Controlled-Release Urea–Hydroxyapatite Nanohybrid for Foliar Nitrogen and Phosphorus Delivery Enhances Biomass and Grain Yield in Wheat (Triticum aestivum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Urea–Hydroxyapatite Nanohybrid (n-UHA)
2.2. Structural and Morphological Characterization
2.2.1. Scanning Electron Microscopy (SEM)
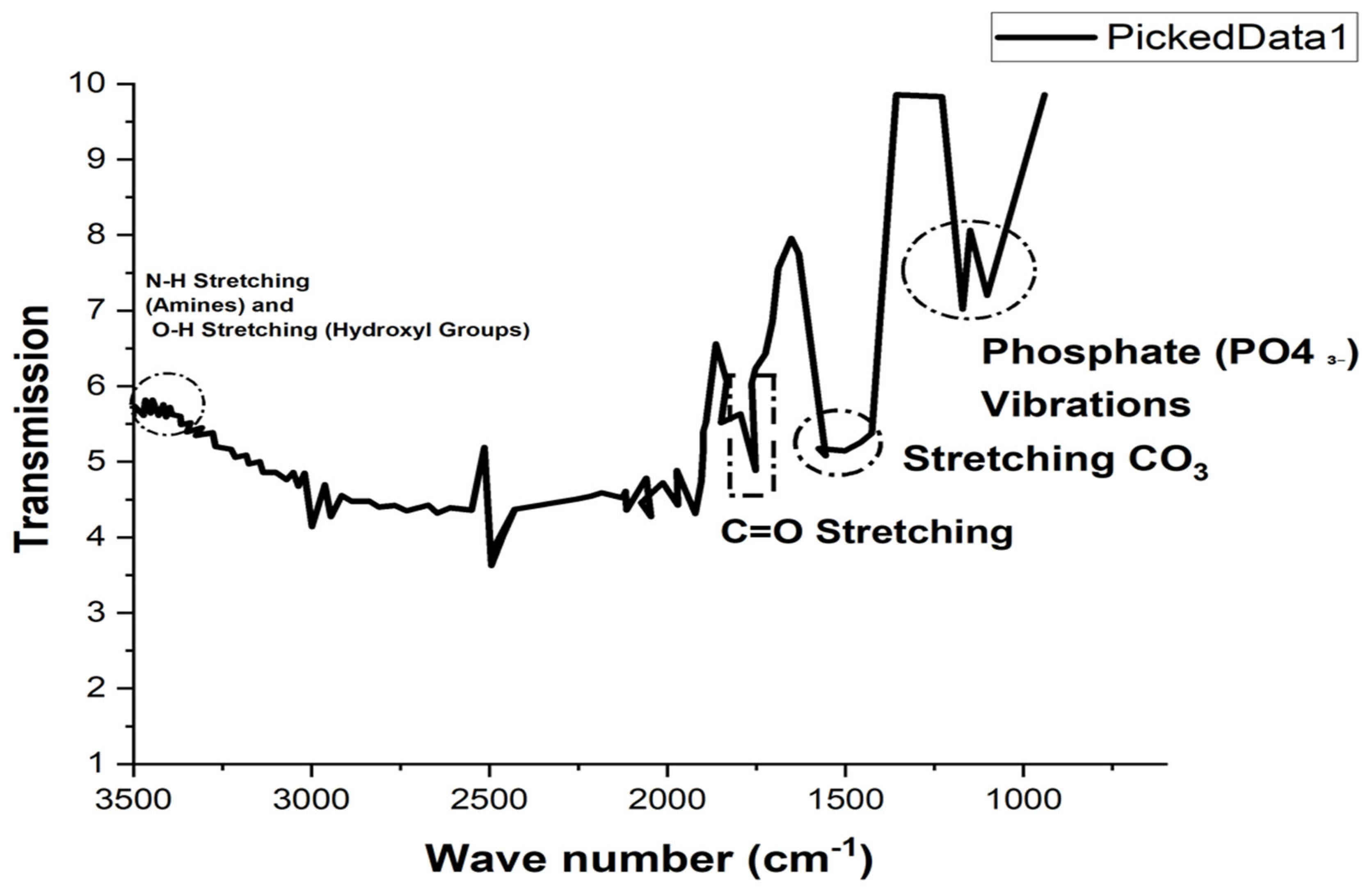
2.2.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.3. Experimental Design and Greenhouse Setup
2.4. Agronomic Management and Growth Conditions
2.5. Plant Sampling and Harvest
2.6. Protein and Spectral Analysis
2.7. Soil Sampling and Soil FC
2.8. Statistical Analysis
3. Results
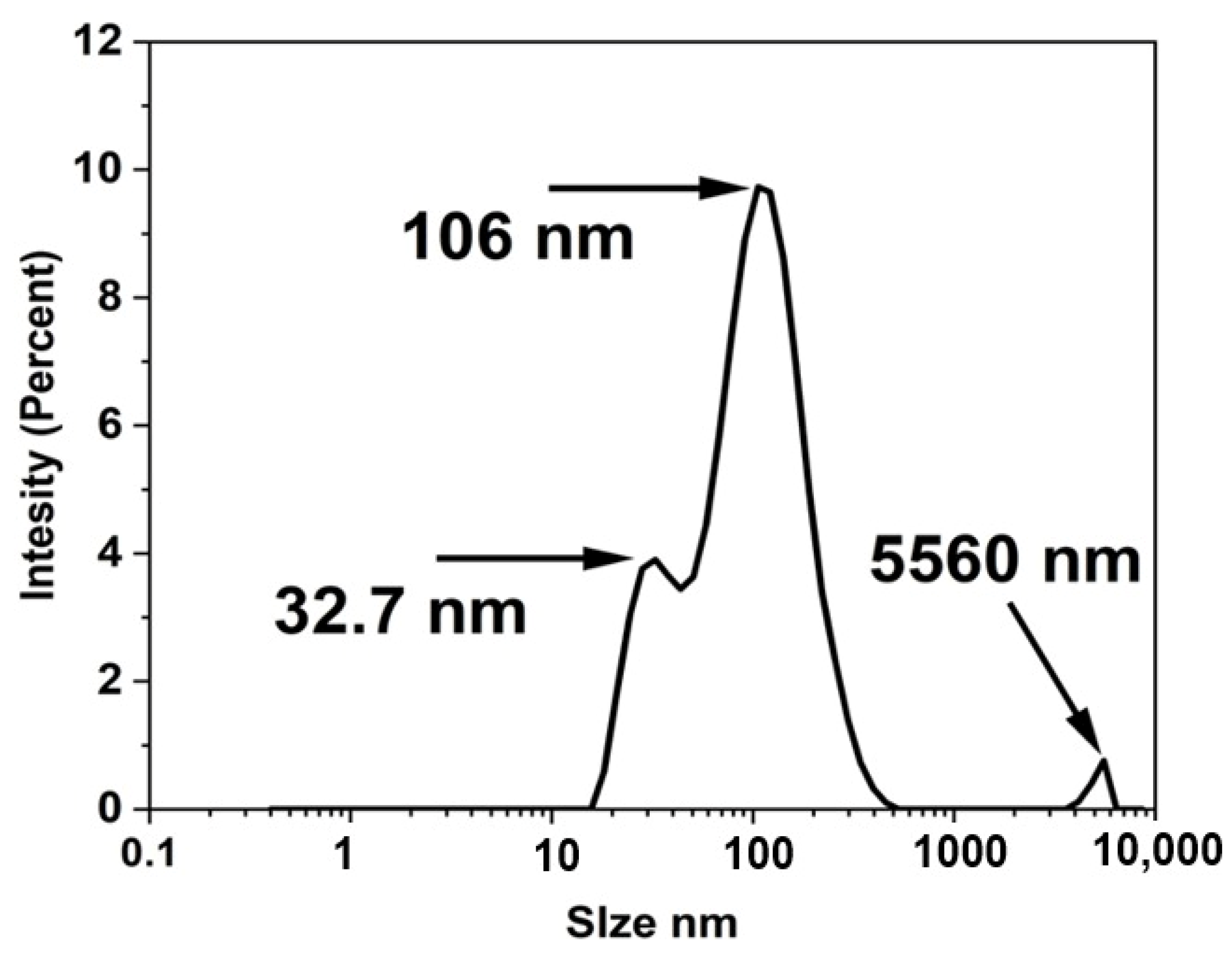
3.1. Physicochemical Characterization of n-UHA
3.2. Field Experiment
3.2.1. Initial Soil Test
3.2.2. Spike Length
3.2.3. Tiller Intensity and Plant Height
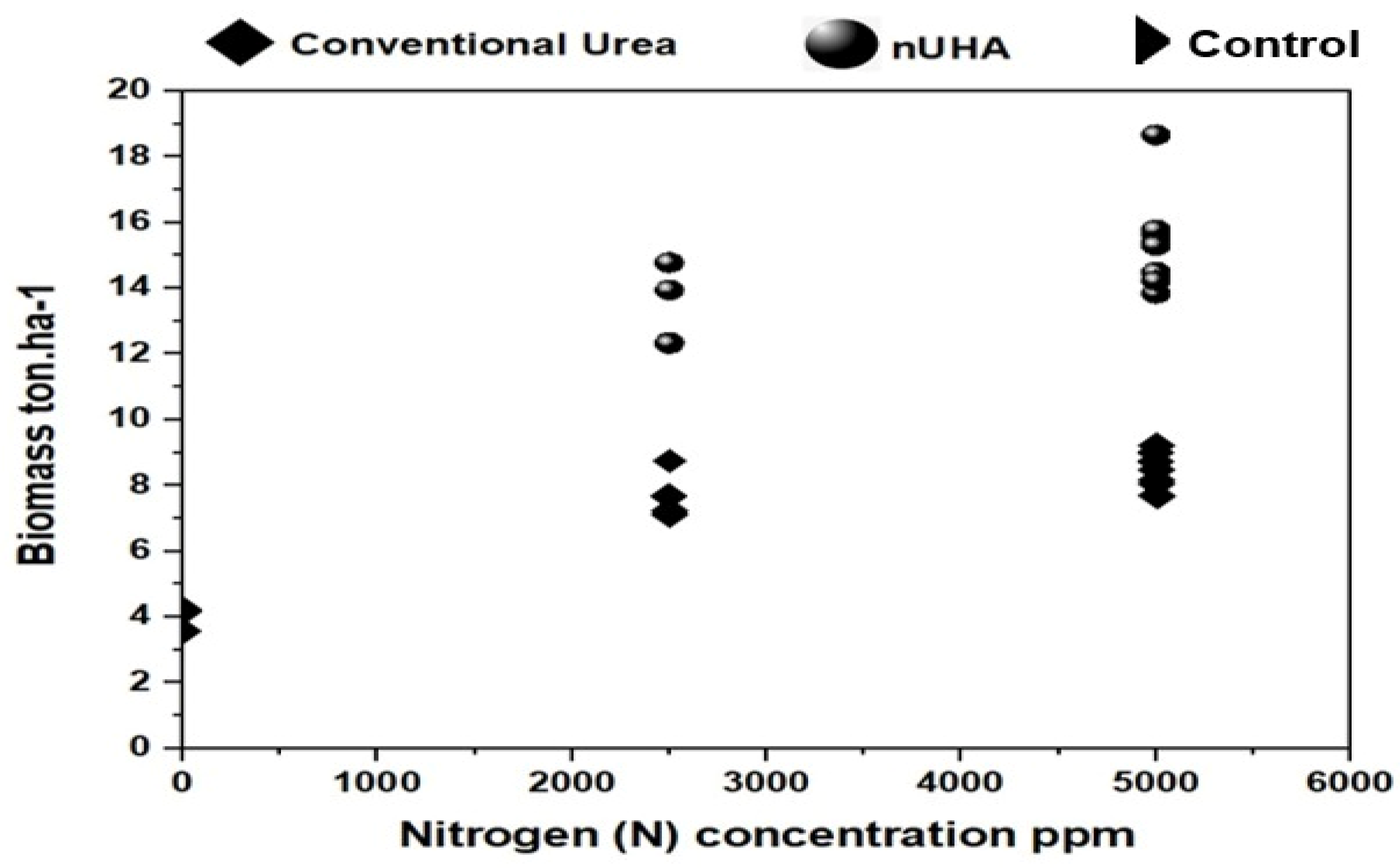
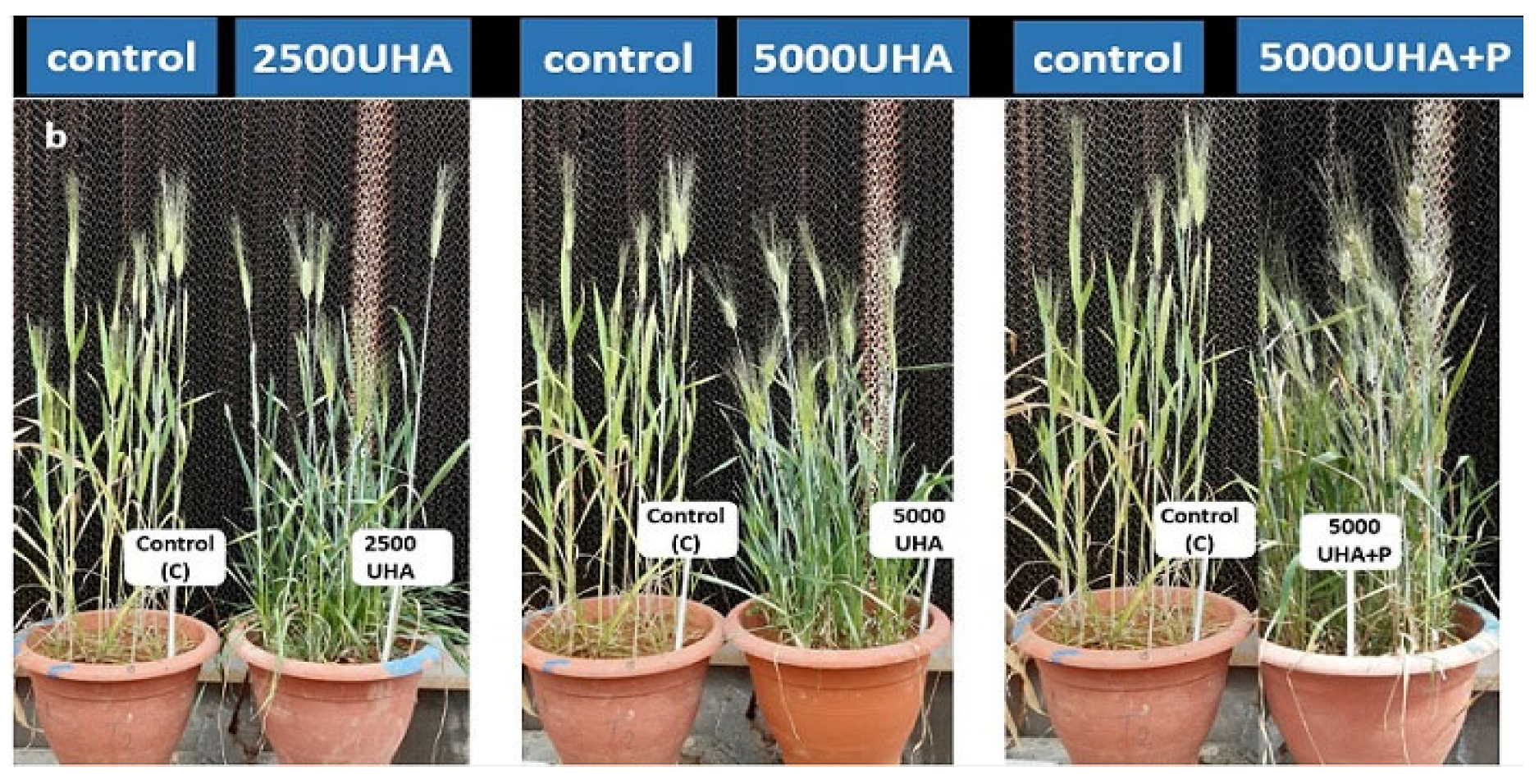
3.2.4. Crop Yield
3.2.5. Protein Content and Chlorophyll Absorbance
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jakhar, A.M.; Aziz, I.; Kaleri, A.R.; Hasnain, M.; Haider, G.; Ma, J.; Abideen, Z. Nano-fertilizers: A sustainable technology for improving crop nutrition and food security. NanoImpact 2022, 27, 100411. [Google Scholar] [CrossRef] [PubMed]
- Easwaran, C.; Moorthy, G.; Christopher, S.R.; Prasanthrajan, M.; Marimuthu, R.; Koothan, V.; Nallusamy, S. Nano hybrid fertilizers: A review on the state of the art in sustainable agriculture. Sci. Total Environ. 2024, 929, 172533. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Yadav, K.; Abd-Elsalam, K.A. Exploring the potential of nanofertilizers for a sustainable agriculture. Plant Nano Biol. 2023, 5, 100044. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, W.; Zhu, G.; Tan, Z.; Huang, L.; Zhang, P.; Gao, L.; Rui, Y. Nano-Pesticides and Fertilizers: Solutions for Global Food Security. Nanomaterials 2024, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamun, M.R.; Hasan, M.R.; Ahommed, M.S.; Khan, Z.H. Nanofertilizers towards sustainable agriculture and environment. Environ. Technol. Innov. 2021, 23, 101658. [Google Scholar] [CrossRef]
- Farooq, T.; Nisa, Z.U.; Hameed, A.; Ahmed, T.; Hameed, A. Priming with copper-chitosan nanoparticles elicit tolerance against PEG-induced hyperosmotic stress and salinity in wheat. BMC Chem. 2022, 16, 23. [Google Scholar] [CrossRef]
- Nongbet, A.; Mishra, A.K.; Mohanta, Y.K.; Mahanta, S.; Ray, M.K.; Khan, M.; Baek, K.-H.; Chakrabartty, I. Nanofertilizers: A smart and sustainable attribute to modern agriculture. Plants 2022, 11, 2587. [Google Scholar] [CrossRef]
- Gade, A.; Ingle, P.; Nimbalkar, U.; Rai, M.; Raut, R.; Vedpathak, M.; Jagtap, P.; Abd-Elsala, A. Nanofertilizers: The next generation of agrochemicals for long-term impact on sustainability in farming systems. Agrochem. 2023, 2, 257–278. [Google Scholar] [CrossRef]
- Abhiram, G. Contributions of Nano-Nitrogen Fertilizers to Sustainable Development Goals: A Comprehensive Review. Nitrogen 2023, 4, 397–415. [Google Scholar] [CrossRef]
- Guha, T.; Gopal, G.; Mukherjee, A.; Kundu, R. Fe3O4-urea nanocomposites as a novel nitrogen fertilizer for improving nutrient utilization efficiency and reducing environmental pollution. Environ. Pollut. 2022, 292 Pt A, 118301. [Google Scholar] [CrossRef]
- Al-Bahri, G.; Alyamani, A.A.; Badran, A.; Hijazi, A.; Nasser, M.; Maresca, M.; Baydoun, E. Enhancing essential grains yield for sustainable food security and bio-safe agriculture through latest innovative approaches. Agronomy 2023, 13, 1709. [Google Scholar] [CrossRef]
- Yu, X.; Keitel, C.; Zhang, Y.; Wangeci, A.N.; Dijkstra, F.A. Global meta-analysis of nitrogen fertilizer use efficiency in rice, wheat and maize. Agric. Ecosyst. Environ. 2022, 338, 108089. [Google Scholar] [CrossRef]
- Borlaug, N.E. The Green Revolution Revisited and the Road Ahead; NobelPrize.org: Stockholm, Sweden, 2002. [Google Scholar]
- Follett, R.F. Innovative 15N microplot research techniques to study nitrogen use efficiency under different ecosystems. Commun. Soil Sci. Plant Anal. 2001, 32, 951–979. [Google Scholar] [CrossRef]
- Cordell, D.; White, S. Tracking phosphorus security: Indicators of phosphorus vulnerability in the global food system. Food Secur. 2015, 7, 337–350. [Google Scholar] [CrossRef]
- Helfenstein, J.; Tamburini, F.; von Sperber, C.; Massey, M.S.; Pistocchi, C.; Chadwick, O.A.; Vitousek, P.M.; Kretzschmar, R.; Frossard, E. Combining spectroscopic and isotopic techniques gives a dynamic view of phosphorus cycling in soil. Nat. Commun. 2018, 9, 3226. [Google Scholar] [CrossRef] [PubMed]
- Aliyat, F.Z.; Maldani, M.; El Guilli, M.; Nassiri, L.; Ibijbijen, J. Isolation and characterization of phosphate solubilizing bacteria from phosphate solid sludge of the Moroccan phosphate mines. Open Agric. J. 2020, 14, 37–50. [Google Scholar] [CrossRef]
- El-Hamshary, O.I.; Bohkari, F.M.; Al-Aklouk, L.A.; Noor, S.O.; Najjar, A.A. Molecular Characterization of some phosphate solubilizing microorganisms. Pharmacophore 2019, 10, 37–51. [Google Scholar] [CrossRef]
- Szameitat, A.E.; Sharma, A.; Minutello, F.; Pinna, A.; Er-Rafik, M.; Hansen, T.H.; Persson, D.P.; Andersen, B.; Husted, S. Nanomaterials as fertilizers for improving plant mineral nutrition and environmental outcomes. Environ. Sci. Nano 2019, 6, 3513–3524. [Google Scholar] [CrossRef]
- Follett, F.; Shuford, J.W.; Taylor, R.W. Effect of sludge treatment, heavy metals, phosphate rate, and pH on soil phosphorus. Commun. Soil Sci. Plant Anal. 1995, 26, 1369–1381. [Google Scholar] [CrossRef]
- Mazrou, Y.S.; Makhlouf, A.H.; Elbealy, E.R.; Salem, M.A.; Farid, M.A.; Awad, M.F.; Hassan, M.M.; Ismail, M. Molecular characterization of phosphate solubilizing fungi Aspergillus niger and its correlation to sustainable agriculture. J. Environ. Biol. 2020, 41, 592–599. [Google Scholar] [CrossRef]
- Soumare, A.; Boubekri, K.; Lyamlouli, K.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L. From isolation of phosphate solubilizing microbes to their formulation and use as biofertilizers: Status and needs. Front. Bioeng. Biotechnol. 2020, 7, 425. [Google Scholar] [CrossRef]
- Walpola, B.; Hettiarachchi, R. Organic manure amended with phosphate solubilizing bacteria on soil phosphorus availability. J. Agric. Sci. 2020, 15, 142–153. [Google Scholar] [CrossRef]
- Solangi, F.; Zhu, X.; Khan, S.; Rais, N.; Majeed, A.; Sabir, M.A.; Iqbal, R.; Ali, S.; Hafeez, A.; Ali, B.; et al. The global dilemma of soil legacy phosphorus and its improvement strategies under recent changes in agro-ecosystem sustainability. ACS Omega 2023, 8, 23271–23282. [Google Scholar] [CrossRef]
- Arsic, M.; Le Tougaard, S.; Persson, D.P.; Martens, H.J.; Doolette, C.L.; Lombi, E.; Schjoerring, J.K.; Husted, S. Bioimaging techniques reveal foliar phosphate uptake pathways and leaf phosphorus status. Plant Physiol. 2020, 183, 1472–1483. [Google Scholar] [CrossRef]
- Fernández, V.; Guzmán, P.; Peirce, C.A.; McLaughlin, M.J. Effect of wheat phosphorus status on leaf surface properties and permeability to foliar-applied phosphorus. Plant Soil 2014, 384, 7–20. [Google Scholar] [CrossRef]
- Mosali, J.; Desta, K.; Teal, R.K.; Freeman, K.W.; Martin, K.L.; Lawles, J.W.; Raun, W.R. Effect of foliar application of phosphorus on winter wheat grain yield, phosphorus uptake, and use efficiency. J. Plant Nutr. 2006, 29, 2147–2163. [Google Scholar] [CrossRef]
- Görlach, B.M.; Henningsen, J.N.; Mackens, J.T.; Mühling, K.H. Evaluation of maize growth following early season foliar P supply of various fertilizer formulations and in relation to nutritional status. Agronomy 2021, 11, 727. [Google Scholar] [CrossRef]
- Girma, K.; Martin, K.L.; Freeman, K.W.; Mosali, J.; Teal, R.K.; Raun, W.R.; Moges, S.M.; Arnall, D.B. Determination of optimum rate and growth stage for foliar-applied phosphorus in corn. Commun. Soil Sci. Plant Anal. 2007, 38, 1137–1154. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, Z.-H.; Zhang, W.-W.; Chen, Y.-H.; Zhu, P.; Peng, C.; Wang, L.; Zhang, S.-X.; Colinet, G. Effect of long-term fertilization on phosphorus fractions in different soil layers and their quantitative relationships with soil properties. J. Integr. Agric. 2022, 21, 2720–2733. [Google Scholar] [CrossRef]
- Ulén, B. A simplified risk assessment for losses of dissolved reactive phosphorus through drainage pipes from agricultural soils. Acta Agric. Scand. Sect. B Soil Plant Sci. 2006, 56, 307–314. [Google Scholar] [CrossRef]
- Fellet, G.; Pilotto, L.; Marchiol, L.; Braidot, E. Tools for nano-enabled agriculture: Fertilizers based on calcium phosphate, silicon, and chitosan nanostructures. Agronomy 2021, 11, 1239. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, X.; Wang, D.; Lu, W.; Ou, Y.; Han, Y.; Zhou, K.; Liu, H.; Fen, W.; Peng, L.; et al. Experimental study on the conduction function of nano-hydroxyapatite artificial bone. Micro Nano Lett. 2010, 5, 19–23. [Google Scholar] [CrossRef]
- Ferraz, M.; Mateus, A.; Sousa, J.; Monteiro, F.J. Nanohydroxyapatite microspheres as delivery system for antibiotics: Release kinetics, antimicrobial activity, and interaction with osteoblasts. J. Biomed. Mater. Res. Part A 2007, 81, 994–1004. [Google Scholar] [CrossRef]
- Han, W.W.T.; Misra, R.D.K. Biomimetic chitosan–nanohydroxyapatite composite scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 1182–1197. [Google Scholar] [CrossRef]
- Mateus, A.Y.P.; Barrias, C.C.; Ribeiro, C.; Ferraz, M.P.; Monteiro, F.J. Comparative study of nanohydroxyapatite microspheres for medical applications. J. Biomed. Mater. Res. Part A 2008, 86, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.H.; Lee, E.J.; Wang, P.; Jun, S.H.; Han, C.M.; Kim, H.E. Functionally gradient chitosan/hydroxyapatite composite scaffolds for controlled drug release. J. Biomed. Mater. Res. B 2009, 90, 275–282. [Google Scholar] [CrossRef]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-based sustainable agriculture and food science: Recent advances and future outlook. Front. Nanotechnol. 2020, 2, 579954. [Google Scholar] [CrossRef]
- Picchi, V.; Gobbi, S.; Fattizzo, M.; Zefelippo, M.; Faoro, F. Chitosan nanoparticles loaded with N-acetyl cysteine to mitigate ozone and other oxidative stresses in durum wheat. Plants 2021, 10, 691. [Google Scholar] [CrossRef]
- Ullah, R.; Sheri, S.; Mohammad, Z.; Afriq Jan, S.; Nafeez, M. Modulating response of sunflower (Helianthus annuus) to induced salinity stress through application of engineered urea functionalized hydroxyapatite nanoparticles. Microsc. Res. Tech. 2022, 85, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Kottegoda, N.; Sandaruwan, C.; Priyadarshana, G.; Siriwardhana, A.; Rathnayake, U.A.; Berugoda Arachchige, D.M.; Kumarasinghe, A.R.; Dahanayake, D.; Karunaratne, V.; Amaratunga, G.A. Urea-hydroxyapatite nanohybrids for slow release of nitrogen. ACS Nano 2017, 11, 1214–1221. [Google Scholar] [CrossRef]
- Gooding, M.; Davies, W. Foliar urea fertilization of cereals: A review. Fertil. Res. 1992, 32, 209–222. [Google Scholar] [CrossRef]
- International Potash Institute (IPI). Role of Potassium Nutrition in Nitrogen Use Efficiency in Cereals; e-ifc Res. Findings; International Potash Institute (IPI): Basel, Switzerland, 2011. [Google Scholar]
- Duncan, E.G.; O’Sullivan, C.A.; Roper, M.M.; Biggs, J.S.; Peoples, M.B. Influence of co-application of nitrogen with phosphorus, potassium and sulphur on the apparent efficiency of nitrogen fertilizer use, grain yield and protein content of wheat: Review. Field Crops Res. 2018, 226, 56–65. [Google Scholar] [CrossRef]
- Pohshna, C.; Mailapalli, D.R. Engineered urea-doped hydroxyapatite nanomaterials as nitrogen and phosphorus fertilizers for rice. ACS Agric. Sci. Technol. 2022, 2, 100–112. [Google Scholar] [CrossRef]
- Raguraj, S.; Wijayathunga, W.M.S.; Gunaratne, G.P.; Amali, R.K.A.; Priyadarshana, G.; Sandaruwan, C.; Karunaratne, V.; Hettiarachchi, L.S.K.; Kottegoda, N. Urea–hydroxyapatite nanohybrid as an efficient nutrient source in Camellia sinensis (tea). J. Plant Nutr. 2020, 43, 2383–2394. [Google Scholar] [CrossRef]
- Szameitat, A.E.; Sharma, A.; Minutello, F.; Pinna, A.; Er-Rafik, M.; Hansen, T.H.; Husted, S. Unravelling the interactions between nanohydroxyapatite and the roots of phosphorus deficient barley plants. Environ. Sci. Nano 2021, 8, 444–459. [Google Scholar] [CrossRef]
- Tariq, A.; Zeng, F.; Graciano, C.; Ullah, A.; Sadia, S.; Ahmed, Z.; Murtaza, G.; Ismoilov, K.; Zhang, Z. Regulation of Metabolites by Nutrients in Plants. In Plant Ionomics; Wiley: Hoboken, NJ, USA, 2023; pp. 1–18. [Google Scholar] [CrossRef]



| Treatment | Nitrogen Concentration | Fertilizer Type | Phosphorus Addition Per Pot | Application Time of Phosphorus |
|---|---|---|---|---|
| T1 | None | Control (no fertilizer) | None | None |
| T2 | 2500 ppm N | Conventional Urea | None | - |
| T3 | 5000 ppm N | Conventional Urea | None | - |
| T4 | 5000 ppm N | Conventional Urea | 0.361 g P (1.2 P2O5) | Split at 5–6 leaf and tillering |
| T5 | 2500 ppm N | Nano-Urea Hydroxyapatite | None | - |
| T6 | 5000 ppm N | Nano-Urea Hydroxyapatite | None | - |
| T7 | 5000 ppm N | Nano-Urea Hydroxyapatite | 0.361 g P | Split at 5–6 leaf and tillering |
| Elments | Weight Ratio (%) |
|---|---|
| N | 37.80 |
| Ca | 18.80 |
| P | 19.50 |
| Sample | pH (Water) | EC (dS/m) | N(Kjeldal) (%) | P Olsen (ppm) | K-Exchangeable (ppm) | Clay (%) | Silt (%) | Sand (%) | Soil Texture |
|---|---|---|---|---|---|---|---|---|---|
| Sample 1 | 8.3 | 2.00 | 0.07 | 2 | 408 | 59.12 | 38.35 | 2.54 | Clayey |
| Sample 2 | 8.2 | 1.85 | 0.08 | 3 | 395 | 57.21 | 40.44 | 2.35 | Clayey |
| Application Method | 0 ppm (±SE) | 2500 ppm N-Urea (±SE) | 5000 ppm N-Urea (±SE) | 5000 ppm N-Urea + * Phosphorus (±SE) | Mean |
|---|---|---|---|---|---|
| ** Conventional Urea (46% N) | 57.47 ± 1.84 e | 65.64 ± 1.45 d | 69.36 ± 1.24 c | 64.74 ± 1.25 d | 64.30 |
| ** n-UHA (37% N) | 57.47 ± 1.84 e | 72.47 ± 0.99 bc | 74.05 ±1.28 b | 78.24 ± 0.94 a | 70.56 |
| Mean | 57.47 | 69.06 | 71.49 | 71.49 |
| Application Method | 0 ppm (±SE) | 2500 ppm N-Urea (±SE) | 5000 ppm N-Urea (±SE) | 5000 ppm N-Urea + ** Phosphorus (±SE) | Mean |
|---|---|---|---|---|---|
| *** Conventional Urea (46% N) | 123.5 ± 14.57 e | 227.5 ± 19.2 d | 240.5 ± 17.4 d | 243.75 ± 18.3 d | 208.68 |
| *** n-UHA (37% N) | 123.5 ± 14.57 e | 542.75 ± 21.3 c | 715.00 ± 19.4 b | 786.5 ± 22.8 a | 541.91 |
| Mean | 123.5 | 385.12 | 477.75 | 515.125 |
| Application Method | 0 ppm (±SE) | 2500 ppm N-Urea (±SE) | 5000 ppm N-Urea (±SE) | 5000 ppm N-Urea + ** Phosphorus (±SE) | Mean |
|---|---|---|---|---|---|
| *** Conventional Urea (46%N) | 91.3 ± 1.11 c | 102.6 ± 1.23 a | 104.12 ± 1.34 a | 104.59 ± 1.43 a | 100.65 |
| *** n-UHA (37%N) | 91.3 ± 1.11 c | 96.49 ± 1.29 b | 96.85 ± 1.27 b | 98.25 ± 1.45 b | 95.72 |
| Mean | 91.3 | 99.54 | 100.42 | 101.42 |
| Application Method | 0 ppm (±SE) | 2500 ppm N-Urea (±SE) | 5000 ppm N-Urea (±SE) | 5000 ppm N-Urea + ** Phosphorus (±SE) | Mean |
|---|---|---|---|---|---|
| *** Conventional Urea (46% N) | 2.73 ± 0.093 d | 5.02 ± 0.073 c | 5.61 ± 0.18 c | 5.49 ± 0.22 c | 4.71 |
| *** n-UHA (37% N) | 2.7 ± 0.093 d | 7.83 ± 0.16 b | 8.78 ± 0.14 b | 10.12 ± 0.19 a | 7.37 |
| Mean | 2.73 | 6.43 | 7.20 | 7.81 |
| Application Method | 0 ppm (±SE) | 2500 ppm N-Urea (±SE) | 5000 ppm N-Urea (±SE) | 5000 ppm N-Urea + ** Phosphorus (±SE) | Mean |
|---|---|---|---|---|---|
| *** Conventional Urea (46% N) | 1.31 ± 0.074 c | 2.67 ± 0.14 b | 2.78 ± 0.17 b | 2.85 ± 0.21 b | 2.40 |
| *** n-UHA (37% N) | 1.31 ± 0.074 c | 5.51 ± 0.20 a | 5.70 ± 0.18 a | 6.22 ± 0.27 a | 4.69 |
| Mean | 1.31 | 4.09 | 4.24 | 4.54 |
| Application Method | 0 ppm (±SE) | 2500 ppm N (±SE) | 5000 ppm N (±SE) | 5000 ppm N + ** P (±SE) | Mean |
|---|---|---|---|---|---|
| *** Conventional Urea (46% N) | 4.05 ± 0.16 e | 7.69 ± 0.19 d | 8.40 ± 0.28 d | 8.35 ± 0.32 d | 7.12 |
| n-UHA (37% N) | 4.05 ± 0.16 e | 13.35 ± 0.29 c | 14.84 ± 0.27 b | 16.34 ± 0.34 a | 12.15 |
| Mean | 4.05 | 10.52 | 11.44 | 12.38 |
| Treatment | Grain | Straw | ||
|---|---|---|---|---|
| CP% | Nitrogen | CP% | Nitrogen | |
| T1 control | 8.1 | 1.3 | 1.5 | 0.24 |
| T2 2500 U | 9.39 | 1.5 | 1.75 | 0.28 |
| T3 5000 U | 8.53 | 1.36 | 1.58 | 0.25 |
| T4 5000 U + P | 9.44 | 1.51 | 1.89 | 0.3 |
| T5 2500 n-UHA | 16.69 | 2.67 | 2.43 | 0.39 |
| T6 5000 n-UHA | 15.63 | 2.5 | 2.58 | 0.41 |
| T7 5000 n-UHA + P | 16.81 | 2.69 | 2.96 | 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Rimawi, M.; Al-Sharif, R.; Ayesh, A.; Mazahrih, N.; Musallam, I.; Al-Tawaha, A.R.; Awabdeh, S.; Al Rjoub, B.; Raya, E.; Awamleh, S. Controlled-Release Urea–Hydroxyapatite Nanohybrid for Foliar Nitrogen and Phosphorus Delivery Enhances Biomass and Grain Yield in Wheat (Triticum aestivum L.). Nitrogen 2025, 6, 72. https://doi.org/10.3390/nitrogen6030072
Al-Rimawi M, Al-Sharif R, Ayesh A, Mazahrih N, Musallam I, Al-Tawaha AR, Awabdeh S, Al Rjoub B, Raya E, Awamleh S. Controlled-Release Urea–Hydroxyapatite Nanohybrid for Foliar Nitrogen and Phosphorus Delivery Enhances Biomass and Grain Yield in Wheat (Triticum aestivum L.). Nitrogen. 2025; 6(3):72. https://doi.org/10.3390/nitrogen6030072
Chicago/Turabian StyleAl-Rimawi, Mayyas, Riyad Al-Sharif, Ayman Ayesh, Naem Mazahrih, Iyad Musallam, Abdel Razzaq Al-Tawaha, Sami Awabdeh, Bayan Al Rjoub, Eva Raya, and Saad Awamleh. 2025. "Controlled-Release Urea–Hydroxyapatite Nanohybrid for Foliar Nitrogen and Phosphorus Delivery Enhances Biomass and Grain Yield in Wheat (Triticum aestivum L.)" Nitrogen 6, no. 3: 72. https://doi.org/10.3390/nitrogen6030072
APA StyleAl-Rimawi, M., Al-Sharif, R., Ayesh, A., Mazahrih, N., Musallam, I., Al-Tawaha, A. R., Awabdeh, S., Al Rjoub, B., Raya, E., & Awamleh, S. (2025). Controlled-Release Urea–Hydroxyapatite Nanohybrid for Foliar Nitrogen and Phosphorus Delivery Enhances Biomass and Grain Yield in Wheat (Triticum aestivum L.). Nitrogen, 6(3), 72. https://doi.org/10.3390/nitrogen6030072







