Enhancing Sustainability in Sugarcane Production Through Effective Nitrogen Management: A Comprehensive Review
Abstract
1. Introduction
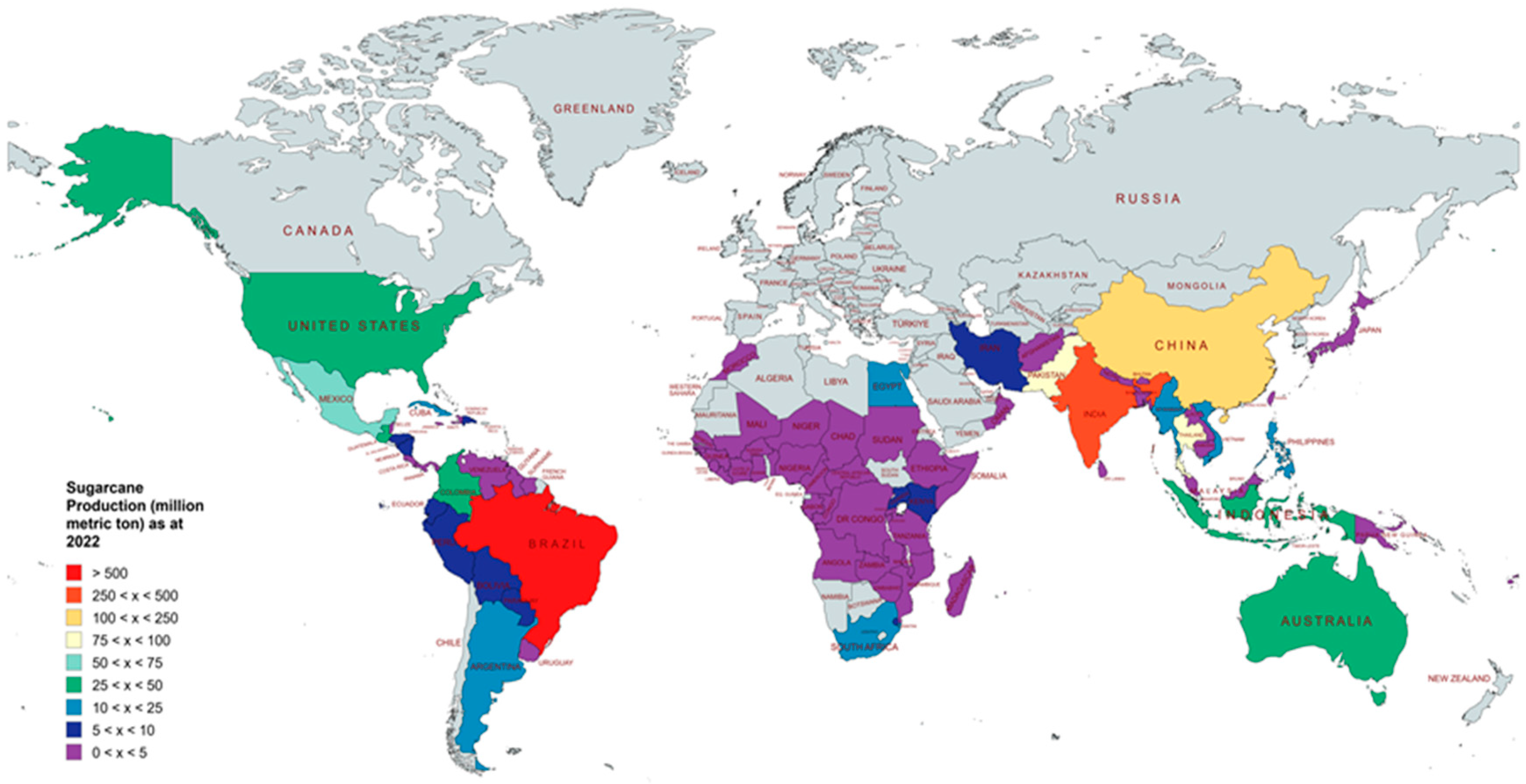
2. Overview of Global Sugarcane Cultivation and Production
3. N Requirement of Sugarcane
4. N Fertiliser Recommendations
| Country | Major N Source | N Fertiliser Recommendations | Reference | |
|---|---|---|---|---|
| Plant Crop (kgN ha−1) | Ratoon Crop (kgN ha−1) | |||
| Brazil | Urea | 40–80 | 100–150 | Otto et al. [31] |
| India | Urea, Ammonium Sulphate | 135–250 | 200 | Shukla et al. [30] |
| Thailand | 200–300 | N/A | Yanai et al. [36] | |
| Australia | Urea, Controlled-release N | 120–160 | 140–180 | Bell et al. [37] |
| South Africa | Urea, Ammonium Nitrate | 80–200 | 100–140 | DOAFF [35] |
| China | Urea | >500 | >500 | Zeng et al. [19] |
| Mexico | Urea, Ammonium Nitrate | 67–112 | 90–135 | Gravois [38] |
| United States | Urea, Ammonium Nitrate, N Solutions | 45–90 | 112–180 | Viator et al. [33] |
| Pakistan | Urea | 173–222 | 173–222 | SCRI [39] |
| Colombia | Urea, Ammonium Nitrate | 67–112 | 90–135 | Gravois [38] |
| Sri Lanka | Urea | 250–300 | 275–325 | SRI [34] |
5. Challenges in N Management Within Sugarcane Farming
5.1. N Losses to the Environment
5.2. Causes for N Losses
5.3. Environmental and Health Consequences of N Losses
6. Sustainable N Management Practices
6.1. Split N Application
| Country | No. of Splits | Split Levels | Main Finding/s | Key Limiting Factor | References |
|---|---|---|---|---|---|
| Brazil | 2 | 50% of the recommendation | Increase in yield | Low soil organic content (SOC) limits N supply | Tenelli et al. [76] |
| 4 | 75%, 13%, 7% and 5% of the recommendation | Increase in sucrose level | Low SOC and mineralisation conditions | Franco et al. [81] | |
| India | 3 | 30, 60 and 90 days after planting | Enhance the quality and quantity of sugarcane for jaggery production | N/A | TNAU [82] |
| 4 | 100% (at planting, 30, 60 and 90 DAP) | Improved shoot population at 120 DAP, stalk population at 240 DAP and millable cane population at harvest | N/A | Lakshmi et al. [84] | |
| 7 | Application rate was 18.99 and 1.64% higher than the recommended level | 23.9% increase in millable stalk count, 10.7% increase in internode length, 82.9% increase in cane-to-top ratio | N/A | Bhilala et al. [83] | |
| 5 | Normal farmer application, 4, 6, 8 and 10 splits | 6 splits N application showed an increase in yield (6 splits > 8 splits > 10 splits > 4 splits > farmer’s practice under drip irrigation) | Flood and furrow irrigation limits the effectiveness of split application | Singh et al. [77] | |
| Pakistan | 2 | 252 kg N ha−1 application rate in 2 equal splits | Higher N rates (336 kg ha−1) also enhanced crop growth rate and leaf area, but had lower NUE. | High temperature limits the growth | Ghaffar et al. [85] |
| Iran | 2 or 3 | 92 kg N ha−1 and an application pattern of 30-30-40% | Increase the juice purity to 90% application | N/A | Koochekzadeh et al. [86] |
6.2. Retention of Crop Residue
6.3. Subsurface Fertiliser Application
6.4. Application Closer to the Root Zone
6.5. Timing of Fertiliser Application
6.6. N Budgeting
6.7. Optimum N Application Rate
6.8. Use of Slow-Release or Controlled-Release N Fertilisers
6.9. Use of Urease Inhibitors
6.10. Use of Nitrification Inhibitors
6.11. Incorporating Biochar
6.12. Precision Agriculture Tools
6.13. Legume Inter or Rotational Cropping
6.14. Application of Biofertilisers
6.15. Site-Specific N Application
6.16. Biotechnology and Genetic Engineering Approach
6.17. Symbiotic Nitrogen Fixation
7. Adopting Simulation Models for N Management
8. Conclusions and Perspectives
- While limited studies have utilised simulation models to aid N management in sugarcane cultivation, such simulation studies have significant potential to serve as supportive tools in N management, as evidenced by their application in other plantation crops. Therefore, there is a need for further simulation studies to be conducted to bolster decision-making processes regarding N management.
- The utilisation of EEFs, including SRFs as well as urease and nitrification inhibitors, remains relatively uncommon within sugarcane agricultural systems. While these methodologies are widely embraced in various other cropping systems, there exists a necessity for further investigations employing recently developed environmentally sustainable EEFs to deepen comprehension of their efficacy within the context of sugarcane cultivation.
- Genetic engineering is in its infancy in the sugarcane industry, with a focus on improving NUE. Only a few studies have reported transgenic sugarcane aimed at enhancing NUE, and no varieties have been released by any country. In the future, genetic improvement through genetic engineering should be a priority.
- Bacteria in a symbiotic relationship with sugarcane have been identified. However, an inoculum containing highly efficient endophytic nitrogen-fixing symbionts has not been developed or released for sugarcane.
- The impact of climate change on the nitrogen cycle in sugarcane systems needs to be thoroughly investigated and understood as a response to increasing extreme weather events.
- Most studies focus on a single or a couple of approaches in improving NUE. Research using an integrated approach to increase NUE in sugarcane production needs to be conducted.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vandenberghe, L.; Valladares-Diestra, K.; Bittencourt, G.A.; Torres, L.Z.; Vieira, S.; Karp, S.G.; Sydney, E.; de Carvalho, J.C.; Soccol, V.T.; Soccol, C.R. Beyond sugar and ethanol: The future of sugarcane biorefineries in Brazil. Renew. Sustain. Energy Rev. 2022, 167, 112721. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAOSTAT). Crops and Livestock Products. 2022. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 15 August 2024).
- Skocaj, D.M.; Everingham, Y.L.; Schroeder, B.L. Nitrogen management guidelines for sugarcane production in Australia: Can these be modified for wet tropical conditions using seasonal climate forecasting? Springer Sci. Rev. 2013, 1, 51–71. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, S.; Chand, M. Nutrient and water management for higher sugarcane production, better juice quality and maintenance of soil fertility-A review. Agric. Rev. 2014, 35, 184–195. [Google Scholar] [CrossRef]
- Hajari, E.; Snyman, S.J.; Watt, M.P. Nitrogen use efficiency of sugarcane (Saccharum spp.) varieties under in vitro conditions with varied N supply. Plant Cell Tissue Organ Cult. (PCTOC) 2015, 122, 21–29. [Google Scholar] [CrossRef]
- McCurdy, M.; Davies, C.; Gunaratnam, A.; Grafton, M.; Bishop, P.; Jeyakumar, P. Instrumentation of a bank of lysimeters: Sensors and sensibility. In Proceedings of the Chemeca, Sydney, Australia, 29 September–2 October 2019. [Google Scholar]
- Gnaratnam, A.; McCurdy, M.; Grafton, M.; Jeyakumar, P.; Bishop, P.; Davies, C. Assessment of nitrogen fertilizers under controlled environment—A lysimeter design. In Proceedings of the Nutrient Loss Mitigations for Compliance in Agriculture, Palmerston North, New Zealand, 12–14 February 2019; Fertilizer and Lime Research Centre, Massey University: Palmerston North, New Zealand; pp. 1–8. [Google Scholar]
- de Castro, S.G.Q.; Decaro, S.T.; Franco, H.C.J.; Graziano Magalhães, P.S.; Garside, A.; Mutton, M.A. Best practices of nitrogen fertilization management for sugarcane under green cane trash blanket in Brazil. Sugar Tech 2017, 19, 51–56. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, Y.; Meng, B.; Zhan, J.; Xi, M.; Deng, Y.; Wu, W.; Lakshmanan, P.; Chen, X.; Zhang, F. High sugarcane yield and large reduction in reactive nitrogen loss can be achieved by lowering nitrogen input. Agric. Ecosyst. Environ. 2024, 369, 109032. [Google Scholar] [CrossRef]
- Deng, Z.; Yin, J.; Eeswaran, R.; Gunaratnam, A.; Wu, J.; Zhang, H. Interacting effects of water and compound fertilizer on the resource use efficiencies and fruit yield of drip-fertigated Chinese wolfberry (Lycium barbarum L.). Technol. Hortic. 2024, 4, e019. [Google Scholar] [CrossRef]
- Abhiram, G.; Eeswaran, R. Legumes for efficient utilization of summer fallow. In Advances in Legumes for Sustainable Intensification; Elsevier: Amsterdam, The Netherlands, 2022; pp. 51–70. [Google Scholar]
- Pan, S.-Y.; He, K.-H.; Lin, K.-T.; Fan, C.; Chang, C.-T. Addressing nitrogenous gases from croplands toward low-emission agriculture. npj Clim. Atmos. Sci. 2022, 5, 43. [Google Scholar] [CrossRef]
- USDA. Production—Sugar. Available online: https://www.fas.usda.gov/data/production/commodity/0612000 (accessed on 20 May 2025).
- Zhao, D.; Li, Y.-R. Climate change and sugarcane production: Potential impact and mitigation strategies. Int. J. Agron. 2015, 2015, 547386. [Google Scholar] [CrossRef]
- Desalegn, B.; Kebede, E.; Legesse, H.; Fite, T. Sugarcane productivity and sugar yield improvement: Selecting variety, nitrogen fertilizer rate, and bioregulator as a first-line treatment. Heliyon 2023, 9, e15520. [Google Scholar] [CrossRef] [PubMed]
- Lofton, J.; Tubaña, B. Effect of nitrogen rates and application time on sugarcane yield and quality. J. Plant Nutr. 2015, 38, 161–176. [Google Scholar] [CrossRef]
- Boschiero, B.N.; Mariano, E.; Torres-Dorante, L.O.; Sattolo, T.M.; Otto, R.; Garcia, P.L.; Dias, C.T.; Trivelin, P.C. Nitrogen fertilizer effects on sugarcane growth, nutritional status, and productivity in tropical acid soils. Nutr. Cycl. Agroecosyst. 2020, 117, 367–382. [Google Scholar] [CrossRef]
- Vieira-Megda, M.X.; Mariano, E.; Leite, J.M.; Franco, H.C.J.; Vitti, A.C.; Megda, M.M.; Khan, S.A.; Mulvaney, R.L.; Trivelin, P.C.O. Contribution of fertilizer nitrogen to the total nitrogen extracted by sugarcane under Brazilian field conditions. Nutr. Cycl. Agroecosyst. 2015, 101, 241–257. [Google Scholar] [CrossRef]
- Zeng, X.-P.; Zhu, K.; Lu, J.-M.; Jiang, Y.; Yang, L.-T.; Xing, Y.-X.; Li, Y.-R. Long-term effects of different nitrogen levels on growth, yield, and quality in sugarcane. Agronomy 2020, 10, 353. [Google Scholar] [CrossRef]
- Wingler, A.; Henriques, R. Sugars and the speed of life—Metabolic signals that determine plant growth, development and death. Physiol. Plant. 2022, 174, e13656. [Google Scholar] [CrossRef]
- Bhatt, R. Resources management for sustainable sugarcane production. In Resources Use Efficiency in Agriculture; Springer: Singapore, 2020; pp. 647–693. [Google Scholar]
- Rozeff, N. A Survey of South Texas Sugarcane Nutrient Studies and Current Fertilizer Recommendations Derived from This Survey. McCormick, L.L., Ed.; American Society of Sugar Cane Technologists: St. Gabriel, LA, USA, 1990; Volume 10, pp. 26–33. [Google Scholar]
- Meyer, J. Sugarcane nutrition and fertilization. In Good Management Practices for the Cane Industry; Verlag Dr. Albert Bartens KG: Berlin, Germany, 2013; pp. 117–168. [Google Scholar]
- Schroeder, B.; Hurney, A.; Wood, A.; Moody, P.; Allsopp, P. Concepts and value of the nitrogen guidelines contained in the Australian sugar industry’s’ SIX EASY STEPS’nutrient management program. In Proceedings of the International Society of Sugar Cane Technologists: Proceedings of the XXVIIth Congress, Veracruz, Mexico, 11 March 2010; pp. 1–13. [Google Scholar]
- Tenelli, S.; Otto, R.; de Castro, S.A.Q.; Sánchez, C.E.B.; Sattolo, T.M.S.; Kamogawa, M.Y.; Pagliari, P.H.; Carvalho, J.L.N. Legume nitrogen credits for sugarcane production: Implications for soil N availability and ratoon yield. Nutr. Cycl. Agroecosyst. 2019, 113, 307–322. [Google Scholar] [CrossRef]
- Bell, M.; Garside, A. Growth and yield responses to amending the sugarcane monoculture: Interactions between break history and nitrogen fertiliser. Crop Pasture Sci. 2014, 65, 287–299. [Google Scholar] [CrossRef]
- Overdahl, C.J.; Rehm, G.W.; Meredith, H.L. Fertilizer Urea; Minnesota Extension Service, University of Minnesota: Saint Paul, MN, USA, 1991. [Google Scholar]
- Templeman, W. Urea as a fertilizer. J. Agric. Sci. 1961, 57, 237–239. [Google Scholar] [CrossRef]
- Alimohammadi, M.; Panahpour, E.; Naseri, A. Assessing the effects of urea and nano-nitrogen chelate fertilizers on sugarcane yield and dynamic of nitrate in soil. Soil Sci. Plant Nutr. 2020, 66, 352–359. [Google Scholar] [CrossRef]
- Shukla, S.K.; Sharma, L.; Awasthi, S.K.; Pathak, A.D. Sugarcane in India: Package of Practices for Different Agro–Climatic Zones; All Indian Coordinated Research Project on Sugarcane, IISR Lucknow, Uttar Pradesh; Maheshwari & Sons: Lucknow, India, 2017; pp. 1–64. [Google Scholar]
- Otto, R.; Franco, H.C.J.; Faroni, C.E.; Vitti, A.C.; de Oliveira, E.C.A.; Sermarini, R.A.; Trivelin, P.C.O. The role of nitrogen fertilizers in sugarcane root biomass under field conditions. Agric. Sci. 2014, 5, 1527–1538. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; Yang, L. Biological nitrogen fixation in sugarcane and nitrogen transfer from sugarcane to cassava in an intercropping system. In Proceedings of the International Society of Sugar Cane Technologists: Proceedings of the XXVIIIth Congress, São Paulo, Brazil, 24–27 June 2013; pp. 204–213. [Google Scholar]
- Viator, H.P.; Johnson, R.M.; Tubana, B.S. How much fertilizer nitrogen does sugarcane need? Sugar J. 2013, 76, 24–26. [Google Scholar]
- SRI. Fertiliser Recommendation for Sugarcane; Division of CN, Sugarcane Research Institute: Lucknow, India, 2024. [Google Scholar]
- DOAFF. Production Guideline, Sugarcane; Department of Agriculture, Forestry and Fisheries: Daejeon, Republic of Korea, 2014. [Google Scholar]
- Yanai, J.; Nakata, S.; Funakawa, S.; Nawata, E.; Katawatin, R.; Kosaki, T. Effect of NPK application on growth, yield and nutrient uptake by sugarcane on a sandy soil in Northeast Thailand. Trop. Agric. Dev. 2010, 54, 113–118. [Google Scholar]
- Bell, M.; Moody, P. Fertilizer N use in the sugarcane industry—An overview and future opportunities. In A Review of Nitrogen Use Efficiency in Sugarcane; Bell, M.J., Ed.; SRA Research Report: Birmingham, UK, 2014; Sugar Research Australia; pp. 305–320. [Google Scholar]
- Gravois, K. Sugarcane Soil Fertility Recommendations for 2024; LSU AgCenter: Shreveport, LA, USA, 2024; pp. 1–5. [Google Scholar]
- SCRI. Sugarcane Nutrition; Sugar Crop Research Institute (SCRI): Daejeon, Republic of Korea, 2024. [Google Scholar]
- Armour, J.; Nelson, P.; Daniells, J.; Rasiah, V.; Inman-Bamber, N. Nitrogen leaching from the root zone of sugarcane and bananas in the humid tropics of Australia. Agric. Ecosyst. Environ. 2013, 180, 68–78. [Google Scholar] [CrossRef]
- Abhiram, G. Contributions of Nano-Nitrogen Fertilizers to Sustainable Development Goals: A Comprehensive Review. Nitrogen 2023, 4, 397–415. [Google Scholar] [CrossRef]
- Abhiram, G.; Grafton, M.; Jeyakumar, P.; Bishop, P.; Davies, C.E.; McCurdy, M. Iron-rich sand promoted nitrate reduction in a study for testing of lignite based new slow-release fertilisers. Sci. Total Environ. 2023, 864, 160949. [Google Scholar] [CrossRef]
- Chen, D.; Suter, H.; Islam, A.; Edis, R.; Freney, J.; Walker, C. Prospects of improving efficiency of fertiliser nitrogen in Australian agriculture: A review of enhanced efficiency fertilisers. Soil Res. 2008, 46, 289–301. [Google Scholar] [CrossRef]
- Thorburn, P.J.; Meier, E.A.; Probert, M.E. Modelling nitrogen dynamics in sugarcane systems: Recent advances and applications. Field Crops Res. 2005, 92, 337–351. [Google Scholar] [CrossRef]
- Scarpare, F.V.; Zotelli, L.d.C.; Barizon, R.; Castro, S.G.Q.d.; Bezerra, A.H.F. Leaching Runoff Fraction for Nitrate and Herbicides on Sugarcane Fields: Implications for Grey Water Footprint. Sustainability 2023, 15, 6990. [Google Scholar] [CrossRef]
- Robertson, F.A.; Thorburn, P.J. Management of sugarcane harvest residues: Consequences for soil carbon and nitrogen. Soil Res. 2007, 45, 13–23. [Google Scholar] [CrossRef]
- Oliveira, B.G.; Lourenço, K.S.; Carvalho, J.L.N.; Gonzaga, L.C.; Teixeira, M.C.; Tamara, A.F.; Soares, J.R.; Cantarella, H. New trends in sugarcane fertilization: Implications for NH3 volatilization, N2O emissions and crop yields. J. Environ. Manag. 2023, 342, 118233. [Google Scholar] [CrossRef]
- Cantarella, H.; Trivelin, P.C.O.; Contin, T.L.M.; Dias, F.L.F.; Rossetto, R.; Marcelino, R.; Coimbra, R.B.; Quaggio, J.A. Ammonia volatilisation from urease inhibitor-treated urea applied to sugarcane trash blankets. Sci. Agríc. 2008, 65, 397–401. [Google Scholar] [CrossRef]
- Denmead, O.; Freney, J.; Jackson, A.; Smith, J.; Saffigna, P.; Wood, A.; Chapman, L. Volatilization of ammonia from urea and ammonium sulfate applied to sugarcane trash in North Queensland. In Proceedings of the Conference of the Australian Society of Sugar Cane Technologists, Townsville, QLD, Australia, 1–4 May 1990; pp. 72–78. [Google Scholar]
- Freney, J.; Denmead, O.; Wood, A.; Saffigna, P. Ammonia loss following urea addition to sugar cane trash blankets. In Proceedings of the 1994 Conference of the Australian Society of Sugar Cane Technologists, Townsville, QLD, Australia, 26–29 April 1994; pp. 114–121. [Google Scholar]
- Friedl, J.; Warner, D.; Wang, W.; Rowlings, D.W.; Grace, P.R.; Scheer, C. Strategies for mitigating N2O and N2 emissions from an intensive sugarcane cropping system. Nutr. Cycl. Agroecosyst. 2023, 125, 295–308. [Google Scholar] [CrossRef]
- Wang, W.; Reeves, S.; Salter, B.; Moody, P.; Dalal, R. Effects of urea formulations, application rates and crop residue retention on N2O emissions from sugarcane fields in Australia. Agric. Ecosyst. Environ. 2016, 216, 137–146. [Google Scholar] [CrossRef]
- Takeda, N.; Friedl, J.; Kirkby, R.; Rowlings, D.; De Rosa, D.; Scheer, C.; Grace, P. Interaction between soil and fertiliser nitrogen drives plant nitrogen uptake and nitrous oxide (N2O) emissions in tropical sugarcane systems. Plant Soil 2022, 477, 647–663. [Google Scholar] [CrossRef]
- Denmead, O.T.; Macdonald, B.; Bryant, G.; Naylor, T.; Wilson, S.; Griffith, D.W.; Wang, W.; Salter, B.; White, I.; Moody, P. Emissions of methane and nitrous oxide from Australian sugarcane soils. Agric. For. Meteorol. 2010, 150, 748–756. [Google Scholar] [CrossRef]
- de Oliveira, M.E.D.; Moraes, S.O. Modeling approaches for agricultural N2O fluxes from large scale areas: A case for sugarcane crops in the state of São Paulo-Brazil. Agric. Syst. 2017, 150, 1–11. [Google Scholar] [CrossRef]
- Degaspari, I.A.M.; Soares, J.R.; Montezano, Z.F.; Del Grosso, S.J.; Vitti, A.C.; Rossetto, R.; Cantarella, H. Nitrogen sources and application rates affect emissions of N 2 O and NH 3 in sugarcane. Nutr. Cycl. Agroecosyst. 2020, 116, 329–344. [Google Scholar] [CrossRef]
- Abhiram, G.; McCurdy, M.; Davies, C.E.; Grafton, M.; Jeyakumar, P.; Bishop, P. An innovative lysimeter system for controlled climate studies. Biosyst. Eng. 2023, 228, 105–119. [Google Scholar] [CrossRef]
- Vasconcelos, A.L.S.; Cherubin, M.R.; Cerri, C.E.; Feigl, B.J.; Reis, A.F.B.; Siqueira-Neto, M. Sugarcane residue and N-fertilization effects on soil GHG emissions in south-central, Brazil. Biomass Bioenergy 2022, 158, 106342. [Google Scholar] [CrossRef]
- Takeda, N.; Friedl, J.; Rowlings, D.; De Rosa, D.; Scheer, C.; Grace, P. No sugar yield gains but larger fertiliser 15N loss with increasing N rates in an intensive sugarcane system. Nutr. Cycl. Agroecosyst. 2021, 121, 99–113. [Google Scholar] [CrossRef]
- Li, Y.-R.; Yang, L.-T. Sugarcane agriculture and sugar industry in China. Sugar Tech 2015, 17, 1–8. [Google Scholar] [CrossRef]
- Prasertsak, P.; Freney, J.; Denmead, O.; Saffigna, P.G.; Prove, B.; Reghenzani, J. Effect of fertilizer placement on nitrogen loss from sugarcane in tropical Queensland. Nutr. Cycl. Agroecosyst. 2002, 62, 229–239. [Google Scholar] [CrossRef]
- Quassi de Castro, S.G.; Costa, V.E.; Quassi de Castro, S.A.; Carvalho, J.L.N.; Borges, C.D.; de Castro, R.A.; Kölln, O.T.; Franco, H.C.J. Fertilizer Application Method Provides an Environmental-Friendly Nitrogen Management Option for Sugarcane. J. Soil Sci. Plant Nutr. 2024, 24, 3195–3208. [Google Scholar] [CrossRef]
- Thorburn, P.J.; Biggs, J.S.; Weier, K.L.; Keating, B.A. Nitrate in groundwaters of intensive agricultural areas in coastal Northeastern Australia. Agric. Ecosyst. Environ. 2003, 94, 49–58. [Google Scholar] [CrossRef]
- Mahmud, K.; Panday, D.; Mergoum, A.; Missaoui, A. Nitrogen losses and potential mitigation strategies for a sustainable agroecosystem. Sustainability 2021, 13, 2400. [Google Scholar] [CrossRef]
- Thorburn, P.J.; Dart, I.K.; Biggs, I.M.; Baillie, C.P.; Smith, M.A.; Keating, B.A. The fate of nitrogen applied to sugarcane by trickle irrigation. Irrig. Sci. 2003, 22, 201–209. [Google Scholar] [CrossRef]
- Rayment, G. Water quality in sugar catchments of Queensland. Water Sci. Technol. 2003, 48, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Vera, I.; Wicke, B.; Hilst, F.v.d. Spatial variation in environmental impacts of sugarcane expansion in Brazil. Land 2020, 9, 397. [Google Scholar] [CrossRef]
- da Silva Paredes, D.; Lessa, A.C.d.R.; de Sant’Anna, S.A.; Boddey, R.M.; Urquiaga, S.; Alves, B.J. Nitrous oxide emission and ammonia volatilization induced by vinasse and N fertilizer application in a sugarcane crop at Rio de Janeiro, Brazil. Nutr. Cycl. Agroecosyst. 2014, 98, 41–55. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Dai, L.; Guo, H.; Huang, Z.; Chen, T.; Huang, Y.; Li, J.; Yang, C.; Abegunrin, T.P. Control of sugarcane planting patterns on slope erosion-induced nitrogen and phosphorus loss and their export coefficients from the watershed. Agric. Ecosyst. Environ. 2022, 336, 108030. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, J.; Hu, M.; Jia, L. Geographical detection of groundwater pollution vulnerability and hazard in karst areas of Guangxi Province, China. Environ. Pollut. 2019, 245, 627–633. [Google Scholar] [CrossRef]
- Fu, T.; Li, C.; Wang, Z.; Qi, C.; Chen, G.; Fu, Y.; Su, Q.; Xu, X.; Liu, W.; Yu, H. Hydrochemical characteristics and quality assessment of groundwater in Guangxi coastal areas, China. Mar. Pollut. Bull. 2023, 188, 114564. [Google Scholar] [CrossRef]
- Sheng, D.; Meng, X.; Wen, X.; Wu, J.; Yu, H.; Wu, M.; Zhou, T. Hydrochemical characteristics, quality and health risk assessment of nitrate enriched coastal groundwater in northern China. J. Clean. Prod. 2023, 403, 136872. [Google Scholar] [CrossRef]
- Chaudhary, I.J.; Chauhan, R.; Kale, S.S.; Gosavi, S.; Rathore, D.; Dwivedi, V.; Singh, S.; Yadav, V.K. Groundwater Nitrate Contamination and its Effect on Human Health: A Review. Water Conserv. Sci. Eng. 2025, 10, 33. [Google Scholar] [CrossRef]
- Mullungal, M.N.; Peediyakkathodi, S.; Bibi, S.; Ratheesh Kumar, C.; Abu-Dieyeh, M. Nutrient contamination in marine environment. In Contaminated Land and Water: Remediation and Management; Springer: New York, NY, USA, 2024; pp. 15–33. [Google Scholar]
- Sowers, K.E.; Pan, W.L.; Miller, B.C.; Smith, J.L. Nitrogen use efficiency of split nitrogen applications in soft white winter wheat. Agron. J. 1994, 86, 942–948. [Google Scholar] [CrossRef]
- Tenelli, S.; Otto, R.; Bordonal, R.O.; Carvalho, J.L.N. How do nitrogen fertilization and cover crop influence soil CN stocks and subsequent yields of sugarcane? Soil Tillage Res. 2021, 211, 104999. [Google Scholar] [CrossRef]
- Singh, H.; Singh, R.; Meena, R.; Kumar, V. Nitrogen fertigation schedule and irrigation effects on productivity and economics of spring sugarcane. Indian J. Agric. Res. 2019, 53, 405–410. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, S.; Jiang, Y.; Lin, Z.; Luo, J.; Li, M.; Guo, J.; Su, Y.; Xu, L.; Que, Y. The physiological and agronomic responses to nitrogen dosage in different sugarcane varieties. Front. Plant Sci. 2019, 10, 406. [Google Scholar] [CrossRef]
- Franco, H.C.J.; Otto, R.; Faroni, C.E.; Vitti, A.C.; de Oliveira, E.C.A.; Trivelin, P.C.O. Nitrogen in sugarcane derived from fertilizer under Brazilian field conditions. Field Crops Res. 2011, 121, 29–41. [Google Scholar] [CrossRef]
- Kingston, G.; Anink, M.; Allen, D. Acquisition of nitrogen by ratoon crops of sugarcane as influenced by waterlogging and split applications. In Proceedings of the 2008 Conference of the Australian Society of Sugar Cane Technologists, Townsville, QLD, Australia, 29 April–2 May 2008; pp. 202–211. [Google Scholar]
- Franco, H.C.J.; Otto, R.; Vitti, A.C.; Faroni, C.E.; Oliveira, E.C.d.A.; Fortes, C.; Ferreira, D.A.; Kölln, O.T.; Garside, A.L.; Trivelin, P.C.O. Residual recovery and yield performance of nitrogen fertilizer applied at sugarcane planting. Sci. Agric. 2015, 72, 528–534. [Google Scholar] [CrossRef]
- TNAU. Nutrient Management: Sugarcane. Available online: https://agritech.tnau.ac.in/agriculture/agri_nutrientmgt_sugarcane.html (accessed on 12 December 2024).
- Bhilala, S.; Rana, L.; Kumar, N.; Kumar, A.; Meena, S.K.; Singh, A. Yield and juice quality in sugarcane influenced by split application of nitrogen and potassium under subtropical climates. Environ. Ecol. 2023, 41, 492–495. [Google Scholar]
- Lakshmi, M.B.; Srilatha, T.; Ramanamurthy, K.; Devi, T.C.; Gouri, V.; Kumari, M. Response of sugarcane to split application of N and K under seedling cultivation. Int. J. Bio-Resour. Stress Manag. 2020, 11, 8–13. [Google Scholar] [CrossRef]
- Ghaffar, A.; Anjum, S.A.; Cheema, M. Effect of nitrogen on growth and yield of sugarcane. J. Am. Soc. Sugar Cane. Technol. 2012, 32, 75. [Google Scholar]
- Koochekzadeh, A.; Fathi, G.; Gharineh, M.; Siadat, S.; Jafari, S.; Alarni-Saeid, K. Impacts of Rate and Split Application ofN Fertilizer on Sugarcane Quality. Int. J. Agric. Res. 2009, 4, 116–123. [Google Scholar] [CrossRef]
- Calcino, D.; Makepeace, P. Fertiliser placement on green cane trash blanketed ratoons in north Queensland. In Proceedings of the Australian Society of Sugar Cane Technologists, Mackay, QLD, Australia; 1988; pp. 125–130. [Google Scholar]
- Agrawal, S.; Saikanth, D.; Mangaraj, A.; Jena, L.; Boruah, A.; Talukdar, N.; Bahadur, R.; Ashraf, S. Impact of crop residue management on crop productivity and soil health: A review. Int. J. Stat. Appl. Math. 2023, SP-8, 599–605. [Google Scholar]
- Madala, H.V.; Lesmes-Vesga, R.A.; Odero, C.D.; Sharma, L.K.; Sandhu, H.S. Effects of planting pre-germinated buds on stand establishment in sugarcane. Agronomy 2023, 13, 1001. [Google Scholar] [CrossRef]
- Wei, Q.; Xu, J.; Liu, Y.; Wang, D.; Chen, S.; Qian, W.; He, M.; Chen, P.; Zhou, X.; Qi, Z. Nitrogen losses from soil as affected by water and fertilizer management under drip irrigation: Development, hotspots and future perspectives. Agric. Water Manag. 2024, 296, 108791. [Google Scholar] [CrossRef]
- Singh, K.; Mishra, S.K.; Brar, A.S. Optimizing Sugarcane and Water Productivity Through Surface and Subsurface Drip Fertigation in Subtropical India. Sugar Tech 2024, 26, 63–76. [Google Scholar] [CrossRef]
- Chen, G.-F.; Tang, Q.-Z.; Li, Y.-R.; Huang, Y.-Y.; Liu, B.; Xu, L.; Huang, H.-R. Effects of Sub-soil Drip Fertigation on Sugarcane in Field Conditions. Sugar Tech 2012, 14, 418–421. [Google Scholar] [CrossRef]
- Asadu, C.O.; Ezema, C.A.; Ekwueme, B.N.; Onu, C.E.; Onoh, I.M.; Adejoh, T.; Ezeorba, T.P.C.; Ogbonna, C.C.; Otuh, P.I.; Okoye, J.O. Enhanced efficiency fertilizers: Overview of production methods, materials used, nutrients release mechanisms, benefits and considerations. Environ. Pollut. Manag. 2024, 1, 32–48. [Google Scholar] [CrossRef]
- Robinson, N.; Brackin, R.; Vinall, K.; Soper, F.; Holst, J.; Gamage, H.; Paungfoo-Lonhienne, C.; Rennenberg, H.; Lakshmanan, P.; Schmidt, S. Nitrate paradigm does not hold up for sugarcane. PLoS ONE 2011, 6, e19045. [Google Scholar] [CrossRef]
- Govindasamy, P.; Muthusamy, S.K.; Bagavathiannan, M.; Mowrer, J.; Jagannadham, P.T.K.; Maity, A.; Halli, H.M.; GK, S.; Vadivel, R.; TK, D. Nitrogen use efficiency—A key to enhance crop productivity under a changing climate. Front. Plant Sci. 2023, 14, 1121073. [Google Scholar] [CrossRef]
- Thorburn, P. Review of Nitrogen Fertiliser Research in the Australian Sugar Industry; CSIRO Sustainable Ecosystems: Canberra, Australia, 2004; pp. 1–104. [Google Scholar]
- Velayudhan, P.K.; Sivalingam, N.; Jha, G.K.; Singh, A.; Pathak, H. Nitrogen budget of Indian agriculture: Trends, determinants and challenges. Environ. Dev. Sustain. 2024, 26, 10225–10242. [Google Scholar] [CrossRef]
- Raun, W.R.; Johnson, G.V. Improving nitrogen use efficiency for cereal production. Agron. J. 1999, 91, 357–363. [Google Scholar] [CrossRef]
- Sanches, G.M.; Otto, R. A novel approach for determining nitrogen requirement based on a new agronomic principle—Sugarcane as a crop model. Plant Soil 2022, 472, 29–43. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Enhancing Nitrogen Use Efficiency in Crop Plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar]
- Abhiram, G.; Grafton, M.; Jeyakumar, P.; Bishop, P.; Davies, C.E.; McCurdy, M. The nitrogen dynamics of newly developed lignite-based controlled-release fertilisers in the soil-plant cycle. Plants 2022, 11, 3288. [Google Scholar] [CrossRef]
- Rathnappriya, R.; Sakai, K.; Okamoto, K.; Kimura, S.; Haraguchi, T.; Nakandakari, T.; Setouchi, H.; Bandara, W. Examination of the effectiveness of controlled release fertilizer to balance sugarcane yield and reduce nitrate leaching to groundwater. Agronomy 2022, 12, 695. [Google Scholar] [CrossRef]
- da Silva, P.C.R.; Paiva, P.E.B.; Charlo, H.C.d.O.; Coelho, V.P.d.M. Slow release fertilizers or fertigation for sugarcane and passion fruit seedlings? Agronomic performance and costs. J. Soil Sci. Plant Nutr. 2020, 20, 2175–2181. [Google Scholar] [CrossRef]
- Soares, J.R.; Cantarella, H.; Vargas, V.P.; Carmo, J.B.; Martins, A.A.; Sousa, R.M.; Andrade, C.A. Enhanced—efficiency fertilizers in nitrous oxide emissions from urea applied to sugarcane. J. Environ. Qual. 2015, 44, 423–430. [Google Scholar] [CrossRef]
- Abhiram, G.; Bishop, P.; Jeyakumar, P.; Grafton, M.; Davies, C.E.; McCurdy, M. Formulation and characterization of polyester-lignite composite coated slow-release fertilizers. J. Coat. Technol. Res. 2023, 20, 307–320. [Google Scholar] [CrossRef]
- Adhikari, K.P.; Saggar, S.; Hanly, J.A.; Guinto, D.F. Urease inhibitors reduced ammonia emissions from cattle urine applied to pasture soil. Nutr. Cycl. Agroecosyst. 2020, 117, 317–335. [Google Scholar] [CrossRef]
- Mira, A.; Cantarella, H.; Souza-Netto, G.J.M.d.; Moreira, L.; Kamogawa, M.Y.; Otto, R. Optimizing urease inhibitor usage to reduce ammonia emission following urea application over crop residues. Agric. Ecosyst. Environ. 2017, 248, 105–112. [Google Scholar] [CrossRef]
- Moreira, L.A.; Otto, R.; Cantarella, H.; Junior, J.L.; Azevedo, R.A.; de Mira, A.B. Urea-versus ammonium nitrate–based fertilizers for green sugarcane cultivation. J. Soil Sci. Plant Nutr. 2021, 21, 1329–1338. [Google Scholar] [CrossRef]
- Gallucci, A.D.; Natera, M.; Moreira, L.A.; Nardi, K.T.; Altarugio, L.M.; de Mira, A.B.; de Almeida, R.F.; Otto, R. Nitrogen-enriched vinasse as a means of supplying nitrogen to sugarcane fields: Testing the effectiveness of N source and application rate. Sugar Tech 2019, 21, 20–28. [Google Scholar] [CrossRef]
- Otto, R.; de Freitas Júnior, J.C.M.; Zavaschi, E.; de Faria, I.K.P.; Paiva, L.A.; Bazani, J.H.; de Mira, A.B.; Kamogawa, M.Y. Combined application of concentrated vinasse and nitrogen fertilizers in sugarcane: Strategies to reduce ammonia volatilization losses. Sugar Tech 2017, 19, 248–257. [Google Scholar] [CrossRef]
- Cerri, C.C.; Maia, S.M.F.; Galdos, M.V.; Cerri, C.E.P.; Feigl, B.J.; Bernoux, M. Brazilian greenhouse gas emissions: The importance of agriculture and livestock. Sci. Agric. 2009, 66, 831–843. [Google Scholar] [CrossRef]
- Signor, D.; Cerri, C.E.P.; Conant, R. N2O emissions due to nitrogen fertilizer applications in two regions of sugarcane cultivation in Brazil. Environ. Res. Lett. 2013, 8, 015013. [Google Scholar] [CrossRef]
- Barth, G.; Otto, R.; Mira, A.B.; Ferraz—Almeida, R.; Vitti, A.C.; Cantarella, H.; Vitti, G.C. Performance of enhanced efficiency nitrogen fertilizers in green—Harvesting sugarcane. Agrosystems Geosci. Environ. 2020, 3, e20015. [Google Scholar] [CrossRef]
- Wen, D.; Valencia, A.; Ordonez, D.; Chang, N.-B.; Wanielista, M. Comparative nitrogen removal via microbial ecology between soil and green sorption media in a rapid infiltration basin for co-disposal of stormwater and wastewater. Environ. Res. 2020, 184, 109338. [Google Scholar] [CrossRef]
- Li, S.; Chen, D.; Wang, C.; Chen, D.; Wang, Q. Reduced nitrification by biochar and/or nitrification inhibitor is closely linked with the abundance of comammox Nitrospira in a highly acidic sugarcane soil. Biol. Fertil. Soils 2020, 56, 1219–1228. [Google Scholar] [CrossRef]
- Migliorati, M.D.A.; Parton, W.J.; Bell, M.J.; Wang, W.; Grace, P.R. Soybean fallow and nitrification inhibitors: Strategies to reduce N2O emission intensities and N losses in Australian sugarcane cropping systems. Agric. Ecosyst. Environ. 2021, 306, 107150. [Google Scholar] [CrossRef]
- Wang, W.; Park, G.; Reeves, S.; Zahmel, M.; Heenan, M.; Salter, B. Nitrous oxide emission and fertiliser nitrogen efficiency in a tropical sugarcane cropping system applied with different formulations of urea. Soil Res. 2016, 54, 572–584. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.; Tang, L.; Heenan, M.; Xu, Z. Effects of nitrification inhibitor and herbicides on nitrification, nitrite and nitrate consumptions and nitrous oxide emission in an Australian sugarcane soil. Biol. Fertil. Soils 2018, 54, 697–706. [Google Scholar] [CrossRef]
- Chen, Y.; Shinogi, Y.; Taira, M. Influence of biochar use on sugarcane growth, soil parameters, and groundwater quality. Soil Res. 2010, 48, 526–530. [Google Scholar] [CrossRef]
- Hamada, K.; Nakamura, S.; Kanda, T.; Takahashi, M. Effects of biochar application depth on nitrate leaching and soil water conditions. Environ. Technol. 2024, 45, 4848–4859. [Google Scholar] [CrossRef]
- Tafti, N.; Wang, J.; Gaston, L.; Park, J.H.; Wang, M.; Pensky, S. Agronomic and environmental performance of biochar amendment in alluvial soils under subtropical sugarcane production. Agrosystems Geosci. Environ. 2021, 4, e20209. [Google Scholar] [CrossRef]
- Eykelbosh, A.J.; Johnson, M.S.; Couto, E.G. Biochar decreases dissolved organic carbon but not nitrate leaching in relation to vinasse application in a Brazilian sugarcane soil. J. Environ. Manag. 2015, 149, 9–16. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, B.; Zhang, Y.; Hu, T.; Lin, Z.; Liu, G.; Wang, X.; Ma, J.; Wang, H.; Jin, H. Biochar application as a tool to decrease soil nitrogen losses (NH3 volatilization, N2O emissions, and N leaching) from croplands: Options and mitigation strength in a global perspective. Glob. Change Biol. 2019, 25, 2077–2093. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Sánchez-Monedero, M.A.; Roig, A.; Hanley, K.; Enders, A.; Lehmann, J. Biochar and denitrification in soils: When, how much and why does biochar reduce N2O emissions? Sci. Rep. 2013, 3, 1732. [Google Scholar] [CrossRef]
- Abbruzzini, T.F.; Zenero, M.D.O.; de Andrade, P.A.M.; Andreote, F.D.; Campo, J.; Cerri, C.E.P. Effects of biochar on the emissions of greenhouse gases from sugarcane residues applied to soils. Agric. Sci. 2017, 8, 869–886. [Google Scholar] [CrossRef]
- Butphu, S.; Rasche, F.; Cadisch, G.; Kaewpradit, W. Eucalyptus biochar application enhances Ca uptake of upland rice, soil available P, exchangeable K, yield, and N use efficiency of sugarcane in a crop rotation system. J. Plant Nutr. Soil Sci. 2020, 183, 58–68. [Google Scholar] [CrossRef]
- Abhiram, G. Slow-Release Fertilisers Control N Losses but Negatively Impact on Agronomic Performances of Pasture: Evidence from a Meta-Analysis. Nitrogen 2024, 5, 1058–1073. [Google Scholar] [CrossRef]
- Shrestha, M.M.; Wei, L. perspectives on the roles of real time nitrogen sensing and IoT integration in smart agriculture. J. Electrochem. Soc. 2024, 171, 027526. [Google Scholar] [CrossRef]
- Amaral, L.R.; Molin, J.P.; Portz, G.; Finazzi, F.B.; Cortinove, L. Comparison of crop canopy reflectance sensors used to identify sugarcane biomass and nitrogen status. Precis. Agric. 2015, 16, 15–28. [Google Scholar] [CrossRef]
- Portz, G.; Molin, J.P.; Jasper, J. Active crop sensor to detect variability of nitrogen supply and biomass on sugarcane fields. Precis. Agric. 2012, 13, 33–44. [Google Scholar] [CrossRef]
- Reyes-Trujillo, A.; Daza-Torres, M.C.; Galindez-Jamioy, C.A.; Rosero-García, E.E.; Muñoz-Arboleda, F.; Solarte-Rodriguez, E. Estimating canopy nitrogen concentration of sugarcane crop using in situ spectroscopy. Heliyon 2021, 7, e06566. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.A.; Fiorio, P.R.; Silva, C.A.A.C.; Demattê, J.A.M.; Silva Barros, P.P.d. Application of vegetative indices for leaf nitrogen estimation in sugarcane using hyperspectral data. Sugar Tech 2024, 26, 160–170. [Google Scholar] [CrossRef]
- Raymond Hunt, E., Jr.; Daughtry, C.S. Chlorophyll meter calibrations for chlorophyll content using measured and simulated leaf transmittances. Agron. J. 2014, 106, 931–939. [Google Scholar] [CrossRef]
- Dinh, T.H.; Watanabe, K.; Takaragawa, H.; Nakabaru, M.; Kawamitsu, Y. Photosynthetic response and nitrogen use efficiency of sugarcane under drought stress conditions with different nitrogen application levels. Plant Prod. Sci. 2017, 20, 412–422. [Google Scholar] [CrossRef]
- Cerqueira, G.; Santos, M.; Marchiori, P.; Silveira, N.; Machado, E.; Ribeiro, R. Leaf nitrogen supply improves sugarcane photosynthesis under low temperature. Photosynthetica 2019, 57, 18–26. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Masoudi, H.; Sajjadiyeh, S.M.; Abdanan Mehdizadeh, S. The determination of Nitrogen Content and Chlorophyll of Sugarcane Crop using Regression Modelling from Color Indices of Aerial Digital Images. Agric. Eng. 2019, 42, 83–98. [Google Scholar]
- You, H.; Zhou, M.; Zhang, J.; Peng, W.; Sun, C. Sugarcane nitrogen nutrition estimation with digital images and machine learning methods. Sci. Rep. 2023, 13, 14939. [Google Scholar] [CrossRef]
- Singh, I.; Srivastava, A.K.; Chandna, P.; Gupta, R.K. Crop sensors for efficient nitrogen management in sugarcane: Potential and constraints. Sugar Tech. 2006, 8, 299–302. [Google Scholar] [CrossRef]
- Mao, Z.-H.; Deng, L.; Duan, F.-Z.; Li, X.-J.; Qiao, D.-Y. Angle effects of vegetation indices and the influence on prediction of SPAD values in soybean and maize. Int. J. Appl. Earth Obs. Geoinf. 2020, 93, 102198. [Google Scholar] [CrossRef]
- Park, S.E.; Webster, T.J.; Horan, H.L.; James, A.T.; Thorburn, P.J. A legume rotation crop lessens the need for nitrogen fertiliser throughout the sugarcane cropping cycle. Field Crops Res. 2010, 119, 331–341. [Google Scholar] [CrossRef]
- Liang, K. Sustainable sugarcane cultivation: The impact of biological nitrogen fixation on reducing fertilizer use. Field Crop 2024, 7. [Google Scholar]
- Gebrewold, A.Z. Review on integrated nutrient management of tea (Camellia sinensis L.). Cogent Food Agric. 2018, 4, 1543536. [Google Scholar] [CrossRef]
- Misra, G. Response of sugarcane to green manuring under North Indian conditions. Indian Sugar 1971, 20, 789–793. [Google Scholar]
- Otto, R.; Pereira, G.L.; Tenelli, S.; Carvalho, J.L.N.; Lavres, J.; de Castro, S.A.Q.; Lisboa, I.P.; Sermarini, R.A. Planting legume cover crop as a strategy to replace synthetic N fertilizer applied for sugarcane production. Ind. Crops Prod. 2020, 156, 112853. [Google Scholar] [CrossRef]
- Khandagave, R. Agronomic management of intercropping in sugarcane and its economic implications. In Proceedings of the International Society of Sugar Cane Technologists: Proceedings of the XXVIIth Congress, Veracruz, Mexico, 7–11 March 2010; p. 63. [Google Scholar]
- Bhander, P.; Bhuiya, M.; Salam, M. Effect of Sesbania rostrata biomass and nitrogen fertilizer on the yield and yield attributes of transplant Amam rice. Progress. Agric. 1998, 9, 89–93. [Google Scholar]
- Shukla, S.; Solomon, S.; Sharma, L.; Jaiswal, V.; Pathak, A.; Singh, P. Green technologies for improving cane sugar productivity and sustaining soil fertility in sugarcane-based cropping system. Sugar Tech 2019, 21, 186–196. [Google Scholar] [CrossRef]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Viaud, P.; Heuclin, B.; Letourmy, P.; Christina, M.; Versini, A.; Mansuy, A.; Chetty, J.; Naudin, K. Sugarcane yield response to legume intercropped: A meta-analysis. Field Crops Res. 2023, 295, 108882. [Google Scholar] [CrossRef]
- Garside, A.; Bell, M. Fallow legumes in the Australian sugar industry: Review of recent research findings and implications for the sugarcane cropping system. In Proceedings of the 2001 Conference of the Australian Society of Sugar Cane Technologists, Mackay, QLD, Australia, 1–4 May 2001; pp. 230–235. [Google Scholar]
- Ambrosano, E.J.; Cantarella, H.; Ambrosano, G.M.B.; Schammas, E.A.; Dias, F.L.F.; Rossi, F.; Trivelin, P.C.O.; Muraoka, T.; Sachs, R.C.C.; Azcón, R. Productivity of sugarcane after previous legumes crop. Bragantia 2011, 70, 810–818. [Google Scholar] [CrossRef]
- Qiu, Z.; Paungfoo—Lonhienne, C.; Ye, J.; Garcia, A.G.; Petersen, I.; Di Bella, L.; Hobbs, R.; Ibanez, M.; Heenan, M.; Wang, W. Biofertilizers can enhance nitrogen use efficiency of sugarcane. Environ. Microbiol. 2022, 24, 3655–3671. [Google Scholar] [CrossRef]
- Yadav, K.K.; Smritikana Sarkar, S.S. Biofertilizers, impact on soil fertility and crop productivity under sustainable agriculture. Environ. Ecol. 2019, 37, 89–93. [Google Scholar]
- Aguado-Santacruz, G.A.; Arreola-Tostado, J.M.; Aguirre-Mancilla, C.; García-Moya, E. Use of systemic biofertilizers in sugarcane results in highly reproducible increments in yield and quality of harvests. Heliyon 2024, 10, e28750. [Google Scholar] [CrossRef]
- de Mendonça, H.V.; Martins, C.E.; da Rocha, W.S.D.; Borges, C.A.V.; Ometto, J.P.H.B.; Otenio, M.H. Biofertilizer replace urea as a source of nitrogen for sugarcane production. Water Air Soil Pollut. 2018, 229, 1–7. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Fertility management of tropical acid soil for sustainable crop production. In Handbook of Soil Acidity; CRC Press: Boca Raton, FL, USA, 2003; pp. 373–400. [Google Scholar]
- Lofton, J.; Tubana, B.S.; Kanke, Y.; Teboh, J.; Viator, H.; Dalen, M. Estimating sugarcane yield potential using an in-season determination of normalized difference vegetative index. Sensors 2012, 12, 7529–7547. [Google Scholar] [CrossRef] [PubMed]
- Sanches, G.M.; Magalhães, P.S.; Kolln, O.T.; Otto, R.; Rodrigues, F., Jr.; Cardoso, T.F.; Chagas, M.F.; Franco, H.C. Agronomic, economic, and environmental assessment of site-specific fertilizer management of Brazilian sugarcane fields. Geoderma Reg. 2021, 24, e00360. [Google Scholar] [CrossRef]
- Landell, M.G.d.A.; Prado, H.d.; Vasconcelos, A.C.M.d.; Perecin, D.; Rossetto, R.; Bidoia, M.A.P.; Silva, M.d.A.; Xavier, M.A. Oxisol subsurface chemical attributes related to sugarcane productivity. Sci. Agric. 2003, 60, 741–745. [Google Scholar] [CrossRef]
- Elwali, A.; Gascho, G. Soil testing, foliar analysis, and DRIS as guides for sugarcane fertilization 1. Agron. J. 1984, 76, 466–470. [Google Scholar] [CrossRef]
- Kumara, A.D.S.; Bandara, D.C. Influence of nitrogen application and varietal differences on selected physiological parameters of sugarcane. Trop. Agric. Res. 2001, 13, 220–230. [Google Scholar]
- Snyman, S.; Hajari, E.; Watt, M.; Lu, Y.; Kridl, J. Improved nitrogen use efficiency in transgenic sugarcane: Phenotypic assessment in a pot trial under low nitrogen conditions. Plant Cell Rep. 2015, 34, 667–669. [Google Scholar] [CrossRef]
- Ye, J.Y.; Tian, W.H.; Jin, C.W. Nitrogen in plants: From nutrition to the modulation of abiotic stress adaptation. Stress Biol. 2022, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Yang, Y.; Yang, Y.; Zhang, X.; Su, Y.; Guo, J.; Que, Y.; Xu, L. Identification of low-nitrogen-related miRNAs and their target genes in sugarcane and the role of miR156 in nitrogen assimilation. Int. J. Mol. Sci. 2022, 23, 13187. [Google Scholar] [CrossRef] [PubMed]
- Garnett, T.; Plett, D.; Heuer, S.; Okamoto, M. Genetic approaches to enhancing nitrogen-use efficiency (NUE) in cereals: Challenges and future directions. Funct. Plant Biol. 2015, 42, 921–941. [Google Scholar] [CrossRef]
- Tiong, J.; Sharma, N.; Sampath, R.; MacKenzie, N.; Watanabe, S.; Metot, C.; Lu, Z.; Skinner, W.; Lu, Y.; Kridl, J. Improving nitrogen use efficiency through overexpression of alanine aminotransferase in rice, wheat, and barley. Front. Plant Sci. 2021, 12, 628521. [Google Scholar] [CrossRef]
- Salesse-Smith, C.E.; Wang, Y.; Long, S.P. Increasing Rubisco as a simple means to enhance photosynthesis and productivity now without lowering nitrogen use efficiency. New Phytol. 2025, 245, 951–965. [Google Scholar] [CrossRef]
- Mehnaz, S. Plant growth-promoting bacteria associated with sugarcane. In Bacteria in Agrobiology: Crop Ecosystems; Springer: New York, NY, USA, 2011; pp. 165–187. [Google Scholar]
- Singh, R.K.; Singh, P.; Li, H.-B.; Song, Q.-Q.; Guo, D.-J.; Solanki, M.K.; Verma, K.K.; Malviya, M.K.; Song, X.-P.; Lakshmanan, P. Diversity of nitrogen-fixing rhizobacteria associated with sugarcane: A comprehensive study of plant-microbe interactions for growth enhancement in Saccharum spp. BMC Plant Biol. 2020, 20, 220. [Google Scholar] [CrossRef] [PubMed]
- James, E.K.; Reis, V.M.; Olivares, F.L.; Baldani, J.I.; Döbereiner, J. Infection of sugar cane by the nitrogen-fixing bacterium Acetobacter diazotrophicus. J. Exp. Bot. 1994, 45, 757–766. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, W.; He, H.; Wang, Z.; Cao, Y. Effects of sugarcane and soybean intercropping on the nitrogen-fixing bacterial community in the rhizosphere. Front. Microbiol. 2021, 12, 713349. [Google Scholar] [CrossRef]
- Colasante, A.; Alfarano, S.; Camacho-Cuena, E.; Gallegati, M. Long-run expectations in a learning-to-forecast experiment: A simulation approach. J. Evol. Econ. 2020, 30, 75–116. [Google Scholar] [CrossRef]
- Stewart, L.; Charlesworth, P.; Bristow, K.; Thorburn, P. Estimating deep drainage and nitrate leaching from the root zone under sugarcane using APSIM-SWIM. Agric. Water Manag. 2006, 81, 315–334. [Google Scholar] [CrossRef]
- van der Laan, M.; Miles, N.; Annandale, J.; Du Preez, C. Identification of opportunities for improved nitrogen management in sugarcane cropping systems using the newly developed Canegro-N model. Nutr. Cycl. Agroecosyst. 2011, 90, 391–404. [Google Scholar] [CrossRef]
- Thorburn, P.J.; Biggs, J.S.; Collins, K.; Probert, M. Using the APSIM model to estimate nitrous oxide emissions from diverse Australian sugarcane production systems. Agric. Ecosyst. Environ. 2010, 136, 343–350. [Google Scholar] [CrossRef]
- Chen, B.; Liu, E.; Tian, Q.; Yan, C.; Zhang, Y. Soil nitrogen dynamics and crop residues. A review. Agron. Sustain. Dev. 2014, 34, 429–442. [Google Scholar] [CrossRef]
- Pasquel, D.; Roux, S.; Richetti, J.; Cammarano, D.; Tisseyre, B.; Taylor, J.A. A review of methods to evaluate crop model performance at multiple and changing spatial scales. Precis. Agric. 2022, 23, 1489–1513. [Google Scholar] [CrossRef]
- Bellocchi, G.; Rivington, M.; Donatelli, M.; Matthews, K. Validation of biophysical models: Issues and methodologies. A review. Agron. Sustain. Dev. 2010, 30, 109–130. [Google Scholar] [CrossRef]
- Donatelli, M.; Magarey, R.D.; Bregaglio, S.; Willocquet, L.; Whish, J.P.; Savary, S. Modelling the impacts of pests and diseases on agricultural systems. Agric. Syst. 2017, 155, 213–224. [Google Scholar] [CrossRef] [PubMed]


| Simulation Model | Prediction | Key Finding | Challenge | Reference |
|---|---|---|---|---|
| APSIM-SWIM | NO3− leaching | The prediction was reasonable | Preferential flow minimises the accuracy | Stewart et al. [173] |
| CANEGRO | NO3− leaching | Prediction accuracy ranged between 0.95 and 0.98 | - | van der Laan et al. [174] |
| APSIM | N2O emission | A close relationship between observed and predicted values | Lower concentrations of N2O highly impact the results | Thorburn et al. [175] |
| DNDC | N2O emission | The IPCC method underestimates the emission compared to the DNDC model | Data availability | de Oliveira et al. [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abhiram, G.; Gopalasingam, T.; Inthujan, J. Enhancing Sustainability in Sugarcane Production Through Effective Nitrogen Management: A Comprehensive Review. Nitrogen 2025, 6, 69. https://doi.org/10.3390/nitrogen6030069
Abhiram G, Gopalasingam T, Inthujan J. Enhancing Sustainability in Sugarcane Production Through Effective Nitrogen Management: A Comprehensive Review. Nitrogen. 2025; 6(3):69. https://doi.org/10.3390/nitrogen6030069
Chicago/Turabian StyleAbhiram, Gunaratnam, Thibiha Gopalasingam, and Jeyarethinam Inthujan. 2025. "Enhancing Sustainability in Sugarcane Production Through Effective Nitrogen Management: A Comprehensive Review" Nitrogen 6, no. 3: 69. https://doi.org/10.3390/nitrogen6030069
APA StyleAbhiram, G., Gopalasingam, T., & Inthujan, J. (2025). Enhancing Sustainability in Sugarcane Production Through Effective Nitrogen Management: A Comprehensive Review. Nitrogen, 6(3), 69. https://doi.org/10.3390/nitrogen6030069






