 3 | 5.02 ** | 21.2 ** | 1346.8 ** | 72.9 ** | 5.46 ** | 5.61 ** | 6.4 ** | 2.36 ** | 213,784.8 ** | 51,327.0 ** |
| S × PS | 3 | 0.07 ** | 0.24 ** | 7.8 ** | 0.02 | 3.05 ** | 0.05 ** | 0.07 * | 0.01 | 2331.2 ** | 296.7 ** |
| Error b | 12 | 0.001 | 0.01 | 0.18 | 0.01 | 0.1 | 0.001 | 0.01 | 0.003 | 125.2 | 27.4 |
| SC | 2 | 5.15 ** | 13.7 ** | 1009.5 ** | 88.1 ** | 71.1 ** | 3.92 ** | 16.0 ** | 4.80 ** | 244,611.9 ** | 59,068.4 ** |
| S × SC | 2 | 0.02 * | 0.12 | 4.1 ** | 0.22 * | 3.13 ** | 0.21 ** | 0.11 ** | 0.002 | 1828.4 ** | 466.1 ** |
| Error c | 8 | 0.004 | 0.03 | 0.29 | 0.03 | 0.22 | 0.002 | 0.01 | 0.002 | 88.6 | 26.7 |
| PS × SC | 6 | 0.12 ** | 0.35 ** | 58.2 ** | 2.8 ** | 3.67 ** | 0.10 ** | 0.53 ** | 0.13 ** | 6033.2 ** | 815.4 ** |
| S × PS × SC | 6 | 0.06 ** | 0.08 ** | 12.2 ** | 0.12 ** | 1.67 ** | 0.05 ** | 0.04 * | 0.01 | 300.0 * | 172.3 ** |
| Error d | 24 | 0.002 | 0.01 | 0.17 | 0.01 | 0.11 | 0.001 | 0.01 | 0.003 | 103.1 | 21.7 |
| S.O.V | D.F | Plant Height (cm) | Leaf Number (n plant−1) | Flag Leaf Area (cm2) | Chlorophyll Content (mg m−2) | Stem Diameter (cm) | Ear Height (cm) |
|---|---|---|---|---|---|---|---|
| S | 1 | 125.9 | 23.5 ** | 80.5 ** | 8881.2 ** | 0.003 | 393.7 ** |
| Error a | 4 | 30.8 | 1.24 | 4.32 | 316.9 | 0.01 | 5.61 |
| PS | 3 | 1106.2 ** | 28.4 ** | 2.79 ** | 147,215.5 ** | 1.89 ** | 1603.0 ** |
| S × PS | 3 | 1.20 | 0.17 | 1.14 | 434.7 ** | 0.02 | 3.73 * |
| Error b | 12 | 1.95 | 0.07 | 0.37 | 24.4 | 0.02 | 1.04 |
| SC | 2 | 5618.9 ** | 25.5 ** | 23.2 ** | 22,647.6 ** | 0.41 ** | 427.1 ** |
| S × SC | 2 | 0.04 | 0.01 | 0.39 | 1087.7 * | 0.03 | 17.0 * |
| Error c | 8 | 14.4 | 0.14 | 0.70 | 211.8 | 0.01 | 4.15 |
| PS × SC | 6 | 8.3 ** | 0.02 | 0.21 | 1467.2 ** | 0.13 ** | 17.8 ** |
| S × PS × SC | 6 | 0.14 | 0.04 | 0.35 | 262.1 ** | 0.07 * | 3.92 ** |
| Error d | 24 | 0.76 | 0.06 | 0.28 | 63.7 | 0.02 | 0.76 |
| Variable | Plant Height (cm) | Leaf Number (n plant−1) | Flag Leaf Area (cm2) | Chlorophyll (mg m−2) | Stem Diameter (cm) | Ear Height (cm) | |
|---|---|---|---|---|---|---|---|
| K2SO4 fertilizer | |||||||
| 0 kg ha−1 | 214.0 ± 3.6 d | 14.97 ± 0.29 d | 35.9 ± 0.47 a | 306.3 ± 4.2 d | 2.16 ± 0.02 d | 96.5 ± 1.06 d | |
| 60 kg ha−1 | 218.8 ± 3.1 c | 15.91 ± 0.26 c | 35.8 ± 0.36 a | 343.0 ± 5.3 c | 2.51 ± 0.02 c | 103.5 ± 1.34 c | |
| 120 kg ha−1 | 226.9 ± 3.2 b | 16.91 ± 0.24 b | 35.3 ± 0.30 b | 414.1 ± 10.7 b | 2.70 ± 0.02 b | 110.8 ± 1.21 b | |
| 180kg ha−1 | 231.4 ± 2.9 a | 17.88 ± 0.25 a | 35.1 ± 0.32 b | 511.7 ± 8.8 a | 2.93 ± 0.09 a | 118.4 ± 0.74 a | |
| Single cross | |||||||
| SC2031 | 229.3 ± 1.29 b | 16.47 ± 0.27 b | 35.5 ± 0.27 b | 394.3 ± 16.4 b | 2.56 ± 0.07 b | 107.0 ± 1.94 b | |
| SC2036 | 233.8 ± 1.57 a | 17.42 ± 0.26 a | 36.5 ± 0.31 a | 424.3 ± 18.4 a | 2.71 ± 0.08 a | 111.6 ± 1.79 a | |
| SC168 | 205.3 ± 1.64 c | 15.36 ± 0.27 c | 34.6 ± 0.26 c | 362.8 ± 15.6 c | 2.45 ± 0.05 c | 103.2 ± 1.71 c | |
| Interaction | |||||||
| 0 kg ha−1 | SC2031 | 222.1 ± 0.55 h | 15.03 ± 0.35 a | 35.8 ± 0.74 a | 311.6 ± 3.8 i | 2.17 ± 0.01 g | 95.5 ± 2.22 j |
| SC2036 | 225.1 ± 1.17 f | 15.97 ± 0.34 a | 35.7 ± 0.56 a | 337.6 ± 4.4 g | 2.18 ± 0.01 g | 100.2 ± 1.22 h | |
| SC168 | 232.9 ± 0.83 d | 16.95 ± 0.28 a | 35.3 ± 0.51 a | 420.3 ± 5.3 e | 2.14 ± 0.05 g | 93.8 ± 0.84 k | |
| 60 kg ha−1 | SC2031 | 236.9 ± 0.65 c | 17.93 ± 0.24 a | 35.1 ± 0.34 a | 507.5 ± 12.5 b | 2.52 ± 0.01 ef | 102.7 ± 1.67 g |
| SC2036 | 224.0 ± 0.85 g | 16.05 ± 0.38 a | 34.7 ± 0.71 a | 321.6 ± 2.6 h | 2.59 ± 0.01 def | 109.5 ± 1.35 e | |
| SC168 | 230.1 ± 0.90 e | 16.87 ± 0.32 a | 35.0 ± 0.48 a | 366.3 ± 3.8 f | 2.43 ± 0.03 f | 98.2 ± 0.84 i | |
| 120 kg ha−1 | SC2031 | 238.8 ± 1.25 b | 17.88 ± 0.25 a | 34.5 ± 0.45 a | 460.9 ± 6.2 d | 2.70 ± 0.02 cd | 111.8 ± 0.91 d |
| SC2036 | 242.1 ± 1.28 a | 18.87 ± 0.28 a | 34.1 ± 0.47 a | 548.2 ± 8.4 a | 2.79 ± 0.02 bc | 115.7 ± 1.03 c | |
| SC168 | 195.8 ± 1.08 i | 13.82 ± 0.32 a | 37.2 ± 0.79 a | 285.8 ± 4.8 j | 2.62 ± 0.02 de | 104.9 ± 1.06 f | |
| 180 kg ha−1 | SC2031 | 201.2 ± 0.98 k | 14.88 ± 0.31 a | 36.7 ± 0.70 a | 325.1 ± 8.8 h | 2.87 ± 0.19 b | 118.1 ± 1.17 b |
| SC2036 | 209.0 ± 1.59 j | 15.89 ± 0.18 a | 36.3 ± 0.39 a | 361.1 ± 9.8 f | 3.30 ± 0.02 a | 121.2 ± 0.95 a | |
| SC168 | 215.2 ± 1.22 i | 16.83 ± 0.30 a | 36.0 ± 0.60 a | 479.3 ± 8.9 c | 2.62 ± 0.09 de | 116.0 ± 0.75 c | |
| Variable | Ear Diameter (cm) | Ear Length (cm) | Grain Yield (g plant−1) | 1000-Grain Weight (g) | Shelling Percentage (%) | Grain Yield (t ha−1) | |
|---|---|---|---|---|---|---|---|
| K2SO4 fertilizer | |||||||
| 0 kg ha−1 | 4.02 ± 0.09 d | 17.50 ± 0.13 d | 168.6 ± 1.21 d | 328 ± 0.23 d | 70.9 ± 0.46 b | 6.57 ± 0.08 d | |
| 60 kg ha−1 | 4.64 ± 0.11 c | 18.29 ± 0.17 c | 179.2 ± 2.00 c | 348 ± 0.43 c | 71.6 ± 0.28 a | 7.03 ± 0.09 c | |
| 120 kg ha−1 | 4.99 ± 0.12 b | 19.13 ± 0.21 b | 184.1 ± 1.80 b | 362 ± 0.44 b | 71.1 ± 0.45 b | 7.49 ± 0.10 b | |
| 180 kg ha−1 | 5.23 ± 0.12 a | 20.02 ± 0.25 a | 188.7 ± 1.79 a | 375 ± 0.50 a | 70.3 ± 0.39 c | 7.86 ± 0.12 a | |
| Single cross | |||||||
| SC2031 | 4.86 ± 0.11 b | 18.65 ± 0.22 b | 181.4 ± 1.92 b | 357 ± 0.42 b | 71.1 ± 0.15 b | 7.27 ± 0.13 b | |
| SC2036 | 5.10 ± 0.13 a | 19.53 ± 0.26 a | 185.9 ± 2.12 a | 370 ± 0.47 a | 72.6 ± 0.22 a | 7.63 ± 0.11 a | |
| SC168 | 4.20 ± 0.09 c | 18.03 ± 0.18 c | 173.2 ± 1.44 c | 332 ± 0.26 c | 69.2 ± 0.25 c | 6.82 ± 0.11 c | |
| Interaction | |||||||
| 0 kg ha−1 | SC2031 | 4.08 ± 0.09 i | 18.17 ± 0.19 e | 181.7 ± 1.25 e | 350 ± 0.19 e | 70.5 ± 0.15 d | 6.95 ± 0.08 g |
| SC2036 | 4.23 ± 0.14 h | 19.02 ± 0.17 c | 186.4 ± 1.85 d | 370 ± 0.18 c | 71.9 ± 0.24 bc | 7.68 ± 0.08 d | |
| SC168 | 3.74 ± 0.15 j | 17.42 ± 0.12 g | 167.6 ± 1.57 j | 330 ± 0.29 i | 71.5 ± 0.11 c | 6.51 ± 0.10 j | |
| 60 kg ha−1 | SC2031 | 4.79 ± 0.02 e | 18.97 ± 0.17 c | 186.0 ± 2.02 d | 337 ± 0.21 h | 70.7 ± 0.26 d | 7.49 ± 0.06 e |
| SC2036 | 5.09 ± 0.07 d | 20.02 ± 0.20 b | 189.9 ± 1.64 c | 379 ± 0.31 b | 69.0 ± 0.25 e | 7.93 ± 0.11 b | |
| SC168 | 4.05 ± 0.12 i | 18.07 ± 0.15 e | 171.3 ± 2.30 h | 368 ± 0.24 d | 70.7 ± 0.51 d | 6.91 ± 0.10 h | |
| 120 kg ha−1 | SC2031 | 5.15 ± 0.08 c | 20.00 ± 0.28 b | 190.5 ± 1.21 b | 379 ± 0.30 b | 68.7 ± 0.24 e | 7.83 ± 0.04 c |
| SC2036 | 5.42 ± 0.13 b | 21.08 ± 0.31 a | 196.0 ± 2.06 a | 397 ± 0.17 a | 68.3 ± 0.24 f | 8.28 ± 0.05 a | |
| SC168 | 4.39 ± 0.06 g | 17.02 ± 0.11 h | 166.8 ± 2.21 k | 317 ± 0.18 k | 73.4 ± 0.31 a | 6.30 ± 0.10 k | |
| 180 kg ha−1 | SC2031 | 5.43 ± 0.15 b | 18.37 ± 0.25 d | 175.4 ± 1.96 g | 338 ± 0.15 g | 72.2 ± 0.49 b | 6.97 ± 0.14 g |
| SC2036 | 5.64 ± 0.13 a | 18.97 ± 0.17 c | 180.3 ± 0.76 f | 348 ± 0.18 f | 73.0 ± 0.26 a | 7.36 ± 0.22 f | |
| SC168 | 4.63 ± 0.06 f | 17.75 ± 0.25 f | 170.0 ± 2.74 i | 326 ± 0.20 j | 71.8 ± 0.36 bc | 6.64 ± 0.08 i | |
| Variable | Protein (%) | Oil (%) | Protein Yield (kg ha−1) | Oil Yield (kg ha−1) | |
|---|---|---|---|---|---|
| K2SO4 fertilizer | |||||
| 0 kg ha−1 | 11.10 ± 0.12 d | 4.49 ± 0.11 d | 731.0 ± 16.1 d | 296.3 ± 10.2 d | |
| 60 kg ha−1 | 11.36 ± 0.13 c | 4.91 ± 0.05 c | 799.5 ± 18.5 c | 345.8 ± 7.8 c | |
| 120 kg ha−1 | 11.94 ± 0.22 b | 5.16 ± 0.09 b | 896.8 ± 24.7 b | 387.2 ± 10.3 b | |
| 180kg ha−1 | 12.43 ± 0.21 a | 5.33 ± 0.11 a | 979.4 ± 28.7 a | 419.8 ± 13.6 a | |
| Single cross | |||||
| SC2031 | 11.41 ± 0.07 b | 4.81 ± 0.07 b | 808.3 ± 21.2 b | 338.2 ± 10.4 b | |
| SC2036 | 12.63 ± 0.17 a | 5.48 ± 0.07 a | 967.0 ± 25.9 a | 419.4 ± 11.3 a | |
| SC168 | 11.08 ± 0.11 c | 4.63 ± 0.07 c | 779.6 ± 16.8 c | 329.4 ± 9.1 c | |
| Interaction | |||||
| 0 kg ha−1 | SC2031 | 10.75 ± 0.02 j | 4.74 ± 0.02 g | 747.1 ± 9.42 h | 329.7 ± 4.60 g |
| SC2036 | 11.10 ± 0.04 h | 4.79 ± 0.02 g | 851.9 ± 12.0 f | 368.0 ± 5.57 e | |
| SC168 | 10.60 ± 0.01 k | 4.06 ± 0.01 i | 690.0 ± 10.8 i | 264.2 ± 3.83 j | |
| 60 kg ha−1 | SC2031 | 11.90 ± 0.05 d | 5.12 ± 0.02 d | 902.7 ± 9.6 d | 388.8 ± 4.08 c |
| SC2036 | 12.05 ± 0.04 c | 5.19 ± 0.01 c | 944.1 ± 17.7 c | 390.6 ± 10.00 c | |
| SC168 | 11.73 ± 0.09 e | 4.92 ± 0.05 f | 810.5 ± 17.2 g | 353.6 ± 5.99 f | |
| 120 kg ha−1 | SC2031 | 13.17 ± 0.08 b | 5.66 ± 0.02 b | 1031.2 ± 10.2 b | 443.2 ± 3.64 b |
| SC2036 | 13.57 ± 0.20 a | 5.94 ± 0.06 a | 1123.7 ± 20.6 a | 491.9 ± 7.00 a | |
| SC168 | 10.98 ± 0.11 i | 4.30 ± 0.02 h | 692.4 ± 18.1 i | 271.2 ± 5.95 i | |
| 180 kg ha−1 | SC2031 | 11.57 ± 0.05 f | 5.02 ± 0.03 e | 807.2 ± 18.8 g | 350.5 ± 8.14 f |
| SC2036 | 11.81 ± 0.07 de | 5.12 ± 0.02 d | 870.3 ± 29.3 e | 377.0 ± 12.2 d | |
| SC168 | 11.27 ± 0.04 g | 4.80 ± 0.01 g | 748.6 ± 10.9 h | 319.0 ± 4.40 h | |
| Effect | SC2031 | SC2036 | SC168 | |
|---|---|---|---|---|
| 1—Leaf number and grain yield/plant | r17 = | 0.94 | 0.944 | 0.992 |
| Direct effect | p17 = | 1.373 | 0.453 | 0.799 |
| Indirect effect via flag leaf area | r12p27 = | −0.169 | −0.47 | −0.654 |
| Indirect effect via chlorophyll | r13p37 = | −0.268 | 0.822 | 0.006 |
| Indirect effect via ear diameter | r14p47 = | 0.944 | 0.347 | 0.624 |
| Indirect effect via ear length | r15p57 = | −0.948 | −0.126 | −0.294 |
| Indirect effect via 1000-grain weight | r16p67 = | 0.007 | −0.082 | 0.512 |
| 2—Flag leaf area and grain yield/plant | r27 = | −0.883 | −0.616 | −0.964 |
| Direct effect | p27 = | 0.173 | 0.56 | 0.661 |
| Indirect effect via leaf number | r12p17 = | −1.336 | −0.38 | −0.79 |
| Indirect effect via chlorophyll | r23p37 = | 0.266 | −0.734 | −0.006 |
| Indirect effect via ear diameter | r24p47 = | −0.901 | −0.224 | −0.619 |
| Indirect effect via ear length | r25p57 = | 0.921 | 0.105 | 0.292 |
| Indirect effect via 1000-grain weight | r26p67= | −0.007 | 0.057 | −0.502 |
| 3—Chlorophyll and grain yield/plant | r37 = | 0.85 | 0.908 | 0.963 |
| Direct effect | p37 = | −0.273 | 0.826 | 0.007 |
| Indirect effect via leaf number | r13p17 = | 1.344 | 0.45 | 0.755 |
| Indirect effect via flag leaf area | r23p27 = | −0.169 | −0.497 | −0.589 |
| Indirect effect via ear diameter | r34p47 = | 0.879 | 0.333 | 0.581 |
| Indirect effect via ear length | r35p57 = | −0.937 | −0.125 | −0.277 |
| Indirect effect via 1000-grain weight | r36p67 = | 0.006 | −0.079 | 0.487 |
| 4—Ear diameter and grain yield/plant | r47 = | 0.993 | 0.997 | 0.991 |
| Direct effect | p47 = | 0.97 | 0.371 | 0.624 |
| Indirect effect via leaf number | r14p17 = | 1.336 | 0.424 | 0.798 |
| Indirect effect via flag leaf area | r24p27 = | −0.161 | −0.338 | −0.655 |
| Indirect effect via chlorophyll | r34p37 = | −0.248 | 0.742 | 0.006 |
| Indirect effect via ear length | r45p57 = | −0.912 | −0.118 | −0.294 |
| Indirect effect via 1000-grain weight | r46p67 = | 0.007 | −0.083 | 0.512 |
| 5—Ear length cm and grain yield/plant | r57 = | 0.921 | 0.947 | 0.989 |
| Direct effect | p57 = | −0.95 | −0.126 | −0.294 |
| Indirect effect via leaf number | r15p17 = | 1.37 | 0.453 | 0.799 |
| Indirect effect via flag leaf area | r25p27= | −0.168 | −0.467 | −0.655 |
| Indirect effect via chlorophyll | r35p37 = | −0.27 | 0.822 | 0.006 |
| Indirect effect via ear diameter | r45p47 = | 0.932 | 0.347 | 0.623 |
| Indirect effect via 1000-grain weight | r56p67 = | 0.007 | −0.082 | 0.511 |
| 6—1000-grain weight and grain yield/plant | r67 = | 0.966 | 0.995 | 0.998 |
| Direct effect | p67 = | 0.007 | −0.084 | 0.513 |
| Indirect effect via leaf number | r16p17 = | 1.354 | 0.438 | 0.796 |
| Indirect effect via flag leaf area | r26p27 = | −0.169 | −0.378 | −0.648 |
| Indirect effect via chlorophyll | r36p37 = | −0.258 | 0.776 | 0.006 |
| Indirect effect via ear diameter | r46p47 = | 0.959 | 0.366 | 0.623 |
| Indirect effect via ear length | r56p57 = | −0.927 | −0.122 | −0.293 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, A.A.; Allam, M.; Radicetti, E.; Mancinelli, R.; Bakheit, B.R. Enhancing Hybrid Maize Performance and Yield Through Potassium Sulfate Fertilization: A Field-Based Assessment. Nitrogen 2025, 6, 104. https://doi.org/10.3390/nitrogen6040104
Mohamed AA, Allam M, Radicetti E, Mancinelli R, Bakheit BR. Enhancing Hybrid Maize Performance and Yield Through Potassium Sulfate Fertilization: A Field-Based Assessment. Nitrogen. 2025; 6(4):104. https://doi.org/10.3390/nitrogen6040104
Chicago/Turabian StyleMohamed, Asmaa A., Mohamed Allam, Emanuele Radicetti, Roberto Mancinelli, and Bahy R. Bakheit. 2025. "Enhancing Hybrid Maize Performance and Yield Through Potassium Sulfate Fertilization: A Field-Based Assessment" Nitrogen 6, no. 4: 104. https://doi.org/10.3390/nitrogen6040104
APA StyleMohamed, A. A., Allam, M., Radicetti, E., Mancinelli, R., & Bakheit, B. R. (2025). Enhancing Hybrid Maize Performance and Yield Through Potassium Sulfate Fertilization: A Field-Based Assessment. Nitrogen, 6(4), 104. https://doi.org/10.3390/nitrogen6040104








